Abstract
Overexpression of epidermal growth factor receptor in breast cancer is associated with estrogen receptor negativity, higher histological grade and larger tumors. The aim of the present study was to evaluate the clinical significance of serum EGFR (sEGFR) in relation to circulating tumor cells (CTCs) in metastatic breast cancer. 252 patients were enrolled in this prospective multicentre study. Blood was drawn before start of a new line of therapy. sEGFR was determined using a sandwich-type ELISA. CTCs were detected using CellSearch. sEGFR was determined in 48 healthy controls and 252 patients, with no significant differences between the two groups. Clinical-pathological parameters did not correlate with sEGFR, irrespective of the cutoff chosen. Patients with sEGFR levels above the 50th and 75th percentile were more likely to present with <5 CTCs per 7.5 ml blood (p = 0.007; p = 0.003). Patients with sEGFR ≥73 ng/ml had significantly longer overall survival than those with sEGFR <73 ng/ml (19.7 vs. 15.2 months; p = 0.007). In the multivariate analysis, presence of ≥5 CTCs, higher grading and higher line of therapy remained independent predictors of shorter OS, while only higher line of therapy and presence of ≥5 CTCs were independent predictors of shorter PFS.
Similar content being viewed by others
Introduction
The family of erbB receptors consists of four closely related transmembrane proteins (erbB1, erbB2, erbB3, erbB4) involved in a network of signalling pathways that have been shown to play a major role in malignant transformation of various epithelial tumors1,2,3,4. In breast cancer (BC), the most extensively studied member of the erbB family is the erbB2 receptor, also known as HER2, which is overexpressed by 15–20% of primary tumors and serves as a target for highly effective antibody-based therapies. While evidence exists to support HER2 activity to be directly linked to enhanced mobility and invasiveness of cancer cells, data on the clinical relevance of the first discovered protein of the erbB family, the erbB1 receptor, is less conclusive5. Commonly referred to as the epidermal growth factor receptor (EGFR), erbB1 undergoes conformational changes upon binding of a specific ligand, inducing downstream signal transduction by various pathways.
EGFR expression in the tumor tissue can be assessed by immunohistochemistry and in situ hybridization, resulting in overexpression rates in BC patients ranging from 6 to 60%, depending on the method used6,7,8,9,10,11. EGFR overexpression has been shown to correlate with higher histological grade and estrogen receptor (ER) negativity; larger and inflammatory tumors are more likely to be EGFR-positive7,11,12. The majority of published studies reported worse clinical outcome in patients with EGFR-overexpressing tumors13,14,15. Since all erbB receptors share a similar structure containing an extracellular ligand-binding domain (ECD) that may be shed from the cell surface into the blood stream, attempts have been made to measure EGFR levels in the serum using enzyme-based assays and evaluate its clinical utility as a biomarker16,17,18. Interestingly, studies have shown that BC patients have lower serum EGFR (sEGFR) levels than age-matched healthy controls, while in other tumor entities, such as glioblastoma and head and neck squamous cell carcinoma, sEGFR levels in patient samples were significantly higher than in controls17,19,20,21,22. Decreased sEGFR levels predicted shorter overall survival in metastatic BC in several trials, however, survival did not correlate with sEGFR in other studies17,18,23,24. Therefore, the prognostic relevance of sEGFR remains to be further clarified.
In the context of blood-based biomarkers, detection of circulating tumor cells (CTCs) is currently the most promising tool to predict prognosis and monitor treatment with a large body of evidence in both early and metastatic BC25,26,27. While small studies have aimed at exploring the relationship between sEGFR and CTCs in lung and colorectal cancer, data on breast cancer are limited. The aim of the present study was to evaluate the clinical significance of sEGFR levels in relation to the CTCs in a large cohort of metastatic BC patients.
Results
Patients’ characteristics
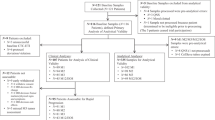
252 patients diagnosed with metastatic breast cancer were included into the analysis. Clinical-pathological data are summarized in Table 1. The median age of patients was 60 years. HER2 was overexpressed by the primary tumor and/or metastasis in 35% of patients; the majority of patients (70%) had a hormone receptor positive tumor. 49.8% of patients presented with ≥ five CTCs per 7.5 ml of peripheral blood. The distribution of patients is summarized in a REMARK diagram (Fig. 1).
sEGFR detection in BC patients and healthy controls
Levels of sEGFR were determined in 48 healthy controls and 252 patients, with no significant differences between the two groups (independent samples t-test; Table 2). Initially, six cutoffs were considered; two were based on the analysis of healthy controls (mean +2 × standard deviation: 97 ng/ml and mean −2 × standard deviation: 29 ng/ml), three on the analysis of the patient cohort (25th percentile: 54 ng/ml, 50th percentile: 62 ng/ml, 75th percentile: 73 ng/ml). In addition, the previously reported cutoff of 45 ng/ml was considered as well17,21. All patients had sEGFR levels above 29 ng/ml, only 9% patients had sEGFR levels above 97 ng/ml and only 9% lower than 45 ng/ml (Table 3). Clinical-pathological parameters, such as hormone receptor and HER2 status, line of therapy, extent of disease and grading, did not correlate with sEGFR levels, irrespective of the cutoff chosen. No correlation was found between serum HER2 levels and sEGFR. Patients with sEGFR levels above the 50th and 75th percentile were more likely to present with <5 CTCs per 7.5 ml blood (p = 0.007 and p = 0.003, respectively; chi-square test). Table 1 shows the results for the cutoff of 73 ng/ml, i.e. 75th percentile.
Univariate survival analysis
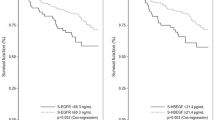
During a median follow up of 19 months, 85 patients died and 183 were diagnosed with progressive disease. Mean overall survival (OS) was 19.7 months (95%-CI: 17.8–21.6 months) in patients with sEGFR ≥73 ng/ml versus 15.2 months (95%-CI: 13.9–16.5) in patients with sEGFR levels <73 ng/ml; median OS has not been reached in either group (p = 0.007) (Fig. 2). No significant impact on OS was observed when other cutoffs were considered (p = 0.057 for 62 ng/ml and p = 0.446 for 54 ng/ml, respectively; Fig. 3). Median progression-free survival (PFS) was 9.4 months (95%-CI: 6.1–12.6) for patients with sEGFR ≥3 ng/ml versus 7.5 months (5.9–9.0 months) with sEGFR levels <73 ng/ml (p = 0.270) (Fig. 2). No significant correlation between PFS and sEGFR was found when other cutoffs were used (Table 3).
As reported previously, positive CTC status was significantly associated with shorter PFS (p = 0.001) and OS (p < 0.001). Patients with sEGFR <73 ng/ml and ≥5 CTCs had the shortest OS (mean 12.3 [95%-CI: 10.6–14.0] months, median 12.3 [95%-CI: 7.5–17.2] months), while patients with sEGFR ≥73 ng/ml and <5 CTCs had the longest OS (mean 21.7 [20.1–23.4] months, median not reached; p < 0.001; Fig. 4).
Multivariate survival analysis
Classical clinical-pathological factors as well as CTC status, sEGFR and sHER2 were included into a multivariate Cox regression analysis. In the multivariate analysis, presence of ≥5 CTCs, higher grading and higher line of therapy remained independent predictors of shorter OS. Only higher line of therapy and elevated CTC counts were independent predictors of shorter PFS in the multivariate analysis (Table 4).
Discussion
To our knowledge, this is the first study to address the clinical relevance of both sEGFR and CTCs in a large cohort of metastatic breast cancer patients. Previous analyses provided contradictory results on the range of normal values of sEGFR, with several studies showing decreased sEGFR levels in cancer patients compared with healthy controls, while others reported increased values in patients or found no significant differences between patients and controls17,18,22,28. Therefore, we performed an analysis of serum samples from 48 age-matched healthy females to determine the clinical utility of various cutoff values.
We found similar sEGFR values in healthy controls and breast cancer patients with a median value of 60.35 and 62.00 ng/ml, respectively. This is in accordance with several other studies18,21,29. Lafky et al. examined sEGFR levels using an acridinium-linked immunosorbent assay in 64 hormone receptor positive metastatic BC patients before start of a new treatment and compared them to 43 matched healthy women with no differences found between the two groups18. Further, Sandri et al. reported similar sEGFR levels in 113 metastatic BC patients and 38 healthy controls using ELISA-based assay21. Similar results were reported in other tumor entities, such as lung cancer30,31, high-grade glioma32 and malignant thymoma33. In malignant pleural mesothelioma22 and head and neck squamous cell carcinoma20 sEGFR levels were found to be significantly higher than in controls. Similarly, Tas et al. detected significantly higher sEGFR levels in BC patients than in healthy controls34. In contrast, Asgeirsson et al. reported significantly decreased sEGFR levels in patients with primary or metastatic breast cancer in comparison to healthy controls (primary BC: median 59 ng/ml, metastatic BC: 31 ng/ml, healthy individuals: 75 ng/ml)1. Decreased sEGFR levels have also been found in patients with ovarian cancer and it has been speculated that sEGFR may have potential as a screening or diagnostic test in this entity35.
Among the cutoff values taken into consideration, we observed the highest prognostic significance when the 75th percentile, e.g. 73 ng/ml, was used. Interestingly, the cutoff previously used by other research groups, 45 ng/ml, had little clinical relevance in our patient cohort17,23 (Table 5). By contrast, 73 ng/ml significantly distinguished between patients with good and poor overall survival (p = 0.007 in the univariate analysis). Müller et al. examined serum samples from 101 patients with metastatic BC before start of chemotherapy using the same assay as in our study17. Patients with sEGFR levels below 45 ng/ml had a non-significant trend towards worse survival, while other cutoffs were less likely to discriminate between favorable and poor outcome. One explanation for this discrepancy might be a different disease setting: while blood samples were collected before first-line chemotherapy in all patients in the abovementioned trial, 61% of patients in our study were scheduled to begin second- or later-line of treatment. Several other trials used 45 ng/ml as cutoff and did not evaluate other values. Souder et al. used a similar cutoff value (44.1 ng/ml) in 535 metastatic BC patients and observed a significantly shorter overall survival in those with decreased sEGFR levels24. In contrast to our study, serum samples were examined before start of first-line endocrine therapy in all patients and only postmenopausal patients were enrolled in this trial. Sandri et al. used 45 ng/ml as cutoff because it was the lowest value in a cohort of 38 healthy controls and observed significantly worse survival in patients with decreased sEGFR21. However, another study using the cutoff of 45 ng/ml reported no significant impact of sEGFR levels on the clinical outcome23.
Although the evidence on the prognostic relevance of sEGFR is not conclusive, the majority of published studies, including the present trial, showed worse clinical outcome in BC patients with low sEGFR. This observation might seem at first surprising, since (over)expression of EGFR in the tumor tissue has been shown to predict poor survival13,14,15,36. The largest analysis of EGFR status in tumor tissue has been reported by Rimawi et al.37. 18% of 2,567 stage I-IIIA BC patients had EGFR-positive tumors; EGFR expression in patients who received adjuvant systemic therapy significantly correlated with worse DFS and OS, whereas no correlation was found in untreated patients, suggesting that EGFR expression may be associated with resistance to some forms of systemic treatment. However, one has to keep in mind that all patients whose tumors were evaluated were diagnosed between 1984 and 1999, explaining why a large proportion (46%) of patients received no systemic therapy. Nieto et al. evaluated tissue EGFR expression in 225 patients with locally advanced BC; patients with EGFR expression had significantly shorter relapse-free and overall survival compared to patients with no expression14. The multivariate analysis confirmed tissue EGFR as an independent prognostic factor. Similar results have been reported in early BC: DiGiovanna et al. demonstrated a negative impact of EGFR expression on the disease-free and disease-specific survival in a large cohort of 802 patients15. Interestingly, EGFR overexpression in this study was linked to activation of HER2, suggesting a potential role for treatment regimens targeting EGFR and HER2 in patients with co-expression of both receptors.
Few studies compared EGFR expression in tumor tissue and sEGFR. Witzel et al. assessed EGFR expression in 76 malignant breast tumors using immunohistochemistry and compared it to EGFR levels in serum determined using ELISA23. Median sEGFR levels at the diagnosis of metastatic BC did not differ between patients with EGFR expression and those with no EGFR expression in their primary tumor.
Different hypotheses have been proposed to explain why higher levels of circulating sEGFR predict longer survival, while expression of tissue EGFR is a negative prognostic factor. Obviously, sEGFR is not a simple surrogate of tumor cell load. Indeed, patients with high levels of CTCs were more likely to have decreased sEGFR in our patient cohort. Since this is the first study to measure both CTCs and sEGFR in metastatic BC, we can only speculate on the possible origins of sEGFR. Obviously, CTCs are not likely to be the main source of sEGFR detected. Since other tissues than the tumor itself may produce sEGFR, serum levels might be influenced by modified regulation through the endocrine or paracrine activity of the tumor23. Baron et al. demonstrated that gonadotropic and steroid hormones may modulate EGFR expression in vivo, leading to higher sEGFR levels in healthy premenopausal women than in age-matched men and postmenopausal women38. Further, it has been speculated that cancer cells with increased malignant potential might show a decreased proteolytic cleavage of the extracellular domain of EGFR17. In the context of methodology, we cannot exclude the possibility that different splice variants of sEGFR are released to the serum affecting the results of different assays used. Indeed, Baron et al. developed an acridinium-linked immunosorbent assay (ALISA) to measure sEGFR and reported a broader range of sEGFR concentrations than using the commercially available ELISA kits. Most importantly, the values obtained by the ALISA showed no association with those obtained using ELISA on identical serum samples38,39. Another possible explanation for the association between decreased sEGFR and worse survival has been discussed recently. Maramotti et al. hypothesized that some soluble forms of EGFR might have a physiological and protective role against cancer40. Indeed, circulating EGFR has been shown to inhibit proliferation and cell migration of non-small cell lung cancer cell lines in vitro 41. Interestingly, this effect could be observed only in wild type lines without EGFR mutations.
The most promising potential clinical application of EGFR detection that has been addressed in previous studies is the possibility to select patients who are likely to respond to EGFR-targeted therapy. Several molecules have been developed to block EGFR, such as cetuximab, or the EGFR tyrosine kinase domain, such as erlotinib and gefitinib. In breast cancer, the only approved therapy that targets EGFR is the oral tyrosine kinase inhibitor lapatinib. Although lapatinib targets the activity of both EGFR and HER2 receptor, only the HER2 overexpression is used to identify patients who might derive treatment benefit. Several studies evaluated the role of EGFR expression in predicting response to therapy. In a cancer cell line-based model, Zhang et al. found that sensitivity to lapatinib was independent of EGFR expression level in HER2-positive breast cancer cells42. Similarly, the EGF103009 trial confirmed that patients with HER2-positive inflammatory BC benefit from lapatinib but no clinical activity was observed in patients with EGFR-positive HER2-negative tumors, suggesting that lapatinib exhibits antitumor effects through HER2 receptor rather than EGFR. Whether sEGFR levels predict or influence the response to EGFR-targeted antibodies, remains unclear. It has been speculated that some isoforms of circulating sEGFR may serve as an alternate target for such treatment. Wilken et al. demonstrated that two EGFR-directed antibodies, cetuximab and panitumumab, recognize and bind sEGFR even at very low doses, suggesting that interactions between sEGFR and antibodies may be relevant in calculating the effective dose of these drugs in cancer patients43.
Several studies aimed at exploring the relevance of EGFR expression of CTCs. EGFR-positive CTCs has been detected in a number of tumor types, such as breast, prostate, colorectal, and lung cancer44. Payne et al. measured CTCs at different time points in a cohort of 33 metastatic BC patients and found consistent positivity over time using the CellSearch system45. Further, two studies examined CTC dynamics in metastatic BC patients treated with tyrosine kinase inhibitors46,47. Agelaki et al. performed CTC monitoring using immunocytochemical staining for HER2 and EGFR in patients treated with lapatinib46. Interestingly, the percentage of HER2-positive CTCs decreased during treatment but more patients harbored EGFR-positive CTCs at disease progression than at time of enrollment. Possibly, EGFR expression might contribute to resistance to lapatinib. These observations are further supported by a case report on a patient with progressive metastatic disease whose prolonged response to lapatinib was reflected by a striking decrease in EGFR-positive CTCs48. Kalykaki et al. reported on 17 metastatic patients with detectable CTCs after the completion of prior treatment who received maintenance therapy with gefitinib47. CTC counts decreased by 73% after the first cycle of gefitinib treatment. In patients initially presenting with EGFR-positive CTCs, most detected CTCs after therapy became EGFR-negative. Beyond metastatic disease, several studies addressed the relevance of EGFR status of CTCs in primary BC. Preliminary data suggest an association between EGFR positivity of CTCs and impaired clinical outcome49. Nadal et al. examined blood samples from 89 patients with localized disease and found a decrease in EGFR-positive CTCs during adjuvant chemotherapy50. Remarkably, patients with hormone receptor positive tumors were significantly more likely to present with EGFR-positive CTCs (33% vs. 9% in hormone receptor negative patients), supporting the biological relevance of the cross-talk between growth factor receptor- and ER-mediated pathways for the development of resistance to endocrine therapy51. It has been hypothesized that another pathway might be of relevance for EGFR expression as well. Kallergi et al. analyzed blood samples from 32 CTC-positive BC patients and showed EGFR to be co-expressed with phosphorylated EGFR, pPI3K and pAkt, implying the importance of an activated pathway in CTCs downstream of EGFR that would involve both Akt and PI3K52.
Conclusions
In our study, sEGFR levels in a large cohort of metastatic breast cancer patients did not differ from those detected in healthy controls. Patients with levels above the 75th percentile (73 ng/ml) had a significantly better overall survival but this association was no longer significant when CTC counts, an established biomarker in metastatic BC, were taken into account. Currently, the potentially most promising indication for EGFR measurements is the prediction of response to therapy with drugs targeting the EGFR or other erbB receptors. In the context of tailored treatment, future studies should clarify whether sEGFR levels or expression of EGFR on CTCs may identify patients likely to benefit from EGFR-targeted molecules.
Methods
252 metastatic breast cancer patients from nine German Breast Cancer Centres were enrolled in this prospective, multicentre, open-label, non-randomized study. Blood was drawn before the start of a new line of therapy. Further inclusion criteria were: age 18 years and older, and first diagnosis of metastatic disease or disease progression before start of a new treatment line. Patients with a second primary malignancy (except in situ carcinoma of the cervix or adequately treated cutaneous basal cell carcinoma) were excluded. Blood samples were collected before start of a new line of therapy chosen according to national and institutional standards. Response to therapy was evaluated by computed tomography every 12 weeks. Informed consent was obtained from all individual participants included in the study.
Quantitative analysis of serum EGFR
sEGFR was quantified by a commercially available ELISA (Oncogene Science, formerly Siemens Medical Solutions Diagnostics, now Wilex Inc., MA, USA). This sandwich-type immunoassay uses a mouse monoclonal capture antibody and an alkaline phosphatase labeled mouse monoclonal as detector. Both capture and detector reagents specifically recognize the ECD of EGFR. The capture antibody recognizes a protein domain on the extracellular portion of EGFR, does not inhibit EGF binding, and does not cross react with erbB-2 oncoprotein or human blood group A antigen. To perform the test, an appropriate volume of specimen is incubated in the wells to allow binding of the antigen by the capture antibody. The immobilized antigen is then exposed to the alkaline phosphatase labeled detector antibody. Addition of substrate to the wells allows the catalysis of a chromogen into a colored product, the intensity of which is proportional to the amount of EGFR which has been bound to the plate. Using a microtiter plate reader, the absorbance of the colored product in the Standards and sample wells can be measured simultaneously. Correlating the absorbance values of samples with the Standards allows the investigator to determine the levels of EGFR in a sample. Samples may be assigned a quantitative value of sEGFR in nanograms per mL (ng/ml) of serum or plasma. For the determination of the cutoff, blood samples from 48 age-matched healthy controls were analyzed (Table 2). The sEGFR concentration was estimated from the standard curve. Each sample, standard and control were analyzed in duplicate.
Detection of other biomarkers
CTCs were detected using the CellSearch™ system (Veridex LLC, NJ, USA). Briefly, 7.5 ml peripheral blood were collected into CellSave Tubes and processed according to manufacturer’s instructions. The assay consists of an immunomagnetic enrichment step employing immunomagnetic beads coated with anti-epithelial cell adhesion molecule (EpCAM) antibody, followed by staining with several antibodies. A circulating tumor cell is defined as a CD45-negative cytokeratin-positive cell with a DAPI-stained nucleus. In the current study, CTC-positive patients were defined as those with at least five tumor cells per 7.5 ml blood. Serum HER2 was determined using a commercially available ELISA (Martell Diagnostic Laboratories, Roseville, MN, USA; formerly Wilex Inc, Cambridge, MA, USA), as described previously16. This test is based on the quantitative measurement of the ECD of the HER2 protein and uses one mouse monoclonal antibody to capture the extracellular domain and another one to detect and quantify it. The assay has been cleared by the Food & Drug Administration (FDA) with the recommended cut-off of 15 ng/ml.
Statistical analysis
Chi-squared test and Fisher’s exact test were used to evaluate the relationship between EGFR detection and clinical-pathological factors. The means between the control group and patients were compared using independent samples t-test. In the survival analysis, following primary end points were considered: (1) death and (2) progression. Survival intervals were measured from the time of blood sampling to the time of death or of the first clinical, histological or radiographic diagnosis of progression. We constructed Kaplan–Meier curves and used the log-rank test to assess the univariate significance of the parameters. Cox regression analysis was used for multivariate analysis. All reported p-values are two-sided. Statistical analysis was performed by SPSS, version 18 (SPSS Inc., Chicago, IL, USA). The analysis was performed according to the REporting recommendations for tumor MARKer prognostic studies (REMARK) criteria on reporting of biomarkers53. The primary question was the prognostic impact of sEGFR in the entire patient cohort.
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethical approval
All procedures performed in this study were in accordance with the ethical standard of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local ethical committees of participation institutions.
Informed consent
Informed consent was obtained from all individual participants included in this study.
References
Asgeirsson, K. S. et al. Serum epidermal growth factor receptor and HER2 expression in primary and metastatic breast cancer patients. Breast Cancer Res 9, R75, https://doi.org/10.1186/bcr1788 (2007).
Abd El-Rehim, D. M. et al. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer 91, 1532–1542, https://doi.org/10.1038/sj.bjc.6602184 (2004).
Sasaki, T., Hiroki, K. & Yamashita, Y. The role of epidermal growth factor receptor in cancer metastasis and microenvironment. Biomed Res Int 2013, 546318, https://doi.org/10.1155/2013/546318 (2013).
Bethune, G., Bethune, D., Ridgway, N. & Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis 2, 48–51 (2010).
Harari, D. & Yarden, Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 19, 6102–6114, https://doi.org/10.1038/sj.onc.1203973 (2000).
Wrba, F., Reiner, A., Ritzinger, E., Holzner, J. H. & Reiner, G. Expression of epidermal growth factor receptors (EGFR) on breast carcinomas in relation to growth fractions, estrogen receptor status and morphological criteria. An immunohistochemical study. Pathol Res Pract 183, 25–29 (1988).
Masuda, H. et al. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 136, 331–345, https://doi.org/10.1007/s10549-012-2289-9 (2012).
Bhargava, R. et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol 18, 1027–1033, https://doi.org/10.1038/modpathol.3800438 (2005).
Suo, Z. et al. EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol 196, 17–25, https://doi.org/10.1002/path.1003 (2002).
Hwangbo, W. et al. EGFR Gene Amplification and Protein Expression in Invasive Ductal Carcinoma of the Breast. Korean J Pathol 47, 107–115, https://doi.org/10.4132/KoreanJPathol.2013.47.2.107 (2013).
Magkou, C. et al. Expression of the epidermal growth factor receptor (EGFR) and the phosphorylated EGFR in invasive breast carcinomas. Breast Cancer Res 10, R49, https://doi.org/10.1186/bcr2103 (2008).
Cabioglu, N. et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol 18, 1021–1029, https://doi.org/10.1093/annonc/mdm060 (2007).
Lee, H. J. et al. Prognostic and predictive values of EGFR overexpression and EGFR copy number alteration in HER2-positive breast cancer. Br J Cancer 112, 103–111, https://doi.org/10.1038/bjc.2014.556 (2015).
Nieto, Y., Nawaz, F., Jones, R. B., Shpall, E. J. & Nawaz, S. Prognostic significance of overexpression and phosphorylation of epidermal growth factor receptor (EGFR) and the presence of truncated EGFRvIII in locoregionally advanced breast cancer. J Clin Oncol 25, 4405–4413, https://doi.org/10.1200/JCO.2006.09.8822 (2007).
DiGiovanna, M. P. et al. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol 23, 1152–1160, https://doi.org/10.1200/JCO.2005.09.055 (2005).
Banys-Paluchowski, M. et al. Clinical Relevance of Serum HER2 and Circulating Tumor Cell Detection in Metastatic Breast Cancer Patients. Anticancer Res 37, 3117–3128, https://doi.org/10.21873/anticanres.11669 (2017).
Muller, V. et al. Prognostic and predictive impact of soluble epidermal growth factor receptor (sEGFR) protein in the serum of patients treated with chemotherapy for metastatic breast cancer. Anticancer Res 26, 1479–1487 (2006).
Lafky, J. M. et al. Serum soluble epidermal growth factor receptor concentrations decrease in postmenopausal metastatic breast cancer patients treated with letrozole. Cancer Res 65, 3059–3062, https://doi.org/10.1158/0008-5472.CAN-05-0067 (2005).
Quaranta, M. et al. Epidermal growth factor receptor serum levels and prognostic value in malignant gliomas. Tumori 93, 275–280 (2007).
Polanska, H. et al. Evaluation of EGFR as a prognostic and diagnostic marker for head and neck squamous cell carcinoma patients. Oncol Lett 12, 2127–2132, https://doi.org/10.3892/ol.2016.4896 (2016).
Sandri, M. T. et al. Serum EGFR and serum HER-2/neu are useful predictive and prognostic markers in metastatic breast cancer patients treated with metronomic chemotherapy. Cancer 110, 509–517, https://doi.org/10.1002/cncr.22825 (2007).
Gaafar, R. et al. Tissue and serum EGFR as prognostic factors in malignant pleural mesothelioma. Lung Cancer 70, 43–50, https://doi.org/10.1016/j.lungcan.2010.01.002 (2010).
Witzel, I. et al. Clinical utility of determination of HER-2/neu and EGFR fragments in serum of patients with metastatic breast cancer. Int J Biol Markers 21, 131–140 (2006).
Souder, C. et al. Serum epidermal growth factor receptor/HER-2 predicts poor survival in patients with metastatic breast cancer. Cancer 107, 2337–2345, https://doi.org/10.1002/cncr.22255 (2006).
Janni, W. et al. Persistence of circulating tumor cells in high risk early breast cancer patients during follow-up care suggests poor prognosis – Results from the adjuvant SUCCESS A trial. 2015 San Antonio Breast Cancer Symposium, S2–03. (2015).
Janni, W. J. et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res 22, 2583–2593, https://doi.org/10.1158/1078-0432.CCR-15-1603 (2016).
Bidard, F. C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15, 406–414, https://doi.org/10.1016/S1470-2045(14)70069-5 (2014).
Baron, A. T. et al. Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 12, 103–113 (2003).
Perez, E. A. et al. A randomized phase II study of sequential docetaxel and doxorubicin/cyclophosphamide in patients with metastatic breast cancer. Ann Oncol 13, 1225–1235 (2002).
Sasaki, H. et al. Elevated serum epidermal growth factor receptor level is correlated with lymph node metastasis in lung cancer. Int J Clin Oncol 8, 79–82, https://doi.org/10.1007/s101470300014 (2003).
Jacot, W., Pujol, J. L., Boher, J. M. & Lamy, P. J. Serum EGF-receptor and HER-2 extracellular domains and prognosis of non-small-cell lung cancer. Br J Cancer 91, 430–433, https://doi.org/10.1038/sj.bjc.6601987 (2004).
Kiviniemi, A. et al. Serum levels of GFAP and EGFR in primary and recurrent high-grade gliomas: correlation to tumor volume, molecular markers, and progression-free survival. J Neurooncol 124, 237–245, https://doi.org/10.1007/s11060-015-1829-7 (2015).
Sasaki, H. et al. Elevated serum epidermal growth factor receptor level in stage IV thymoma. Surg Today 34, 477–479, https://doi.org/10.1007/s00595-003-2722-0 (2004).
Tas, F., Bilgin, E., Karabulut, S. & Duranyildiz, D. Clinical significance of serum epidermal growth factor receptor (EGFR) levels in patients with breast cancer. Cytokine 71, 66–70, https://doi.org/10.1016/j.cyto.2014.09.001 (2015).
Baron, A. T. et al. Soluble epidermal growth factor receptor: a biomarker of epithelial ovarian cancer. Cancer Treat Res 149, 189–202, https://doi.org/10.1007/978-0-387-98094-2_9 (2009).
Buchholz, T. A. et al. Epidermal growth factor receptor expression correlates with poor survival in patients who have breast carcinoma treated with doxorubicin-based neoadjuvant chemotherapy. Cancer 104, 676–681, https://doi.org/10.1002/cncr.21217 (2005).
Rimawi, M. F. et al. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer 116, 1234–1242, https://doi.org/10.1002/cncr.24816 (2010).
Baron, A. T. et al. A preliminary study of serum concentrations of soluble epidermal growth factor receptor (sErbB1), gonadotropins, and steroid hormones in healthy men and women. Cancer Epidemiol Biomarkers Prev 10, 1175–1185 (2001).
Baron, A. T. et al. A sandwich type acridinium-linked immunosorbent assay (ALISA) detects soluble ErbB1 (sErbB1) in normal human sera. J Immunol Methods 219, 23–43 (1998).
Maramotti, S. et al. Soluble Epidermal Growth Factor Receptors (sEGFRs) in Cancer: Biological Aspects and Clinical Relevance. Int J Mol Sci 17, https://doi.org/10.3390/ijms17040593 (2016).
Lococo, F. et al. Preliminary Evidence on the Diagnostic and Molecular Role of Circulating Soluble EGFR in Non-Small Cell Lung Cancer. Int J Mol Sci 16, 19612–19630, https://doi.org/10.3390/ijms160819612 (2015).
Zhang, D. et al. Activity of lapatinib is independent of EGFR expression level in HER2-overexpressing breast cancer cells. Mol Cancer Ther 7, 1846–1850, https://doi.org/10.1158/1535-7163.MCT-08-0168 (2008).
Wilken, J. A., Baron, A. T. & Maihle, N. J. The epidermal growth factor receptor conundrum. Cancer 117, 2358–2360, https://doi.org/10.1002/cncr.25805 (2011).
Yap, T. A., Lorente, D., Omlin, A., Olmos, D. & de Bono, J. S. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res 20, 2553–2568, https://doi.org/10.1158/1078-0432.CCR-13-2664 (2014).
Payne, R. E. et al. Measurements of EGFR expression on circulating tumor cells are reproducible over time in metastatic breast cancer patients. Pharmacogenomics 10, 51–57, https://doi.org/10.2217/14622416.10.1.51 (2009).
Agelaki, S. et al. Efficacy of Lapatinib in Therapy-Resistant HER2-Positive Circulating Tumor Cells in Metastatic Breast Cancer. PLoS One 10, e0123683, https://doi.org/10.1371/journal.pone.0123683 (2015).
Kalykaki, A. et al. Elimination of EGFR-expressing circulating tumor cells in patients with metastatic breast cancer treated with gefitinib. Cancer Chemother Pharmacol 73, 685–693, https://doi.org/10.1007/s00280-014-2387-y (2014).
Liu, Z. et al. Eradication of EGFR-positive circulating tumor cells and objective tumor response with lapatinib and capecitabine. Cancer Biol Ther 10, 860–864, https://doi.org/10.4161/cbt.10.9.13323 (2010).
Serrano, M. J. et al. EMT and EGFR in CTCs cytokeratin negative non-metastatic breast cancer. Oncotarget 5, 7486–7497, https://doi.org/10.18632/oncotarget.2217 (2014).
Nadal, R. et al. Biomarkers characterization of circulating tumour cells in breast cancer patients. Breast Cancer Res 14, R71, https://doi.org/10.1186/bcr3180 (2012).
Osborne, C. K., Shou, J., Massarweh, S. & Schiff, R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 11, 865s–870s (2005).
Kallergi, G. et al. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res 10, R80, https://doi.org/10.1186/bcr2149 (2008).
McShane, L. M. et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93, 387–391, https://doi.org/10.1038/sj.bjc.6602678 (2005).
Rocca, A. et al. Perioperative serum VEGF and extracellular domains of EGFR and HER2 in early breast cancer. Anticancer Res 29, 5111–5119 (2009).
Acknowledgements
The DETECT study was supported by a research grant from Roche Pharma AG, Germany and by Adnagen AG, Germany. ELISA kits were provided at no cost by Oncogene Science, a former part of Siemens Medical Solutions Diagnostics and now part of Wilex. The funding agencies had no role in study design or collection, analysis, and interpretation of data nor in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
T.F., W.J., B.A., P.A.F., S.K.B., K.P., B.R., S.R., E.F.S. and V.M. designed and conducted the study. M.B.P. analyzed and interpreted the data, performed statistical analysis and prepared the manuscript. T.F., I.W., S.R. and V.M. analyzed and interpreted the data and were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banys-Paluchowski, M., Witzel, I., Riethdorf, S. et al. Evaluation of serum epidermal growth factor receptor (EGFR) in correlation to circulating tumor cells in patients with metastatic breast cancer. Sci Rep 7, 17307 (2017). https://doi.org/10.1038/s41598-017-17514-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17514-8
This article is cited by
-
A generalizable nanopore sensor for highly specific protein detection at single-molecule precision
Nature Communications (2023)
-
Correlations between serum cetuximab and EGFR-related markers, and skin disorders in head and neck cancer patients
Cancer Chemotherapy and Pharmacology (2021)
-
Dysregulated EGFR pathway in serum in early-stage breast cancer patients: A case control study
Scientific Reports (2020)
-
Prognostic impact of serum levels of EGFR and EGFR ligands in early-stage breast cancer
Scientific Reports (2020)
-
The prognostic relevance of urokinase-type plasminogen activator (uPA) in the blood of patients with metastatic breast cancer
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.