Abstract
It is well-established that baroreflex sensitivity is essential for blood pressure control, and also plays a key role in the modulation of disease-induced metabolic alterations. In order to investigate the role of the baroreflex in the cardiometabolic and inflammatory derangements promoted by fructose overload, Wistar rats underwent sinoaortic denervation (SAD) or sham surgery and were studied 90 days after receiving tap water (Den and Ctrl) or a 10% fructose solution (Fruc and Den-Fruc). All experimental groups showed marked and similar degree of baroreflex impairment compared to Ctrl. As expected, fructose overload effectively induced metabolic syndrome; however, when it was associated with SAD, several alterations were attenuated. While Fruc rats displayed increased sympathetic modulation and tone and reduced vagal modulation compared to Ctrl animals, Den-Fruc rats showed greater vagal tone and modulation when compared to the Fruc group. Moreover, the Den-Fruc group showed augmented expression of β1 adrenergic receptors and TNF/IL-10 ratio and reduction of β2 in the left ventricle. The increase in vagal function was correlated with improved insulin sensitivity (r2 = 0.76), and decreased abdominal fat (r2 = −0.78) and β2 receptors (r2 = −0.85). Our results showed that: (1) chronic fructose overload induced severe baroreflex impairment, i.e. in a similar magnitude to that observed in SAD rats, which is accompanied by cardiometabolic dysfunctions; (2) the compensatory enhancement in parasympathetic function in SAD rats submitted to fructose intake may point out the possibility of use of approaches that improve vagal function as therapeutic target to attenuate fructose-induced cardiometabolic dysfunctions.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a multi-factorial cluster of metabolic and cardiovascular derangements and impaired molecular mechanisms that plays an important role in cardiovascular disease. MetS is thought to increase overall mortality by 50% and cardiovascular death by 150% compared to subjects without the condition1,2. Therefore, a better understanding of not only the altered metabolic and cardiovascular function resulting from MetS but also the mechanisms that mediate these alterations is of importance.
MetS, which may be experimentally induced by chronic and excessive fructose intake3, is characterized by several metabolic derangements, such as weight gain, dyslipidemia, increased blood pressure, and insulin resistance4, as well as autonomic dysfunction, e.g., reduced heart rate variability and increased blood pressure variability5, decreased parasympathetic activity6 and baroreflex impairment7. It has previously been demonstrated that autonomic dysfunction occurs before metabolic alterations in fructose-fed experimental animals8. This suggests that the cardiovascular autonomic nervous system plays a key role as a modulator of fructose-induced changes. Indeed, parasympathetic and baroreflex dysfunction have been associated with fructose-induced metabolic (Brito et al.6; Moraes-Silva et al.7), and oxidative stress9 alterations.
The baroreflex is a moment-to-moment autonomic modulator of the cardiovascular system, and baroreflex impairment has been considered a major risk marker for cardiovascular disease and mortality10. Chronic sinoaortic denervation (SAD), an experimental procedure that abolishes baroreflex control, is used to study baroreflex-induced feedback pathways due to its key role in sympathetic and parasympathetic nervous system modulation11,12. Interestingly, twenty days after baroreceptor deafferentation, the parasympathetic efferent pathway undergoes functional and cellular adaptations, leading to increased vagal responsiveness12. Reinforcing the role of vagal function in homeostasis, its anti-inflammatory potential has been demonstrated in several studies13,14,15. Further investigation of how the baroreflex modulates autonomic feedback pathways may help understanding the onset and progression of fructose-induced MetS.
Since MetS compromises the baroreceptor reflex16, not only is adequate autonomic-controlled buffering of blood pressure oscillations impaired but also the regulation of different metabolic parameters. Therefore, we aimed to demonstrate that baroreflex-mediated autonomic control of the circulation is a pivotal mechanism in cardiovascular, inflammatory and metabolic alterations induced by chronic and excessive fructose intake.
Results
Sinoaortic denervation induces baroreflex impairment
SAD efficacy was demonstrated by the significant impairments measured in baroreflex activity and sensitivity in the experimental groups when compared to the Ctrl group (Table 1). Groups which underwent SAD surgery displayed marked decreases in alpha index, in the total number of pressure change events (total ramps), in the total number of spontaneous baroreflex responses (BR ramps), and in bradycardia and tachycardia reflex indexes. Interestingly, the baroreflex deficit observed in the Fruc group was similar to that observed in denervated rats (Table 1).
Fructose overload induces metabolic syndrome
Measurements of body weight, abdominal adipose tissue and metabolic parameters, such as lipid profile and glycaemia are shown in Table 2. As expected, Fruc rats presented increased body weight and abdominal adipose tissue weight compared to the Ctrl rats. The Den group presented decreased body weight compared to Ctrl and Fruc groups.However, the Den-Fruc group displayed augmented body weight compared to Ctrl and Den groups. While Den rats presented abdominal adipose tissue weight comparable to Ctrl, the Den-Fruc group increased abdominal fat in 30% compared to Ctrl and 54% when compared to Den groups.
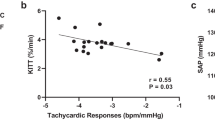
In terms of the metabolic profile, the fasting glycaemia were similar among the four groups. Fructose consumption caused hypercholesterolemia and hypertriglyceridemia in Fruc compared to Ctrl group. However, the Den-Fruc group presented just an increase in triglyceride levels compared to Ctrl group. Moreover, total cholesterol was reduced in Den-Fruc group compared to the Fruc group (Table 2). In addition, a reduction in kITT index was observed in Fruc group in comparison with the Ctrl and Den-Fruc groups, indicating insulin resistance.
Autonomic nervous system adaptations are differently impacted by SAD and fructose overload
Selective blockers were used to evaluate autonomic tonus to the heart. Propranolol, a β1 receptor blocker, was used to interrupt sympathetic effector response, while methylatropine, a muscarinic antagonist, was used to inhibit parasympathetic activity. The combination of these two drugs affected intrinsic heart rate, which was not different between studied groups (Fig. 1A). The fructose groups showed increase in sympathetic tone when compared to Ctrl group (Fig. 1B). As for parasympathetic tone, we found that SAD was able to increase it; however, this increase was only statiscally significant when comparing Den-Fruc to Fruc group (Fig. 1C).
Autonomic blockade. Intrinsic heart rate (1A), sympathetic (1B) and vagal tone (1C) obtained by autonomic blockade with propranolol and methylatropine, respectively. Control (Ctrl), Fructose overload (Fruc), sinoaortic denervation (Den), and Den associated with fructose overload (Den-Fruc). *p < 0.05 vs. Ctrl; #p < 0.05 vs. Fruc by Bonferroni post-hoc, ANOVA (1A p = 0.240; 1B p = 0.006; 1C p = 0.018).
Regarding hemodynamics, the Den-Fruc group displayed a higher heart rate compared to all other groups. Systolic blood pressure increased in both groups treated with fructose compared to Ctrl rats, and was higher in Den-Fruc than in Ctrl and Den animals (Table 3).
Blood pressure variability was increased in Den when compared to Ctrl group, and was even greater in the Den-Fruc group. However, only the Fruc group demonstrated increased vascular sympathetic modulation (LF SBP) (Table 3).
Considering heart rate variability, SAD (Den and Den-Fruc vs. Ctrl group) promoted a reduction in total variability, represented by VAR PI; and consequently a reduction in the specific interest spectrum band, absolute LF and HF PI (Table 3). However, when we analyzed the representative percentage of total modulation, it became clear that fructose ingestion (Fruc group) led to increased sympathetic modulation to the heart (LF PI) compared to the Ctrl group. This alteration was not observed in Den-Fruc group. It should be emphasized that Fruc group presented a decrease in parasympathetic modulation (HF PI, nu) compared to Ctrl group. Moreover, SAD resulted in an elevation in parasympathetic modulation to the heart (Den and Den-Fruc vs. Ctrl group). Taken together, these findings indicate an increased sympathovagal balance in the Fruc group and decreased sympathovagal balance in both Den and Den-Fruc when compared to Ctrl group (Table 3).
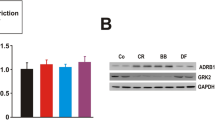
Comparing β adrenergic receptor expression in the left ventricle between groups, fructose overload (Fruc group) promoted an increase in both β1 and β2 receptors compared to Ctrl group, while the association between SAD and fructose ingestion (Den-Fruc group) resulted in a significant increase in β1, along with a decrease in β2 receptors in the left ventricle (Fig. 2).
β Receptors expression. Quantification of β1 (2A) and β2 (2B) receptors in the left ventricle (LV). Percentage of change compared to control group. Control (Ctrl), Fructose overload (Fruc), sinoaortic denervation (Den), and Den associated with fructose overload (Den-Fruc). *p < 0.05 vs. Ctrl; #p < 0.05 vs. Fruc; $p < 0.05 vs. Den by Bonferroni post-hoc, ANOVA (2A p < 0.001; 2B p < 0.001).
Fructose overload and/or SAD induced inflammation in cardiac tissue
Examining the cardiac inflammatory profile, Fruc rats showed an increase in NFkB and IL-10, with a reduced IL-6 and TNFα/IL-10 ratio compared to Ctrl rats. Both denervated groups, however, demonstrated increased TNFα and IL-6, without any changes in NFkB and IL-10 values, which in turn increased the TNFα/IL-10 ratio compared to Ctrl group (Figs 3, 4).
Cardiac immunohistochemistry. Nuclear factor kappa B (NFκB) (3A) and interleukin-6 (IL-6) (3B) by immunohistochemistry in cardiac tissue. Control (Ctrl), Fructose overload (Fruc), sinoaortic denervation (Den), and Den associated with fructose overload (Den-Fruc). *p < 0.05 vs. Ctrl; #p < 0.05 vs. Fruc by Bonferroni post-hoc, ANOVA (3A p = 0.005; 3B p < 0.001).
Cardiac inflammation. Tumor necrosis factor alpha (TNFα) (4A) interleukin-10 (IL-10) (4B) and the TNFα/IL-10 ratio (4C) by ELISA in cardiac tissue. Control (Ctrl), Fructose overload (Fruc), sinoaortic denervation (Den), and Den associated with fructose overload (Den-Fruc). *p < 0.05 vs. Ctrl; #p < 0.05 vs. Fruc by Bonferroni post-hoc, ANOVA (4A p = 0.018; 4B p = 0.039; 4C p = 0.023).
Association analyses
To determine to what extent studied parameters were co-associated, correlation analyses were performed (Table 4). Increased sympathetic tone was positively correlated with increases in the cardiac β2 receptor population. Interestingly, this event was more evident in the Den group (r2 = 0.93), while in Den-Fruc group the correlation was negative. On the other hand, vagal tone was negatively correlated with cardiac β2 receptor density. Once more, this correlation was stronger in Den and Den-Fruc groups. Moreover, higherincreased insulin sensitivity was correlated with an increase in cardiac parasympathetic modulation, especially in Den rats. This can be seen in Table 4, which shows that the higher the insulin sensitivity, the lower the sympathovagal balance.
Discussion
The central aim of this study was to investigate the role of the baroreflex as a modulating mechanism of fructose-induced adaptations (and MetS). SAD surgery was effective in this regard: both Den and Den-Fruc groups presented significantly diminished heart rate responses to blood pressure changes, as evidenced by the sequence method and the spontaneous baroreflex evaluated by alpha index17,18.
Fructose overload was also effective in inducing a metabolic syndrome phenotype. A range of effects of fructose intake in animal models have been described according to concentrations, exposure time, administration, gender, age, species and others19. In our study, 90 days of 10% fructose ingestion in drinking water increased body weight, abdominal fat, total cholesterol, triglycerides (Table 2) and systolic blood pressure (Table 3), while decreasing kITT, thus characterizing MetS.
Interestingly, fructose consumption reduced baroreflex sensitivity and activity similarly to (Table 1) SAD. Our group has already demonstrated that fructose overload is capable of promoting baroreflex impairment7. In the present study, we found that this deficiency in Fruc rats is similar to that caused by SAD surgery, suggesting that long-term excessive fructose intake can be as harmful as SAD to baroreflex function.
Altered autonomic responses to fructose and SAD were the main observations of the present study. Based on the results of autonomic blockade with propranolol and methylatropine, sympathetic tone was increased in both groups treated with fructose (Fig. 1B). This was expected, since sympathetic hyperactivity is correlated with increased adipose tissue, dyslipidemia and hypertension20,21. In addition, fructose may act directly on the central nervous system, thus increasing sympathetic activity22. Additionally, the Fruc group demonstrated increased sympathetic modulation in both absolute and percent values compared to Ctrl (Table 3). This resulted in increased sympathovagal balance, increased peripheral sympathetic modulation and blood pressure. This elevation in blood pressure may be linked not only to sympathetic hyperactivity but also to baroreflex dysfunction23.
Isolated SAD produced a reduction in the total modulation (VAR IP) and a consequent decrease in the absolute band of sympathetic and parasympathetic modulation. Additionally, we found an increased dominance of parasympathetic modulation in the Den group, thus reducing the sympathovagal balance. Although there was no change in SBP, VAR SBP was severely augmented, as is characteristic of this model24,25.
Surprisingly, when Fruc and SAD were experimentally combined, alterations caused by fructose consumption, such as increased abdominal fat, cholesterol, and insulin resistance were attenuated, together with smaller increases in body weight and SBP compared to the Fruc only group. Therefore, we may ask, how can SAD attenuate fructose-induced metabolic derangements?
Despite the fact that there is a parasympathetic hyperresponsiveness after chronic SAD in control rats12, we firstly believed that, after 90 days, SAD-induced impairments would already be established in Fruc-Den rats, as observed in other SAD-associated conditions26,27,28. Conversely, we observed that parasympathetic compensation was still present, together with attenuation in some fructose-induced alterations. This finding suggests that severe baroreflex impairment in fructose-fed rats may induce a vagal compensatory mechanism to modulate cardiometabolic impairments caused by chronic fructose overload.
Another relevant detail is that vagal function is associated with kITT, as shown by Kreier et al.29 and reinforced by the correlation presented in this study (r2 = 0.76). Sinaiko et al.30 have demonstrated in humans that insulin sensitivity at 13 years of age was predictive of metabolic disorders found 6 years later. Higher rates of obesity, increased blood pressure, cholesterol and triglycerides were found in this population. The same effect may be observed in our animals, as both denervated groups presented insulin sensitivity values similar to the control group. This may have contributed to the attenuation of the metabolic effects promoted by fructose in the Den-Fruc group, which displayed a reduction of abdominal fat, cholesterol and blood pressure.
While the relationship between insulin resistance and vagal activity may justify the attenuation of metabolic changes, the interaction between cardiac inflammation and beta adrenoceptors with vagal activity may account for the other observed results. Isolated denervation did not alter gene expression of β1 and β2 receptors, in accordance with the desensitization of β adrenoceptors after long exposures to enhanced sympathetic activity31. Conversely, in the Fruc group, secondary to increased sympathetic modulation and tonic effect to the heart, an increase in β1 receptor expression is needed to ensure autonomic responsiveness32. As a compensatory response, there was also an increase in β2 receptor gene expression, and this may be related to the prevention of the increase in heart rate in these animals and to the reduction, although not significant, of intrinsic heart rate, thus preventing changes in heart rate variability. Reinforcing the role of sympathetic tonus in this response, a positive correlation between this parameter and β2 receptors was found for Fruc rats (r2 = 0.68).
In the combined SAD and fructose group (Den-Fruc), increased heart rate was observed compared to all other groups (Table 3). Since this group demonstrated a greater sympathetic tone to the heart compared to other groups without reducing intrinsic heart rate, as did the Fruc group, this change was expected. The Den-Fruc group also showed a significant increase in the expression of β1; however, as the vagal tone and modulation were already elevated, there was no compensatory response mediated by β2 receptors. The increase in cardiac β1 receptor expression may also explain this heart rate increment in Den-Fruc, since it is well-known that β1 receptors are more important for positive chronotropism than β2 receptors33. We should note that both sympathetic and vagal tonus demonstrated strong negative correlations (r2 < −0.85) with β2 receptor concentration for Den-Fruc rats, showing that when both sympathetic and vagal tonus are increased, the gene expression of β2 receptors is decreased.
In this regard, the gene expression of cardiac β2 receptors may play a role in the cardiac inflammatory profile. Distinct inflammatory responses among the groups were found in the cardiac muscle. Fructose consumption seems to lead to a profile of inflammation related to metabolic residues. The increase in NFkB indicates tissue injury, which may be associated with the oxidative stress caused by residues in fructose metabolism34. This may be due to the preferential use of fructose as the main energy source, rather than glucose35. There was also an increase in the anti-inflammatory cytokine IL-10, which has been found to have a direct association with increased NFkB36, thus indicating an attempt to maintain the balance between pro and anti-inflammatory cytokines. This condition was observed when the TNFα/IL-10 ratio was analyzed, and a very significant reduction of this inflammatory balance was found. Another contributing factor may be the increase in cardiac β2 receptors in Fruc animals, since its activation through the parasympathetic nervous system improved the anti-inflammatory profile15.
Conversely, denervated groups had a different inflammatory pattern. We observed an impressive increase in IL-6, indicating the activation of immune system cells. This probably occurs due to a greater infiltration of these cells caused by increased blood pressure variability37. It is known that the higher the blood pressure variability, the higher the risk of target organ damage, regardless of the blood pressure values38, thus generating a greater inflammatory response. In fact, both SAD groups demonstrated an increase in the inflammatory profile, as demonstrated by the enhanced TNFα/IL-10 ratio. It should also be stressed that this inability to increase the anti-inflammatory response may be associated with the fact that the NFkB values remained unchanged. In addition, there is evidence of a weak relationship between parasympathetic and the inflammatory profile in these groups. Despite the increased vagal tone and vagal modulation values, there was no improvement in the anti-inflammatory profile in our hands. This may be due to the reduction in β2 receptors, as demonstrated in the Den-Fruc group.
In summary, the main results from our experiments are the following: (1) chronic fructose overload induced changes in cardiometabolic parameters accompanied by severe baroreflex impairment, i.e. in a similar magnitude to that observed in chronic denervated rats; (2) chronic SAD enhances parasympathetic activity, corroborating previous studies in control rats12; (3) this vagal enhancement is also present when SAD is concomitantly associated with chronic fructose intake, and is strongly associated with favorable cardiac and metabolic profile changes. Nonetheless, it does not mean that SAD itself is beneficial in metabolic syndrome; actually, it shows that, in the presence of a metabolic pathological stimulus, such as fructose overload, the parasympathetic compensation induced by baroreflex deficiency may be longer and to a greater magnitude than what was observed in the study of Soares et al.12, in which the increased vagal responsiveness was present 20 days after SAD. The vagal attempt to counterbalance the systemic metabolic alterations induced by fructose in the presence of complete baroreflex dysfunction may point out the possibility of use of approaches that improve vagal function as therapeutic target to attenuate fructose-induced cardiometabolic dysfunctions. Moreover, our findings reinforce the role of the baroreflex in physiological and pathological adaptations, and this may be a target mechanism in the onset and development of MetS.
Methods
Bioethical Statement
We declare that all methods used in this protocol were carried out in accordance with relevant guidelines and regulations. All experiments were carried out after approval by Ethical Committee for Animal Use from the University of Sao Paulo Medical School under protocol number 274/11 of 07/13/2011.
Animal model and groups
We used male Wistar rats (Rattus norvegicus) weighing around 150 g at the beginning of the protocol. Animals were kept in the Heart Institute (InCor) Animal Shelter, four specimens per cage with free movement, and access to food and water ad libitum. The temperature was kept at 20–22 °C and the light-dark cycle was 12–12 h.
MetS was induced by fructose overload (100 g/l in drinking water)39. Our four experimental groups were assigned as follows: the control group (Ctrl), monitored for 90 days (n = 10); the fructose group (Fruc), receiving 10% solution of fructose from the beginning to the end of the 90-day follow-up period (n = 10); the sinoaortic denervation group (Den), which underwent a surgical removal of the baroreceptor afferent fibers at day 1 and was monitored for 90 days (n = 8); and the sinoaortic denervated fructose group (Den-Fruc), in which rats were denervated at day 1 and received the 10% fructose solution for 90 days.
Surgical procedures
For sinoaortic denervation surgery, animals were anesthetized with isoflurane (1.5% on O2 flow). They were placed in supine position and an incision was made in the neck, exposing the carotid artery. The baroreceptor fibers were identified and cut at sympathetic nerve, while the Hering nerve and laryngeal nerve were cut at carotid bifurcation. This procedure was performed on both sides, as described by Krieger et al.17 Krieger17.
At the end of the 90-day protocol, animals underwent catheterization of the femoral artery and vein. Once more, they were anesthetized with isoflurane (2.5% on O2 flow) due to their weight gain. Polyethylene cannulas were inserted into the femoral artery (PE-10, with internal diameter of 0.1 mm) and into the femoral vein (PE-50, with internal diameter of 0.5 mm). Both cannulas were connected to a larger tube (Tygon, with an internal diameter of 0.8 mm). The cannulas were filled with heparinized saline and fixed in the femoral vessels using cotton suture wire, and the extremity with the larger diameter was externalized in the dorsal cervical area and fixed to the skin using cotton suture wire. The externalized portion of the cannula was kept closed by using stainless steel pins. After surgery, animals were treated with a single injection of penicillin (Benzetacil, Fontoura- Wyeth, 30.000 U/kg) and analgesic (Dipyrone, 0.35 g/kg).
Hemodynamics
After surgical preparation, the animals were placed in individual cages with free access to food and water (or water containing 10% fructose) for 48 hours for recovery. On the first day of the experiment, the rats were connected to the computerized acquisition systems by connecting the arterial cannula to a polyvinyl tube (Tygon), which was attached to an electromagnetic transducer (Kent Instruments). This transducer was linked to an amplifier (General Purpose Amplifier-Stemtech, Inc.) and connected to a computer with an analog to digital converter board (DataQ/Windaq, sampling rate of 2000 Hz per channel), which allows real-time data acquisition of pulse waves and heart rate and posterior software analysis11.
The animals remained connected for half an hour to the system for adaptation, and signal acquisition was then started. A basal period was recorded for 30 minutes. Pharmacological autonomic blockade was performed to evaluate sympathetic and parasympathetic tonus. For this purpose, one injection of propranolol (8 mg/kg) and/or methylatropine (4 mg/Kg) was randomly administrated in the cannula attached to the femoral vein. After a short period wait for drug action, a new recording was performed for 10 minutes. Twenty-four hours after the first blockade, the procedure was repeated in reverse order of the drug-delivery. Therefore, we managed to isolate the propranolol and methylatropine effects on intrinsic heart rate, enabling us to measure the parasympathetic and sympathetic tonus to the heart40.
Analysis of blood pressure signals was performed using an algorithm implemented in Windaq/DataQ, which was associated with an acquisition system. This software allowed the detection of the maximal value in the blood pressure curve, beat to beat, providing the values of systolic blood pressure (SBP). Heart rate was determined from the pulse interval (PI) between two systolic peaks.
Heart rate and blood pressure variability
Heart rate variability was measured by linear methods in time and frequency domains by using the Cardioseries® v2.4 software. Temporal series of PI and SBP (30 minutes of baseline recording) were analyzed in time domain, obtaining the total variance of PI (VAR PI) and the variance of SBP (VAR SBP). Mean square root of differences between consecutives PI (RMSSD) was also evaluated.
For the frequency domain, the interpolated waves of these same baseline periods were divided into segments of 512 beats, with an overlap of 50%, and were processed by Fast Fourier Transform. One spectrum was obtained for each segment and the oscillatory components were quantified in two different frequencies: low frequency (LF) from 0.20 to 0.75 Hz and high frequency (HF) from 0.75 to 3.00 Hz. The results are represented by absolute values (ms2 and mmHg2), the percentage of total spectrum (%) and normalized units (nu) (percentage of LF and HF bands only). The very low oscillations (<0.20 Hz) were considered non-stationary7.
Baroreflex
Baroreflex activity and sensitivity were measured in two different ways using the Cardioseries® v2.4 software: the alpha index of LF band, and the spontaneous baroreflex by the sequence method. The first one was calculated by the square root of the ratio between the absolute value of LF PI by the absolute value of LF SBP, correlating the absolute values of LF for the heart with absolute LF for the vessels, when they were consistent with each other. The second one required finding 4 points with consecutive increases or decreases in blood pressure associated with a consecutive 4 points of decreasing or increasing heart rate, respectively. We evaluated the total number of events (total ramps), the number of events presenting a heart rate response (BR ramps), the ratio between BR ramps and total ramps (BEI) and the gain, which represents the baroreflex sensitivity for increasing or decreasing blood pressure41.
Metabolic profile
After 90 days and before the cannulation procedure, the animals were weighed and underwent 4 hours of fasting. Then, a small cut on the tail tip was performed to collect a few drops of blood. Glycaemia (Accu-check, Roche®), total cholesterol and triglycerides (Accutrend, Roche®) were measured using reactive tapes. The insulin tolerance test (ITT) was performed by injecting 0.75 U/kg of insulin on the tail vein. Glycaemia was measured at 0, 4, 8, 12, and 16 minutes after insulin administration. These values were used to calculate the glycaemia decay by constant of insulin tolerance test (kITT)42. After all procedures, the animals were euthanized by decapitation. The abdominal adipose tissue and hearts were collected and weighed.
PCR analysis of β1 and β2 receptors gene
Cardiac tissue was separated between atrium and ventricles and 100 mg of the left ventricle was treated with 1.0 ml of Trizol® reagent (InvitrogenTM). The samples were homogenized and total RNA was extracted. Concentrations were measured (Thermo Scientific) at a wavelength of 260–280 nm. We obtained an aliquot of 3 µg of each RNA sample and treated it with Turbo DNA-Free (Ambion) to exclude possible contamination by DNA. Reverse transcription was carried out using SuperScriptTM II Reverse Transcriptase (InvitrogenTM) for proteins of interest. Finally, we ran a real time PCR using β-actin and GAPDH as housekeeping proteins. Quantification was undertaken using the difference between target proteins minus the average of two housekeeping proteins. To compare the results, we normalized the Ctrl group at 100% and created a relation for the other groups.
Cardiac inflammatory mediators
Cardiac cytokines were quantified in the left ventricle using a commercial ELISA kits from R&D Systems (Minneapolis, MN, USA). We used markers for interleukin 10 (IL-10) and tumor necrosis factor alpha (TNFα). The results are presented as the ratio of marked proteins to the total amount of protein43. For interleukin 6 (IL-6) and nuclear factor kappa B (NFκB) measurements, we used histological sections of the left ventricles. We performed a circulatory perfusion of potassium chloride (14 mmol/l), followed by buffered formaldehyde (3.7%). After histological preparation, the slides were incubated with a complex containing primary and secondary antibodies to the proteins of interest43. The measurements were performed by colorimetric quantification (Image J®) obtained by 20 photos per slide in a 400× increase.
Statistics
All data were submitted to D’Agostino and Pearson omnibus normality test. They are expressed as mean and standard error of mean. To determine significance level, we used ANOVA with Bonferroni post-hoc test. Association between variables was evaluated using Pearson’s correlation analysis. P value < 0.05 was considered statistically significant.
References
Lakka, H. M. et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama 288, 2709–2716 (2002).
Girman, C. J. et al. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). The American journal of cardiology 93, 136–141 (2004).
Richelsen, B. Sugar-sweetened beverages and cardio-metabolic disease risks. Current opinion in clinical nutrition and metabolic care, https://doi.org/10.1097/MCO.0b013e328361c53e (2013).
Elliott, S. S., Keim, N. L., Stern, J. S., Teff, K. & Havel, P. J. Fructose, weight gain, and the insulin resistance syndrome. The American journal of clinical nutrition 76, 911–922 (2002).
Farah, V. et al. Nocturnal hypertension in mice consuming a high fructose diet. Autonomic neuroscience: basic & clinical 130, 41–50, https://doi.org/10.1016/j.autneu.2006.05.006 (2006).
Brito, J. O. et al. Parasympathetic dysfunction is associated with insulin resistance in fructose-fed female rats. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica… [et al.] 41, 804–808 (2008).
Moraes-Silva, I. C. et al. Preventive role of exercise training in autonomic, hemodynamic, and metabolic parameters in rats under high risk of metabolic syndrome development. J Appl Physiol (1985) 114, 786–791, https://doi.org/10.1152/japplphysiol.00586.2012 (2013).
De Angelis, K., Senador, D. D., Mostarda, C., Irigoyen, M. C. & Morris, M. Sympathetic overactivity precedes metabolic dysfunction in a fructose model of glucose intolerance in mice. American journal of physiology. Regulatory, integrative and comparative physiology 302, R950–957, https://doi.org/10.1152/ajpregu.00450.2011 (2012).
Brito-Monzani, J. O. et al. Hypertension induces additional cardiometabolic impairments and attenuates aerobic exercise training adaptations in fructose-fed ovariectomized rats. Hypertension research: official journal of the Japanese Society of Hypertension 41, 88–95, https://doi.org/10.1038/hr.2017.94 (2018).
Rovere, M. T. L., Bigger, J. T., Marcus, F. I., Mortara, A. & Schwartz, P. J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. The Lancet 351, 478–484, https://doi.org/10.1016/s0140-6736(97)11144-8 (1998).
Irigoyen, M. C. et al. Changes of renal sympathetic activity in acute and chronic conscious sinoaortic denervated rats. Hypertension 26, 1111–1116 (1995).
Soares, P. P. et al. Effects of rat sinoaortic denervation on the vagal responsiveness and expression of muscarinic acetylcholine receptors. Journal of cardiovascular pharmacology 47, 331–336, https://doi.org/10.1097/01.fjc.0000205982.67653.26 (2006).
Tracey, K. J. Reflex control of immunity. Nature reviews. Immunology 9, 418–428, https://doi.org/10.1038/nri2566 (2009).
Huston, J. M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. The Journal of experimental medicine 203, 1623–1628, https://doi.org/10.1084/jem.20052362 (2006).
Vida, G. et al. beta2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 25, 4476–4485, https://doi.org/10.1096/fj.11-191007 (2011).
Seravalle, G. & Grassi, G. Sympathetic Nervous System, Hypertension, Obesity and Metabolic Syndrome. High blood pressure & cardiovascular prevention: the official journal of the Italian Society of Hypertension 23, 175–179, https://doi.org/10.1007/s40292-016-0137-4 (2016).
Krieger, E. M. Neurogenic Hypertension in the Rat. Circulation research 15, 511–521 (1964).
Rodrigues, F. L., de Oliveira, M., Salgado, H. C. & Fazan, R. Jr. Effect of baroreceptor denervation on the autonomic control of arterial pressure in conscious mice. Experimental physiology 96, 853–862, https://doi.org/10.1113/expphysiol.2011.057067 (2011).
Abdulla, M. H., Sattar, M. A. & Johns, E. J. The Relation between Fructose-Induced Metabolic Syndrome and Altered Renal Haemodynamic and Excretory Function in the Rat. International journal of nephrology 2011, 934659, https://doi.org/10.4061/2011/934659 (2011).
Mancia, G. & Grassi, G. The autonomic nervous system and hypertension. Circulation research 114, 1804–1814, https://doi.org/10.1161/CIRCRESAHA.114.302524 (2014).
da Silva, A. A., do Carmo, J., Dubinion, J. & Hall, J. E. The role of the sympathetic nervous system in obesity-related hypertension. Current hypertension reports 11, 206–211 (2009).
Klein, A. V. & Kiat, H. The mechanisms underlying fructose-induced hypertension: a review. Journal of hypertension 33, 912–920, https://doi.org/10.1097/HJH.0000000000000551 (2015).
Grassi, G. Sympathetic overdrive and cardiovascular risk in the metabolic syndrome. Hypertension research: official journal of the Japanese Society of Hypertension 29, 839–847, https://doi.org/10.1291/hypres.29.839 (2006).
Irigoyen, M. C. & Krieger, E. M. Baroreflex control of sympathetic activity in experimental hypertension. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica… [et al.] 31, 1213–1220 (1998).
Zhang, Z. Q., Barres, C. & Julien, C. Involvement of vasodilator mechanisms in arterial pressure lability after sino-aortic baroreceptor denervation in rat. The Journal of physiology 482(Pt 2), 435–448 (1995).
Mostarda, C. et al. n: role in cardiac remodelling and mortality. Clin Exp Pharmacol Physiol 37, 447–452, https://doi.org/10.1111/j.1440-1681.2009.05327.x (2010).
Mostarda, C. et al. Baroreflex deficiency induces additional impairment of vagal tone, diastolic function and calcium handling proteins after myocardial infarction. American journal of translational research 6, 320–328 (2014).
Chen, L. et al. Baroreflex deficiency aggravates atherosclerosis via alpha7 nicotinic acetylcholine receptor in mice. Vascular pharmacology 87, 92–99, https://doi.org/10.1016/j.vph.2016.08.008 (2016).
Kreier, F. et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat–functional implications. The Journal of clinical investigation 110, 1243–1250, https://doi.org/10.1172/JCI15736 (2002).
Sinaiko, A. R. et al. Influence of insulin resistance and body mass index at age 13 on systolic blood pressure, triglycerides, and high-density lipoprotein cholesterol at age 19. Hypertension 48, 730–736, https://doi.org/10.1161/01.HYP.0000237863.24000.50 (2006).
Vasquez, E. C. & Krieger, E. M. Decreased chronotropic responses to adrenergic stimulation following sinoaortic denervation in the rat. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica… [et al.] 15, 377–387 (1982).
Tran, L. T., MacLeod, K. M. & McNeill, J. H. Selective alpha(1)-adrenoceptor blockade prevents fructose-induced hypertension. Molecular and cellular biochemistry 392, 205–211, https://doi.org/10.1007/s11010-014-2031-5 (2014).
Santos, I. N. et al. Evidence for two atypical conformations of beta-adrenoceptors and their interaction with Gi proteins. European journal of pharmacology 513, 109–118, https://doi.org/10.1016/j.ejphar.2005.03.008 (2005).
Yang, K., Feng, C., Lip, H., Bruce, W. R. & O’Brien, P. J. Cytotoxic molecular mechanisms and cytoprotection by enzymic metabolism or autoxidation for glyceraldehyde, hydroxypyruvate and glycolaldehyde. Chemico-biological interactions 191, 315–321, https://doi.org/10.1016/j.cbi.2011.02.027 (2011).
Sakai, M., Oimomi, M. & Kasuga, M. Experimental studies on the role of fructose in the development of diabetic complications. The Kobe journal of medical sciences 48, 125–136 (2002).
Saraiva, M. & O’Garra, A. The regulation of IL-10 production by immune cells. Nature reviews. Immunology 10, 170–181, https://doi.org/10.1038/nri2711 (2010).
Kim, K. I. et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circulation journal: official journal of the Japanese Circulation Society 72, 293–298 (2008).
Irigoyen, M. C. et al. Hypertension, Blood Pressure Variability, and Target Organ Lesion. Current hypertension reports 18, 31, https://doi.org/10.1007/s11906-016-0642-9 (2016).
Dai, S. & McNeill, J. H. Fructose-induced hypertension in rats is concentration- and duration-dependent. Journal of pharmacological and toxicological methods 33, 101–107 (1995).
Negrao, C. E., Moreira, E. D., Santos, M. C., Farah, V. M. & Krieger, E. M. Vagal function impairment after exercise training. J Appl Physiol (1985) 72, 1749–1753 (1992).
Oka, H., Mochio, S., Yoshioka, M., Morita, M. & Inoue, K. Evaluation of baroreflex sensitivity by the sequence method using blood pressure oscillations and R-R interval changes during deep respiration. European neurology 50, 230–243, 73865 (2003).
Bonora, E. et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. The Journal of clinical endocrinology and metabolism 68, 374–378 (1989).
Bezerra, O. C. et al. Cholinergic Stimulation Improves Oxidative Stress and Inflammation in Experimental Myocardial Infarction. Scientific reports 7, 13687, https://doi.org/10.1038/s41598-017-14021-8 (2017).
Acknowledgements
Special thanks to Dr. Katia De Angelis and Dr. Erik Kistler who helped make this last version even better. FAPESP 2011/04111-6 and FAPESP 2013/23418-0.
Author information
Authors and Affiliations
Contributions
F.S. designed the protocol, performed the experiments and analyzed the results. E.D.M. was responsible for surgical techniques. F.S. and I.C.M.S. wrote the manuscript. M.C.I. designed the protocol, supervised and reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
dos Santos, F., Moraes-Silva, I.C., Moreira, E.D. et al. The role of the baroreflex and parasympathetic nervous system in fructose-induced cardiac and metabolic alterations. Sci Rep 8, 10970 (2018). https://doi.org/10.1038/s41598-018-29336-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29336-3
This article is cited by
-
Parental fructose consumption induces early baroreflex dysfunction in offspring: impact on arterial pressure and on insulin resistance
International Journal of Obesity (2024)
-
Nicotinamide attenuates streptozotocin-induced diabetes complications and increases survival rate in rats: role of autonomic nervous system
BMC Endocrine Disorders (2021)
-
Acute renal denervation normalizes aortic function and decreases blood pressure in spontaneously hypertensive rats
Scientific Reports (2020)
-
TRPC6 participates in the development of blood pressure variability increase in sino-aortic denervated rats
Heart and Vessels (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.