Abstract
FOLFIRINOX has been one of the first-line options for advanced pancreatic cancer, even though it induces significant adverse effects. Several institutions have begun using modified FOLFIRINOX to decrease its side effects and increase its tolerability. We systematically investigated the outcome from patients who initially received modified FOLFIRINOX as a chemotherapy regimen for advanced pancreatic cancer. We used the random-model generic inverse variance method to analyse the binary data with 95% confidence intervals (CIs). Eleven studies were included in the meta-analysis with 563 total patients. The 6-month and 1-year overall survival (OS) rates of locally advanced pancreatic cancer (LAPC) were 90.9% and 76.2%. The 6-month and 1-year progression-free survival (PFS) rates of LAPC were 81.5% and 48.5%. The 6-month and 1-year OS rates of metastatic pancreatic cancer (MPC) were 79.7% and 47.6%. The 6-month and 1-year PFS rates of MPC were 56.3% and 20.6%. The following rates were also calculated: complete response rate (CR): 2.9%; partial response rate (PR): 35.9%; stable disease rate (SD): 41.2%; overall response rate (OR): 34.6%; disease control rate (DCR): 76.7%; progressive disease: 23.1%; and grade III/IV adverse events (AEs): neutropenia 23.1%, febrile neutropenia 4.8%, thrombocytopenia 4.8%, anaemia 5.7%, fatigue 11.5%, nausea 9.1%, diarrhoea 10.1%, vomiting 5.7%, neuropathy 3.8%, and increased ALT 5.7%. In conclusion, modified FOLFIRINOX could provide comparative survival benefits with fewer adverse events compared to the conventional dosage.
Similar content being viewed by others
Introduction
Pancreatic cancer (PC) has one of the highest cancer mortality rates in the world1. In 2017, the estimated number of deaths from pancreatic cancer was 43,090 in the United States; further, the 5-year relative survival rate was only 8%, and that of the distant stage was only 3%2. Pancreatic cancer is currently the third leading cause of cancer-related deaths in the United States3 and will become the second leading cause in 20304. Because most cases are diagnosed at late stages as either metastatic or locally advanced5,6,7,8, curative surgical resection can be performed in only 15–20% of cases9,10.
Other than surgical resection, systemic chemotherapy is the only major treatment that can improve survival for patients with locally advanced or metastatic pancreatic cancer. Twenty years ago, gemcitabine (GEM) replaced 5-fluorouracil (5-FU) as the main chemotherapeutic drug for treating advanced pancreatic cancer because a modest survival increase (5.65 vs 4.41 months) and more clinical benefits were found in a Phase III clinical trial11. Since then, gemcitabine monotherapy had been the gold standard for pancreatic cancer. Later, numerous clinical trials combined gemcitabine with other anti-tumour agents to increase the anti-tumour effects, but most such studies were unable to demonstrate the superiority of or a significant improvement in OS for gemcitabine combination therapy; only gemcitabine combined with capecitabine and erlotinib have shown promise5,6,12,13.
Recently, in the PRODIGE 4/ACCORD 11 randomized trials, a four-drug regimen called FOLFIRINOX, consisting of folinic acid, 5-fluorouracil, irinotecan and oxaliplatin, was demonstrated to prolong overall survival compared to gemcitabine monotherapy (11.1 months vs 6.8 months). These results suggested that this combined regimen should be used in clinical practice as a first-line option for advanced pancreatic cancer patients14. Shortly thereafter, a regimen of gemcitabine and albumin-bound paclitaxel was shown to have statistically significant survival benefits in OS and PFS, thus providing another choice for treating advanced pancreatic cancer15. However, FOLFIRINOX appears to be more effective than GEM/NAB-P16. Although FOLFIRINOX is a first-line option for patients with metastatic pancreatic cancer, there is a controversy about whether the survival benefits of the four-drug combination regimen outweigh the associated toxicities17. The significant adverse effects induced by this regimen include neutropenia, thrombocytopenia, febrile, diarrhoea neutropenia, febrile neutropenia, thrombocytopenia, diarrhoea, and neuropathy, which limit its usage and require stopping chemotherapy during treatment. Therefore, FOLFIRINOX is usually prescribed for patients ≤76 years old who have a good performance status (ECOG 0 or 1)14. To decrease the side effects and increase its tolerability, several institutions have used modified FOLFIRINOX. We conducted a systematic review and meta-analysis to assess the effectiveness and toxicities of modified FOLFIRINOX for patients with advanced pancreatic cancer compared to the conventional dosage.
Methods
Literature search
A systematic search was conducted to find eligible articles. Two investigators independently searched for prospective or retrospective studies (phase I-III trials, cohort studies, or case series) using Embase, PubMed, Web of Science, Scopus, and Cochrane without an upper-limit date until December 31, 2017. The search criteria included studies of advanced pancreatic cancer patients at any age who received any type of modified FOLFIRINOX in initial chemotherapy without language restrictions and no consideration of subsequent treatment. The preceding original regimen of FOLFIRINOX contained oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 180 mg/m2, 5-fluorouracil (5-FU) bolus 400 mg/m2 and 5-fluorouracil (5-FU) 2400 mg/m2. Modified FOLFIRINOX was defined as at least one of the drugs was reduced and/or the removal of 5-FU bolus in FOLFIRINOX14.
The search strategy was as follows: ((‘folinic acid’/exp AND fluorouracil/exp AND irinotecan/exp AND oxaliplatin/exp AND ‘drug combination’/exp) or (Folfirinox):ab,ti) and (‘pancreas cancer’/de OR ‘pancreas tumor’/de OR ‘pancreas adenoma’/de OR ‘pancreas adenocarcinoma’/de OR ‘pancreas carcinoma’/de OR ‘pancreas islet cell carcinoma’/de OR (pancrea* NEAR/3 (cancer* OR neoplas* OR tumo* OR adenocarcinom* OR carcinom* OR adenom*)):ab,ti). For the detailed search strategy, see the supplement.
After removing duplicate articles, two investigators independently reviewed the abstracts. Studies were excluded if the study type was a review/meta-analysis, case report, comment, letter to the editor, or irrelevant literature. Differences between the investigators were resolved by a third-party investigator’s opinion. Full articles were then selected for further assessment, and articles with only abstracts were excluded. Other exclusion criteria included studies that used a regimen other than modified FOLFIRINOX, did not include the initial usage of modified FOLFIRINOX or dose adjusted by physician’s judgement without a specific time or presented the same patient cohort in another study. For details of the excluded articles, see the Supplement.
Data extraction
General information was extracted from the foregoing selected publications and included the name of article, first authors, the name of journal, year of publication, study design, participating centres, country, observation sites, beginning and ending time, tumour stage, the composition of the modified FOLFIRINOX, its usage, number of patients, the ratio of males and females, average age, duration of follow-up, and performance status.
Survival was evaluated by the OS (6-month and 1-year) and PFS (6-month and 1-year) for the LAPC and MPC groups, which were extracted from the selected publications. If the survival rates were not directly available from the articles or authors, Engauge Digitizer was used to pool survival data from the Kaplan–Meier survival curve in each selected publication, especially for advanced pancreatic cancer reports for which the OS and PFS rates were not provided18. We chose the complete response (CR), partial response (PR), overall response (OR), stable disease (SD), disease control ratio (DCR), and progressive rate to evaluate the objective response to chemotherapy. The adverse events were calculated when they achieved grade III/IV. Grade III/IV toxicity includes neutropenia, febrile neutropenia, thrombocytopenia, anaemia, fatigue, vomiting, nausea, diarrhoea, neuropathy, and increased ALT.
Statistical analysis
First, we used the Critical Appraisal Skill Program (CASP) to evaluate each study (supplement). The CASP is a critical appraisal tool that is used in observational studies to assess the methodological quality of the individual studies. Binary data were meta-analysed with the random-model generic inverse variance method. We used random-effects rather than fixed-effects models because of the heterogeneity in the initial treatment of advanced pancreatic cancer. We used the odds ratio as the effect measure method and then changed it to probability. The I2 statistics reflected the heterogeneity: I2 = 0% indicated no heterogeneity, I2 = (0%,25%) indicated low heterogeneity, I2 = [25%,50%) indicated mild heterogeneity, I2 = [50%,75%) indicated moderate heterogeneity, and I2 = [75%,100%] indicated high heterogeneity19. All analyses were performed in Review Manager version 5.3 and Excel 2010.
Results
Study search
Figure 1 is a flow diagram that shows the selection process for the searched studies. We searched all databases that are available. There were 4772 related studies identified from the initial literature search; 2541 studies were eliminated because of duplications. Only 70 studies were eligible upon abstract screening. After full-text screening, only 11 studies remained, and they were included in the final analysis20,21,22,23,24,25,26,27,28,29,30.
In these 11 studies, there were 563 patients, including 333 MPC and 230 LAPC. The number of patients who were treated with modified FOLFIRINOX ranged from 10 to 137. The average age in each study ranged from 60 to 65 years old (Table 1). Most patients’ performance status was 0 or 1, and a small portion had a score of 223. Most of the studies removed the 5-FU bolus, but two studies reduced the dose from 400 mg/m2 to 300 mg/m2 27,29. There was an overlap of population in one study26,31. The most usage of continuous infusion 5-FU was 2400 mg/m2, but one study increased it to 2800 mg/m2 or 3200 mg/m2 and eliminated the 5-FU bolus28. The most frequently used dose of oxaliplatin was the same as the normal FOLFIRINOX regimen, but two studies used 63.75 mg/m2 and 68 mg/m2 29,31. The dosage of irinotecan ranged from 135 mg/m2 to 180 mg/m2. For the detailed modified FOLFIRINOX regimens, see Table 2.
Survival date
We divided advanced pancreatic cancer into LAPC and MPC to analyse the survival date because of the different prognoses between them. The pooled 6-month and 1-year OS rates of LAPC were 90.9 (95% CI 82.7–95.1%. I2 = 0%, P for Heterogeneity: 0.82) and 76.2% (95% CI 64.5–84.9%. I2 = 37%, P for Heterogeneity: 0.19). The pooled 6-month and 1-year PFS rates of LAPC were 81.5% (95% CI 69.3–89.6%. I2 = 46%, P for Heterogeneity: 0.10) and 48.5% (95% CI 38.7–58.2%. I2 = 27%, P for Heterogeneity: 0.23). The pooled 6-month and 1-year OS rates of MPC were 79.7% (95% CI 74.6–84.1%. I2 = 0%, P for Heterogeneity: 0.56) and 47.6% (95% CI 36.3–58.8%. I2 = 68%, P for Heterogeneity: 0.004). The pooled 6-month and 1-year PFS rates of MPC were 56.3% (95% CI 49.2–63.1%. I2 = 26%, P for Heterogeneity: 0.23) and 20.6% (95% CI 13.8–29.1%. I2 = 54%, P for Heterogeneity: 0.04) (Fig. 2).
Response rates
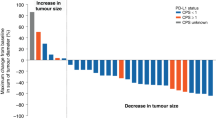
The pooled complete response rate (CR) was 2.9% (95% CI 1.0–10.7%. I2 = 37%, P for Heterogeneity: 0.21). The pooled partial response rate (PR) was 35.9% (95% CI 30.6–41.2%. I2 = 5%, P for Heterogeneity: 0.39). The pooled stable disease rate (SD) was 41.2% (95% CI 29.1–54.5%. I2 = 79%, P for Heterogeneity: <0.0001). The pooled overall response rate (OR) was 34.6% (95% CI 27.5–42.5%. I2 = 44%, P for Heterogeneity: 0.08). The pooled disease control rate (DCR) was 76.7% (95% CI 68.4–83.4%. I2 = 54%, P for Heterogeneity: 0.04). The pooled progressive disease was 23.1% (95% CI 16.7–31.5%. I2 = 54%, P for Heterogeneity: 0.04) (Fig. 3 and Table 3).
Adverse events
There were 344 grade III/IV adverse events in our study (Table 4). Figure 4 shows the pooled event rates for grade III/IV adverse events. The pooled grade III/IV incidences of neutropenia, febrile neutropenia, thrombocytopenia, and anaemia were 23.1% (95% CI 11.5–41.2%. I2 = 89%, P for Heterogeneity: <0.00001), 4.8% (95% CI 1.0–16%. I2 = 70%, P for Heterogeneity: 0.02), 4.8% (95% CI 2.9–8.3%. I2 = 0%, P for Heterogeneity: 0.88), and 5.7% (95% CI 2.9–9.9%. I2 = 36%, P for Heterogeneity: 0.18).
The pooled incidences of non-haematological AEs were as follows: fatigue 11.5% (95% CI 7.4–16.7%. I2 = 0%, P for Heterogeneity: 0.80), nausea 9.1% (95% CI 5.7–15.3%. I2 = 33%, P for Heterogeneity: 0.19), diarrhoea 10.1% (95% CI 7.4–15.3%. I2 = 32%, P for Heterogeneity: 0.17), vomiting 5.7% (95% CI 2.9–12.3%. I2 = 66%, P for Heterogeneity: 0.008), neuropathy 3.8% (95% CI 2.0–7.4%. I2 = 10%, P for Heterogeneity: 0.35), and increased ALT 5.7% (95% CI 2.9–11.5%. I2 = 54%, P for Heterogeneity: 0.09) (Fig. 5).
Discussion
Our systematic review and meta-analysis considered 11 studies, which contained 563 patients with advanced pancreatic cancer treated with modified FOLFIRINOX. Previously, FOLFIRINOX was used to treat advanced pancreatic adenocarcinoma and demonstrated a better therapeutic benefit than gemcitabine (GEM)32. Although the dosage of FOLFIRINOX was reduced, the 12-month survival rate was still much higher than those of gemcitabine and its combinational regimen, with the first at 76.2% in LAPC and 47.6% in MPC, compared to 18–37.2%11,33,34,35,36. Since then, many clinical studies have been assessed the treatment of advanced pancreatic cancer by using modified FOLFIRINOX. Compared to the preceding original regimen of FOLFIRINOX, the OS and PFS at 6 and 12 months for modified FOLFIRINOX were nearly equivalent14,20,37,38. Similar to the data obtained for OS and PFS, as mentioned above, the response rate of modified FOLFIRINOX was also comparable to that of the original regimen14,20,37,38. Nevertheless, the favourable overall survival after modified FOLFIRINOX might be partly attributable to patient selection from many non-randomized studies.
For the adverse events, the pooled rates of grade III/IV adverse events were lower than those of the FOLFIRINOX group; some were even lower than the GEM group14,39,40, such as anaemia, fatigue and vomiting. Concomitantly, a prospective phase II study of dose-attenuated treatment found that modified FOLFIRINOX could significantly reduce the occurrence of vomiting and fatigue41. As we know, in practice, when patients experience serious adverse events during continuous FOLFIRINOX chemotherapy, the strategy for physicians is to reduce the dosage or even stop the chemotherapy. Therefore, modified FOLFIRINOX is a good choice at the beginning of therapy, particularly for those with poor performance status. Modified FOLFIRINOX provides a relatively mild intervention and thus induces lower adverse events, thereby ensuring the continuity of chemotherapy. Interestingly, there was a great difference between the Asian group and Euromerican group in neutropenia (48.5% [20.6%, 77.4%] vs 10.7% [2.9%, 31.3%]). This may be due to different genetic traits between the ethnic groups.
In general, the modified FOLFIRINOX regimen could provide good survival benefits for patients with advanced pancreatic cancer by increasing the OS and PFS and causing fewer adverse events. Our findings suggest that the dosage attenuation of initial FOLFIRINOX improves its tolerability without compromising its efficacy. Compared to the original regimen of FOLFIRINOX, modified FOLFIRINOX may be more applicable for patients with poor performance status. However, there were multiple combinations of the four drugs in which the 5-FU bolus was removed; which combination is the best for different ethnic groups or different healthy conditions remains a significant question. Clinical trials are still needed to justify the best combination for modified FOLFIRINOX. At last, although most of the studies that we chose were non-randomized and some even had a retrospective design that might bring bias, the current meta-analysis could provide constructive information for clinicians and patients.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 136, E359–E386, https://doi.org/10.1002/ijc.29210 (2015).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin 67, 7–30, https://doi.org/10.3322/caac.21387 (2017).
Society, A. C. Cancer facts and figures 2016. Atlanta (GA): American Cancer Society (2016).
Rahib, L. et al. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Research 74, 2913–2921, https://doi.org/10.1158/0008-5472.can-14-0155 (2014).
Warsame, R. & Grothey, A. Treatment options for advanced pancreatic cancer: a review. Expert review of anticancer therapy 12, 1327–1336 (2012).
Stathis, A. & Moore, M. J. Advanced pancreatic carcinoma: Current treatment and future challenges. Nature Reviews Clinical Oncology 7, 163–172, https://doi.org/10.1038/nrclinonc.2009.236 (2010).
Hariharan, D., Saied, A. & Kocher, H. M. Analysis of mortality rates for pancreatic cancer across the world. HPB 10, 58–62, https://doi.org/10.1080/13651820701883148 (2008).
Petersen, G. M. et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiology Biomarkers and Prevention 15, 704–710, https://doi.org/10.1158/1055-9965.EPI-05-0734 (2006).
Li, D., Xie, K., Wolff, R. & Abbruzzese, J. L. Pancreatic cancer. Lancet (London, England) 363, 1049–1057, https://doi.org/10.1016/S0140-6736(04)15841-8 (2004).
Fogel, E. L. et al. A Multidisciplinary Approach to Pancreas Cancer in 2016: A Review. The American journal of gastroenterology 112, 537–554, https://doi.org/10.1038/ajg.2016.610 (2017).
Burris, I. H. A. et al. Improvements in survival and clinical benefit with gemcitabine as first- line therapy for patients with advanced pancreas cancer: A randomized trial. Journal of Clinical Oncology 15, 2403–2413 (1997).
Cunningham, D. et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. Journal of Clinical Oncology 27, 5513–5518, https://doi.org/10.1200/JCO.2009.24.2446 (2009).
Moore, M. J. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 25, 1960–1966, https://doi.org/10.1200/JCO.2006.07.9525 (2007).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England Journal of Medicine 364, 1817–1825, https://doi.org/10.1056/NEJMoa1011923 (2011).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New England Journal of Medicine 369, 1691–1703, https://doi.org/10.1056/NEJMoa1304369 (2013).
Chan, K. et al. A Bayesian meta-analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer. PloS one 9, e108749, https://doi.org/10.1371/journal.pone.0108749 (2014).
Gresham, G. K., Wells, G. A., Gill, S., Cameron, C. & Jonker, D. J. Chemotherapy regimens for advanced pancreatic cancer: A systematic review and network meta-analysis. BMC cancer 14 (2014).
Gupta, J. et al. Kaplan-meier survival curves: A potential source of data for systematic reviews. Value in Health 15, A459–A460, https://doi.org/10.1016/j.jval.2012.08.1465 (2012).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. British Medical Journal 327, 557–560 (2003).
Ghorani, E. et al. Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience. Oncology 89(5), 281–287 (2015).
Mahaseth, H. et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 42, 1311–1315 (2013).
Blazer, M. et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Annals of surgical oncology 22, 1153–1159, https://doi.org/10.1245/s10434-014-4225-1 (2015).
Nanda, R. H., El-Rayes, B., Maithel, S. K. & Landry, J. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. Journal of surgical oncology 111, 1028–1034 (2015).
Takeda, Y. et al. FOLFIRINOX Combination Chemotherapy in Patients with Metastatic or Recurrent Pancreatic Cancer–A Single Institution Experience. Gan to kagaku ryoho. Cancer & chemotherapy 42, 2360–2363 (2015).
Chllamma, M. K. et al. FOLFIRINOX for advanced pancreatic cancer: the Princess Margaret Cancer Centre experience. British journal of cancer 115, 649–654, https://doi.org/10.1038/bjc.2016.222 (2016).
Li, X. et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer letters 406, 22–26, https://doi.org/10.1016/j.canlet.2017.07.012 (2017).
Stein, S. M. et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. British journal of cancer 114, 737–743, https://doi.org/10.1038/bjc.2016.45 (2016).
Vivaldi, C. et al. First-line treatment with FOLFOXIRI for advanced pancreatic cancer in clinical practice: Patients’ outcome and analysis of prognostic factors. International journal of cancer 139, 938–945 (2016).
Vočka, M. & Petruzelka, L. Modified FOLFIRINOX in the treatment of pancreatic cancer-efficiency and toxicity. Gastroenterologie a Hepatologie 70, 413–417 (2016).
Yoshida, K. et al. A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget 8, 111346–111355, https://doi.org/10.18632/oncotarget.22795 (2017).
Bai, X. et al. [Modified FOLFIRINOX for advanced pancreatic cancer: a tertiary center experience from China]. Zhonghua wai ke za zhi [Chinese journal of surgery] 54, 270–275, https://doi.org/10.3760/cma.j.issn.0529-5815.2016.04.006 (2016).
Conroy, T. et al. Irinotecan Plus Oxaliplatin and Leucovorin-Modulated Fluorouracil in Advanced Pancreatic Cancer—A Groupe Tumeurs Digestives of the Fédération Nationale des Centres de Lutte Contre le Cancer Study. Journal of Clinical Oncology 23, 1228–1236, https://doi.org/10.1200/jco.2005.06.050 (2005).
Murad, A. M. et al. Phase II trial of the use of gemcitabine and 5-fluorouracil in the treatment of advanced pancreatic and biliary tract cancer. American journal of clinical oncology 26, 151–154, https://doi.org/10.1097/01.coc.0000017525.15572.52 (2003).
Ulrich-Pur, H. et al. A phase II trial of biweekly high dose gemcitabine for patients with metastatic pancreatic adenocarcinoma. Cancer 88, 2505–2511 (2000).
Scheithauer, W. et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Annals of oncology: official journal of the European Society for Medical Oncology 14, 97–104 (2003).
Heinemann, V. et al. Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Annals of oncology: official journal of the European Society for Medical Oncology 11, 1399–1403 (2000).
Peddi, P. F. et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP: Journal of the pancreas 13, 497–501, https://doi.org/10.6092/1590-8577/913 (2012).
Rombouts, S. J. et al. FOLFIRINOX in Locally Advanced and Metastatic Pancreatic Cancer: A Single Centre Cohort Study. Journal of Cancer 7, 1861–1866, https://doi.org/10.7150/jca.16279 (2016).
Miyashita, K. et al. Investigation of the tolerability of FOLFIRINOX in patients with unresectable advanced pancreatic cancer: Single-institution experience in Japan. Journal of Clinical Oncology 33, 487–487 (2015).
Marthey, L. et al. FOLFIRINOX for Locally Advanced Pancreatic Adenocarcinoma: Results of an AGEO Multicenter Prospective Observational Cohort. Annals of surgical oncology (2014).
Ginocchi, L. et al. Modified FOLFOXIRI in Advanced Pancreatic Cancer. Jop Journal of the Pancreas 23, 238–238 (2012).
Author information
Authors and Affiliations
Contributions
Hongxuan Tong designed the study and performed the literature search, analysis, quality evaluation, interpretation of data and drafting. Zhu Fan and Biyuan Liu performed the study search, quality evaluation and picture processing. Tao Lu contributed to general management. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tong, H., Fan, Z., Liu, B. et al. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: a systematic review and meta-analysis. Sci Rep 8, 8666 (2018). https://doi.org/10.1038/s41598-018-26811-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26811-9
This article is cited by
-
Pancreatic adenocarcinoma third line systemic treatments: a retrospective cohort study
BMC Cancer (2024)
-
Oxaliplatin-induced peripheral neuropathy can be minimized by pressurized regional intravascular delivery in an orthotopic murine pancreatic cancer model
Discover Oncology (2022)
-
Das Pankreaskarzinom
Der Onkologe (2022)
-
Thymidine phosphorylase induction by ionizing radiation antagonizes 5-fluorouracil resistance in human ductal pancreatic adenocarcinoma
Radiation and Environmental Biophysics (2022)
-
Meta-analysis and indirect treatment comparison of modified FOLFIRINOX and gemcitabine plus nab-paclitaxel as first-line chemotherapy in advanced pancreatic cancer
BMC Cancer (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.