Abstract
In standing, coordinated activation of lower extremity muscles can be simplified by common neural inputs to muscles comprising a functional synergy. We examined the effect of task difficulty on common inputs to agonist-agonist (AG-AG) pairs supporting direction specific reciprocal muscle control and agonist-antagonist (AG-ANT) pairs supporting stiffness control. Since excessive stiffness is energetically costly and limits the flexibility of responses to perturbations, compared to AG-ANT, we expected greater AG-AG common inputs and a larger increase with increasing task difficulty. We used coherence analysis to examine common inputs in three frequency ranges which reflect subcortical/spinal (0–5 and 6–15 Hz) and corticospinal inputs (6–15 and 16–40 Hz). Coherence was indeed higher in AG-AG compared to AG-ANT muscles in all three frequency bands, indicating a predilection for functional synergies supporting reciprocal rather than stiffness control. Coherence increased with increasing task difficulty, only in AG-ANT muscles in the low frequency band (0–5 Hz), reflecting subcortical inputs and only in AG-AG group in the high frequency band (16–40 Hz), reflecting corticospinal inputs. Therefore, common neural inputs to both AG-AG and AG-ANT muscles increase with difficulty but are likely driven by different sources of input to spinal alpha motor neurons.
Similar content being viewed by others
Introduction
From a neuromuscular perspective, standing balance is maintained through coordinated activation of multiple lower extremity muscles, organized in functional synergies1,2,3. Neural control can be simplified by synchronized or common inputs which activate the muscles comprising a functional synergy as a single unit, instead of separate neural signals to each muscle3,4,5,6. Biomechanically, in order to maintain balance, the body’s center of mass (COM) dynamics must be appropriately controlled relative to the base of support (BOS) i.e., the contact area between the body and the support surface7,8. If the BOS becomes smaller, the COM has to be confined within a smaller area, thereby increasing task difficulty. Such an increase in task difficulty is evidenced by an increase in center of pressure (COP) amplitude and velocity8,9. The co-ordination of leg muscle activation is related to COP movements1,2 and consequently task difficulty. Therefore, we examined how an increase in task difficulty influences the common inputs which can support the aforementioned co-ordination.
During voluntary or anticipatory COM movements, EMG co-variance shows a reciprocal pattern i.e., groups of anterior or posterior muscles are activated alternately, and not simultaneously2,3,9. When task difficulty increases, two or more agonist (AG-AG) muscles may be co-activated to increase torque3,10,11, but agonists and antagonists are usually activated separately. Additionally, in some difficult situations like standing on an unstable surface, synergies comprising agonist-antagonist (AG-ANT) muscles can emerge2. Co-activation of antagonistic muscles can increase the stiffness of a joint, which in turn can reduce displacement in response to perturbations, without the need for active neural control involving a feedback loop. However, when task difficulty increases, greater stiffness increases the likelihood of losing balance in response to perturbations12,13. Therefore, though we expect both AG-AG and AG-ANT common inputs to increase with task difficulty, we expect the strength of common inputs to be greater in AG-AG compared to AG-ANT muscles.
The EMG signal contains spectral information about motor neuron firing and the motor unit action potentials6,14. Common presynaptic inputs to the motor neuron pools of two or more muscles can synchronize their firing frequency. The strength of such synchronization becomes apparent in the coherence which is a measure of correlation in the frequency domain, between trains of action potentials discharged by motor neurons innervating two muscles. Therefore, common neural inputs to different muscles can be inferred based on EMG-EMG coherence15,16. Neural inputs to muscles at different frequencies are characteristic of activity in different brain areas and circuits. Therefore, the source of the presynaptic common inputs (spinal, subcortical or corticospinal) can be inferred based on the frequencies at which coherences emerge16. In standing, coherence has been reported in the 0–5 Hz and 6–15 Hz bands, both of which reflect subcortical inputs though 6–15 Hz may also have some corticospinal contributions14,17. As task difficulty increases, corticospinal excitability of leg muscles increases18,19,20, suggesting greater cortical involvement in balance control. Therefore, we hypothesize that in difficult standing tasks, coherence will also emerge at higher frequencies (>15 Hz), reflecting corticospinal inputs16.
The primary purpose of this study was to determine how common inputs to AG-AG and AG-ANT muscle pairs, in 3 frequency bands (low: 0–5 Hz, medium: 6–15 Hz and high: 16–40 Hz), change when task difficulty is manipulated by decreasing the BOS in standing. We expect AG-AG coherence to be greater than AG-ANT coherence, and with increasing task difficulty, we hypothesize a larger increase in AG-AG compared to AG-ANT coherence. We expect high frequency coherence reflecting corticospinal inputs to emerge only in the more difficult tasks. Given a lack of previous data, it is premature to predict whether AG-AG and AG-ANT common inputs will be differentially driven by subcortical or corticospinal inputs. Our data will help to clarify how common inputs can simplify the co-ordination of lower extremity muscles in standing. Specifically, we aim to determine whether common neural inputs from cortical and subcortical sources favor reciprocal or stiffness control, using EMG-EMG coherence.
Methods
Participants
Twenty healthy young adults (21.0 ± 1.3 y, 9F) without current lower extremity injury, or neurological and orthopedic conditions known to impair standing balance, volunteered for the study. Data were acquired during a single 45 min long lab visit. The Medical Ethical Committee of the University Medical Center Groningen approved the study protocol and informed consent document, and the study was conducted according to the Declaration of Helsinki21. We determined foot dominance22 using 3 questions about use preference.
Procedures
Participants completed four tasks in random order, with 2–3 min rest between tasks: (1) wide stance (feet shoulder width apart); (2) narrow stance (feet together); (3) tandem stance (dominant foot posterior), and (4) one leg stance (dominant foot). For each task, participants performed two, 45-s-long trials. Participants wore socks, crossed their arms across the chest and focused their vision on a cross displayed on a projection screen at a distance of ~3 m.
Data Acquisition
Wireless sensors (dimensions – 37*26*15 mm, electrode material – silver; Trigno™ Wireless System, Delsys, Natick, MA, USA) were used to record EMG from 6 muscles on the dominant side: soleus (Sol); lateral gastrocnemius (LG); tibialis anterior (TA); peroneus longus (PL); biceps femoris (BF), and rectus femoris (RF). The signal was amplified 1000 times and sampled at 5.0 kHz using data acquisition interface and software (Power 1401 and Signal v5.11, Cambridge Electronic Design Ltd, Cambridge UK).
The net ground reaction forces act on a point, within the BOS, called the COP8. Dynamics of COP movements provide insight into the neuromuscular control which ensures that COM movements relative to the BOS are controlled in a manner that minimizes the risk of a fall. Therefore, COP data were acquired to confirm whether the BOS limitation did in fact increase task difficulty illustrated by an increase in COP velocity and area. COP location was calculated using moment data obtained from 2 force plates (Bertec 4060-08, Columbus, OH, USA) embedded in the floor, sampled at 200 Hz and acquired using a custom LabVIEW script (v2015, National Instruments, Austin, TX, USA).
Data Analysis
From each 45 s trial, the middle 30 s of EMG and COP data were selected. The EMG was first visually inspected for any artifacts. Data for one participant was excluded due to noise confirmed by spectral analysis which showed high power across a large range of frequencies. For the rest of the participants, the data were bandpass filtered using a 4th order dual pass Butterworth filter with 20 and 500 Hz cut-offs. A combination of computational and experimental approaches14,23 show that motor unit firing information is more easily discernable in rectified data, especially when the motor unit action potentials vary in shape, as is expected in surface EMG. Therefore, we rectified the data using the Hilbert transform because it improves the distinction between the motor unit firing (which we aim to examine) and high frequency voltage fluctuations in the motor unit action potentials14,24. Subsequently, the two trials were concatenated to obtain a 60 s long record.
In order to examine the EMG levels during each task, the concatenated time series for each muscle was integrated (iEMG)4,5 and the iEMG in narrow, tandem and one leg stance were normalized by the iEMG in wide stance. Therefore, the levels of activation in the other standing tasks were represented as multiples of activation in wide stance (Fig. 1). Thereafter, the normalized iEMG for 6 muscles were organized in a vector representing the EMG pattern for each task4,5,25. To determine whether the EMG patterns in the difficult tasks were similar to wide stance (or not), we estimated the cosine of the angle between the muscle activation vectors for wide stance and the other tasks. High cosine values (close to 1) indicate that a similar EMG pattern is used in the other standing tasks compared to wide stance4,5,25. This analysis allowed us to confirm that the relative activation levels of the different muscles were similar across tasks and the selected muscles were relevant for all our tasks. Cross-spectrum (fxy) of pairs of muscles and auto-spectrum (fxx and fyy) of individual muscles was determined using Welch’s periodogram method. Estimates were obtained using 1 s (5000 data points) long Hanning windows without overlap, resulting in 1 Hz frequency resolution. Intermuscular coherence was estimated by normalizing the squared cross-spectrum by the product of the auto-spectra26 –
Single-pair coherence was estimated for the following AG-AG pairs: Sol-LG, Sol-PL, LG-PL; and AG-ANT pairs: Sol-TA, LG-TA, PL-TA, RF-BF. Coherence is reported in the 0–55 Hz range and considered to be significant if it exceeds the confidence limit (at α = 0.05) for the number of segments (L) used to estimate the spectrum27 –
TA-PL coherence was significant across all frequencies indicating cross-talk28, and was therefore not included in any further analysis. For the remaining pairs, pooled coherence was estimated separately for AG-AG and AG-ANT pairs using the following equation29 –
where, k is the number of pairs pooled together (3 each for AG-AG and AG-ANT), and Li is the total number of segments used for estimating the spectrum.
COP data were lowpass filtered using a 4th order dual pass Butterworth filter with 5 Hz cut-off, and the two trials were concatenated. COP velocity (COPvel) and area (COParea) were calculated to characterize COP dynamics in each task.
Coherence data for one participant was excluded due to noisy EMG, and COP data was not available for 2 participants due to technical problems. Therefore, the final coherence analysis included 19 participants and the COP analysis included 17 participants.
Statistics
Repeated measures ANOVAs were used to test for effect of task difficulty on all the COP outcomes. The COP area data were log transformed since it was not normally distributed. Six repeated measures ANOVAs were used to examine the effect of task difficulty on activation level (iEMG) of each muscle. Both single pair and pooled coherence were Fisher transformed and subsequently integrated in 3 separate frequency bands – 0–5 Hz (low), 6–15 Hz (med) and 16–40 Hz (high). These frequency bands were chosen based on the significant regions observed in our data, previously reported standing data17,30,31, and probable neural origin16,28 of coherent signals to different muscles. For pooled coherence (expressed as z-score*Hz), separate 2*4 repeated measures ANOVAs were run for each frequency band, to test for main effects of muscle group (AG-AG and ANT-ANT) and task difficulty (wide, narrow, tandem and one leg), and muscle group by task interaction. Since the data were not normally distributed, log transformation was applied before running the ANOVAs. The significance level was set at α = 0.05. Post-hoc paired t-tests were run to examine whether coherence in any of the difficult tasks differed significantly from wide stance. These tests were done separately for the AG-AG and AG-ANT groups leading to 3 pairwise comparisons for each group and Bonferroni adjusted significance level of α = 0.017. Further post-hoc tests were used to examine the difference between AG-AG and AG-ANT group, separately in each task. Since only one comparison was made in each task, correction of the alpha level was not required.
For single-pair coherence, 3*4 repeated measures ANOVAs were run separately for each frequency band and for the AG-AG and AG-ANT pairs. Coherence in none of the AG-ANT pairs exceeded the significance level in the high frequency range. Therefore, no further statistical analyses were run for these data. Post-hoc t-tests were used to compare wide stance with the other tasks separately for each pair and to compare between muscle pairs separately for each task. This allowed us to examine differences between individual muscle pairs within the AG-AG and AG-ANT groups. In both cases, 3 paired t-tests were conducted and the Bonferroni corrected alpha value was 0.017. Post-hoc tests were not computed for comparisons in which the coherence in both tasks or both muscle pairs did not exceed the significance level (see Fig. 2). ANOVA effect sizes were estimated using partial eta squared, with <0.25, 0.26–0.63 and >0.63 considered small, medium and large effect sizes respectively32,33. For post-hoc tests, Cohen’s d was used and 0.21–0.50, 0.51–0.79 and >0.79 were considered small, medium and large effect sizes respectively34. Coherence values were inverse z-transformed for the figures.
Results
Center of pressure
Table 1 shows the main effect of task on COP velocity and area (p < 0.001), which increased with increasing task difficulty.
EMG activation levels and patterns
The activation level of each muscle (iEMG) increased with increasing task difficulty (p < 0.001; Fig. 1). Additionally, in all tasks the EMG pattern was similar to the pattern in wide stance. Specifically, the cosine of the angles between the muscle activation vectors for wide stance and the other tasks were - narrow (0.94 ± 0.07), tandem (0.93 ± 0.07) and one leg (0.88 ± 0.08).
Pooled coherence
In all frequency bands (Figs 2, 3 and 5), coherence was higher in AG-AG compared to AG-ANT group (muscle group main effect). Additionally, in the low and high frequency bands there was a task main effect and an interaction between task difficulty and muscle group (Figs 4 and 5). Post hoc paired t-tests revealed that in the low frequency band, only AG-ANT coherence was higher in one leg compared to wide, t(18) = −3.00, p = 0.008, Cohen’s d = 0.68, mean difference = 0.1 z-score*Hz; and lower in narrow compared to wide t(18) = 2.85, p = 0.011, Cohen’s d = 0.65, mean difference = 0.01 z-score*Hz. Additionally, AG-AG coherence was higher than AG-ANT coherence only in wide (t(18) = 3.41, p = 0.003, Cohen’s d = 0.92, ∆ = 0.88 z-score*Hz) and narrow (t(18) = 3.53, p = 0.002, Cohen’s d = 1.32, ∆ = 1.58 z-score*Hz) stance. In the high frequency band, AG- AG coherence was higher in one leg compared to wide t(18) = −4.19, p = 0.001, Cohen’s d = 0.96, mean difference = 0.24 z-score*Hz; while AG-ANT coherence was lower in narrow compared to wide t(18) = 3.82, p = 0.001, Cohen’s d = 0.88, mean difference = 0.08 z-score*Hz. Additionally, AG-AG coherence was higher than AG-ANT coherence in narrow (t(18) = 3.74, p = 0.001, Cohen’s d = 1.05, ∆ = 0.98 z-score*Hz), tandem (t(18) = 4.97, p < 0.001, Cohen’s d = 1.21, ∆ = 1.08 z-score*Hz) and one leg (t(18) = 4.80, p < 0.001, Cohen’s d = 1.12, ∆ = 0.86 z-score*Hz) stance. Note that in both the low and high frequency bands, the lower coherence in narrow compared to wide stance is statistically significant, but the mean differences are much smaller than the increase from wide to one leg stance and cannot be meaningfully interpreted. In the medium frequency band, there was a main effect of task but a relatively small effect size. Additionally, none of the post-hoc tests were significant indicating that coherence in any of the difficult tasks did not differ from the control task i.e., wide stance. Table 2 shows the p-values, F values, degrees of freedom and effect sizes (partial eta squared) for the ANOVAs.
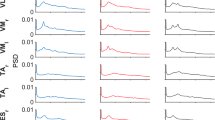
Effect of muscle group (AG-AG or AG-ANT) on coherence. Left panels depict individual muscle pair coherence and right panels show coherence pooled across the AG-AG (Sol-LG, Sol-PL, LG-PL) and AG-ANT (Sol-TA, LG-TA, RF-BF) muscles pairs. Solid lines depict AG-AG muscles and broken lines depict AG-ANT muscles.
Non-pooled coherence
In the AG-AG group, the main effect of task, main effect of muscle pair and the interaction were significant in all 3 frequency bands (Table 3). In the AG-ANT group, the main effect of task, main effect of muscle pair and the interaction were significant in the low and medium frequency bands (Table 4). In some tasks, the coherence for some muscle pairs did not exceed the significance level (see Fig. 2). Post-hoc tests were not computed if coherence in both pairs or tasks did not exceed the significance level.
Post-hoc tests for the differences between individual muscle pairs, in each task are shown Tables 5 and 6. In the AG-AG group, in tandem and one leg stance, LG-PL coherence was higher than the other 2 pairs, and Sol-LG coherence was higher than Sol-PL. In the AG-ANT group, in tandem and one leg stance, TA-Sol and TA-LG coherence was higher than RF-BF coherence.
Post-hoc tests for the differences between tasks, for each individual pair are shown in Tables 7 and 8. In the AG-AG group, LG-PL coherence was higher in one leg and tandem, compared to wide stance. Also, Sol-PL coherence was lower in narrow and tandem, compared to wide stance possibly due to a peak observed in wide stance at approximately 10 Hz.
Discussion
We examined the effects of task difficulty on common neural inputs to lower extremity AG-AG and AG-ANT muscles in standing, in healthy young adults. The increase in COP velocity and area confirmed that the experimental manipulations increased task difficulty. In agreement with the hypothesis, we found higher coherence in AG-AG compared to AG-ANT pairs in the three frequency bands. Coherence in the difficult one leg task was higher than wide stance, only in the AG-ANT group in the low frequency band (0–5 Hz), reflecting common subcortical inputs, and only in AG-AG group in the high frequency band (16–40 Hz), reflecting common corticospinal inputs. Therefore, common neural inputs to both AG-AG and AG-ANT muscles increase with difficulty but are likely driven by different sources of input to spinal alpha motor neurons. Our data supports the argument that common neural inputs to groups of muscles simplify the co-ordination of lower extremity muscles to control standing balance. Biomechanically, we expected the ankle muscles to be more important for maintaining balance in our experimental tasks. We included some knee muscles because previous muscle synergy analyses showed that knee muscles are also important for COP control in standing1,2. However, RF-BF coherence was consistently low, possibly because these muscles are not as functionally relevant as the ankle muscles for the chosen tasks. Therefore, our conclusions regarding AG-ANT common input are limited to the ankle muscles. Further studies are required to determine if other groups/pairs of knee muscles receive common inputs in standing tasks.
Common inputs to alpha motor neurons can arise from supraspinal inputs, afferent feedback or spinal connections between motor neuron pools31. Even though the exact neurophysiological origin of 0–5 coherence between unilateral muscles is not known, it is maintained in patients with cortical and capsular strokes35,36, suggesting a subcortical source16. Coherence in the 6–15 Hz band may have contributions from both cortical and subcortical sources and there is some evidence that it arises from neural networks comprising the cerebellum, sensorimotor cortex, inferior olive and thalamus16,37,38. In the 16–40 Hz range, EMG-EMG coherence is diminished in spinal cord injury patients28 and EMG is coherent with cortical activity recorded using EEG or MEG37,39, providing strong evidence for a corticospinal origin. A limitation of this method is that increase in EMG- EMG coherence cannot be directly interpreted as an increase in the level of co-activation as quantified using EMG amplitude. High EMG-EMG coherence indicates that motor units in both muscles receive neural inputs at the same frequencies14. Though such neural inputs may not necessarily arrive at both muscles at the same time, increase in AG-ANT coherence does suggest that the neural inputs can support a co-ordination pattern that increases stiffness. Given this physiological background, in the following sections we discuss the relevance of common neural inputs for the coordination of lower extremity muscles in standing. In agreement with the hypothesis, we found that AG-AG coherence which supports direction specific reciprocal muscle control is usually higher that AG-ANT coherence which supports stiffness control. However, low frequency coherence reflecting subcortical common inputs were almost equivalent in the AG-AG and AG-ANT groups in the two most difficult tasks (Fig. 5). This finding must be interpreted in conjunction with the observations in other frequency bands. In both the medium and high frequency bands AG-AG coherence is consistently higher than AG-ANT coherence. It is thus clear that there is a bias towards functional synergies which can create direction specific torques to counteract gravitational torques. However, when there is a need to increase AG-ANT co-activation, it is likely supported by sub-cortical inputs to alpha motor neurons. On the other hand, task related increases in AG-AG coherence are presumably driven primarily by corticospinal inputs.
In standing, 0–5 Hz coherence is observed between bilateral homologous muscles17,31,40 and unilateral muscles acting on different joints4,5,41 and our study adds to the limited evidence for 0–5 Hz coherence between unilateral muscles acting at the same joint17. We found no effect of task difficulty on AG-AG coherence pooled across three pairs, but in agreement with previous data30 we found that pooled AG-ANT coherence increases when task difficulty increases due to reductions in BOS. The examination of individual muscle pairs (i.e., non-pooled coherence) supports the findings from pooled data in general. However, some further nuances become apparent. Compared to other AG-AG pairs, LG-PL coherence is relatively higher while Sol-PL coherence is relatively low, especially in the difficult tasks (Table 5). Additionally, task difficulty related increase in coherence is seen only in the LG-PL pair. Though Sol and PL are both plantarflexors, Sol is an invertor and PL is an evertor, making them antagonists in the mediolateral (ML) direction. On the other hand, LG and PL are agonists in both anteroposterior (AP) and ML directions. These findings are in line with a previous study10 which examined coherence between different pairs of plantarflexors in standing and found the highest coherence between medial gactrocnemius (MG) and soleus, which are both invertors. Also, in the 0–5 Hz range, lower extremity EMG is coherent with COP movements10,42 suggesting that inputs to particular pairs of muscle may be synchronized based on the direction of the torques they produce. In other words, coherence between specific muscle pairs may be related to the direction in which their activation shifts the COP. Indeed, LG or PL activation shifts the center of pressure (COP) medially, while MG or SL shift the COP laterally10,43. The stronger coherence between LG-PL and MG-Sol in difficult tasks provides evidence for task specific evertor/invertor synergies, which are not required for counteracting the smaller gravitational torques in wide and narrow stance. Similarly, the increase in AG-ANT coherence is driven by TA and Sol which are both invertors. Therefore, our findings support the argument that functional synergies, formed through common neural inputs to different muscles, are specific to the biomechanical demands of the task. A limitation of the present and previous studies is that surface EMG may not accurately reflect motor unit coherence at low frequencies (<5 Hz) and high contraction intensities, as suggested by recent experimental data44. However, since surface EMG underestimates low frequency common inputs, task difficulty related effects may in fact be more prominent if intra-muscular recordings are used.
In our data, 6–15 Hz AG-AG coherence is apparent in all the tasks, while there is little or no AG-ANT coherence in any task. Also, there is no effect of task difficulty on either AG-AG or AG-ANT coherence. However, we do observe a peak in AG-AG coherence at ~10 Hz in wide and narrow stance, but not in tandem and one leg stance. Coherence in the 8–12 Hz (with a peak at ~10 Hz) range has previously been reported between bilateral homologous muscles40,45. Though we tested coherence between unilateral muscles, the peak in EMG power at 10 Hz is present in the two tasks which require symmetrical activity in both legs. However, it disappears in tandem and one leg stance when the two legs have different biomechanical configurations and consequently muscle activations. Indeed, analysis of non-pooled coherence shows that Sol-PL coherence is lower in tandem, compared to wide stance. Sol-PL coherence is also lower in narrow compared to wide. The 10 Hz peak is also apparent in narrow stance (Fig. 2), although it is smaller than the peak in wide stance. However, visual inspection of the graph (Fig. 2) shows that the peak is present in narrow stance also, although it is smaller than the peak in wide stance. Therefore, we hypothesize that 10 Hz coherence reflects synchronization of muscle activation between the legs. Obata et al. found a small 10 Hz peak in coherence between unilateral MG-Sol, but only when vision was occluded in bipedal stance17. In our data, the peak is apparent in all three AG-AG pairs and therefore the conflicting findings cannot be attributed to the specific muscle pair. They pooled data across all the participants and used EMG normalized to unit variance, possibly accounting for the differences. Also, it must be noted that 10 Hz oscillations are widespread in the neuromotor system and likely have a multifactorial origin46. Further work is required to clarify the reasons for differences in the peaks between tasks, but this observation further emphasizes the biomechanical task specificity of functional synergies.
In standing, high frequency AG-AG coherence becomes apparent only when task difficulty and consequently muscle activation increases. This finding is in line with previous reports examining the effects of BOS manipulations and leaning tasks on coherent inputs to lower extremity muscles10,47. It is also in agreement with previous TMS and EEG studies which demonstrate increasing cortical involvement in standing balance control as task difficulty increases18,19,48,49,50. However, a caveat must be added. Currently available measurement techniques allow easier recording of cortical compared to subcortical activity. Since the M1 receives inputs from multiple brain areas, including prefrontal areas, cerebellum and basal ganglia, our findings (and those of TMS and EEG studies) do not rule out the possibility that task-related changes observed in M1 activity are in fact driven by inputs to M1 from other brain areas. Further studies are required to determine if other brain areas drive the synchronization of M1 outputs.
High frequency AG-ANT coherence was not present in any task. Individual motor neurons within the primary motor cortex (M1) may activate multiple AG-AG muscles51, possibly though branched descending inputs to spinal motor neuron pools innervating different muscles6. Additionally, M1 neurons show strong directional tuning, i.e., they are activated only during movements in one direction51. In fact, some M1 neurons also have a inhibitory effects on antagonistic movements52. Therefore, the properties of descending inputs from individual M1 neurons to multiple muscles favor coherent AG-AG activation. However, the possibility of synchronized activation of multiple M1 neurons, with differing directional tunings, cannot be ruled out. Additionally, common corticospinal inputs may also arise from other areas like the premotor and supplementary motor areas. Further studies are required to determine whether AG-ANT coherence driven by corticospinal inputs may emerge in other types of tasks and movements. In summary, we demonstrated that common neural input is a likely mechanism underlying the task-specific coordination of lower extremity muscles in standing. This argument is strengthened by the observation that the pattern of coherence reflects the biomechanical demands of each task. Additionally, AG-AG synchronization can be driven by both cortical and subcortical inputs, but when task difficulty increases, corticospinal involvement increases. Conversely, task related changes in AG-ANT synchronization are driven mainly by subcortical inputs.
Data Availability
Data generated or analysed during this study are included in the Supplementary Information files of this article.
References
Krishnamoorthy, V., Goodman, S., Zatsiorsky, V. & Latash, M. L. Muscle synergies during shifts of the center of pressure by standing persons: identification of muscle modes. Biol. Cybern. 89, 152–161 (2003).
Krishnamoorthy, V., Latash, M. L., Scholz, J. P. & Zatsiorsky, V. M. Muscle modes during shifts of the center of pressure by standing persons: Effect of instability and additional support. Exp. Brain Res. 157, 18–31 (2004).
Imagawa, H., Hagio, S. & Kouzaki, M. Synergistic co-activation in multi-directional postural control in humans. J. Electromyogr. Kinesiol. 23, 430–437 (2013).
Danna-Dos-Santos, A. et al. Multi-muscle control during bipedal stance: An EMG-EMG analysis approach. Exp. Brain Res. 232, 75–87 (2014).
Danna-Dos-Santos, A. et al. The influence of visual information on multi-muscle control during quiet stance: a spectral analysis approach. Exp. brain Res. 233, 657–669 (2015).
Semmler, J. G. Motor unit synchronization and neuromuscular performance. Exerc. Sport Sci. Rev. 30, 8–14 (2002).
Winter, D. A. Biomechanics and motor control of human movement. (John Wiley & Sons, 2009).
Winter, D. A., Prince, F., Frank, J. S., Powell, C. & Zabjek, K. F. Unified theory regarding A/P and M/L balance in quiet stance. J. Neurophysiol. 75, 2334–2343 (1996).
Amiridis, I. G., Hatzitaki, V. & Arabatzi, F. Age-induced modifications of static postural control in humans. Neurosci. Lett. 350, 137–140 (2003).
Watanabe, T., Saito, K., Ishida, K., Tanabe, S. & Nojima, I. Coordination of plantar flexor muscles during bipedal and unipedal stances in young and elderly adults. Exp. brain Res. 1–11 (2018).
Yang, W. C., Cheng, C. H., Wang, H. K., Lin, K. H. & Hsu, W. L. Multi-muscle coordination during a challenging stance. Eur. J. Appl. Physiol. 115, 1959–1966 (2015).
Reeves, N. P., Narendra, K. S. & Cholewicki, J. Spine stability: the six blind men and the elephant. Clin. Biomech. 22, 266–274 (2007).
Grüneberg, C., Bloem, B. R., Honegger, F. & Allum, J. H. J. The influence of artificially increased hip and trunk stiffness on balance control in man. Exp. brain Res. 157, 472–485 (2004).
Boonstra, T. W. & Breakspear, M. Neural mechanisms of intermuscular coherence: implications for the rectification of surface electromyography. J. Neurophysiol. 107, 796–807 (2011).
De Luca, C. J. & Erim, Z. Common drive in motor units of a synergistic muscle pair. J. Neurophysiol. 87, 2200–2204 (2002).
Grosse, P., Cassidy, M. J. & Brown, P. EEG–EMG, MEG–EMG and EMG–EMG frequency analysis: physiological principles and clinical applications. Clin. Neurophysiol. 113, 1523–1531 (2002).
Obata, H., Abe, M. O., Masani, K. & Nakazawa, K. Modulation between bilateral legs and within unilateral muscle synergists of postural muscle activity changes with development and aging. Exp. brain Res. 232, 1–11 (2014).
Papegaaij, S., Taube, W., Hogenhout, M., Baudry, S. & Hortobágyi, T. Age-related decrease in motor cortical inhibition during standing under different sensory conditions. Front. Aging Neurosci. 6, 126 (2014).
Nandi, T., Fisher, B. E., Hortobágyi, T. & Salem, G. J. Increasing mediolateral standing sway is associated with increasing corticospinal excitability, and decreasing M1 inhibition and facilitation. Gait Posture 60, 135–140 (2018).
Tokuno, C. D., Taube, W. & Cresswell, A. G. An enhanced level of motor cortical excitability during the control of human standing. Acta Physiol. 195, 385–395 (2009).
Association, G. A. of the W. M. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 81, 14 (2014).
Hebbal, G. V. & Mysorekar, V. R. Evaluation of some tasks used for specifying handedness and footedness. Percept. Mot. Skills 102, 163–164 (2006).
Yao, B., Salenius, S., Yue, G. H., Brown, R. W. & Liu, J. Z. Effects of surface EMG rectification on power and coherence analyses: an EEG and MEG study. J. Neurosci. Methods 159, 215–223 (2007).
Myers, L. J. et al. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J. Neurosci. Methods 124, 157–165 (2003).
Poston, B., Danna-Dos Santos, A., Jesunathadas, M., Hamm, T. M. & Santello, M. Force-Independent Distribution of Correlated Neural Inputs to Hand Muscles During Three-Digit Grasping. J Neurophysiol 104, 1141–1154 (2010).
Halliday, D. M. et al. A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog. Biophys. Mol. Biol. 64, 237–278 (1995).
Rosenberg, J. R., Amjad, A. M., Breeze, P., Brillinger, D. R. & Halliday, D. M. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog. Biophys. Mol. Biol. 53, 1–31 (1989).
Norton, J. A. & Gorassini, M. A. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J. Neurophysiol. 95, 2580–2589 (2006).
Amjad, A. M., Halliday, D. M., Rosenberg, J. R. & Conway, B. A. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J. Neurosci. Methods 73, 69–79 (1997).
García-Massó, X., Pellicer-Chenoll, M., Gonzalez, L. M. & Toca-Herrera, J. L. The difficulty of the postural control task affects multi-muscle control during quiet standing. Exp. brain Res. 234, 1977–1986 (2016).
Boonstra, T. W. et al. Muscle networks: Connectivity analysis of EMG activity during postural control. Sci. Rep. 5, 17830 (2015).
Ferguson, C. J. An effect size primer: A guide for clinicians and researchers. Prof. Psychol. Res. Pract. 40, 532 (2009).
Richardson, J. T. E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 6, 135–147 (2011).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4, 1–12 (2013).
Farmer, S. F., Bremner, F. D., Halliday, D. M., Rosenberg, J. R. & Stephens, J. A. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J. Physiol. 470, 127–155 (1993).
Nielsen, J. B. et al. Reduction of common motoneuronal drive on the affected side during walking in hemiplegic stroke patients. Clin. Neurophysiol. 119, 2813–2818 (2008).
Mima, T. & Hallett, M. Corticomuscular coherence: a review. J. Clin. Neurophysiol. 16, 501 (1999).
Schnitzler, A., Timmermann, L. & Gross, J. Physiological and pathological oscillatory networks in the human motor system. J. Physiol. 99, 3–7 (2006).
Halliday, D. M., Conway, B. A., Farmer, S. F. & Rosenberg, J. R. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci. Lett. 241, 5–8 (1998).
Boonstra, T. W. et al. Low-alcohol doses reduce common 10-to 15-Hz input to bilateral leg muscles during quiet standing. J. Neurophysiol. 100, 2158–2164 (2008).
Degani, A. M., Leonard, C. T. & Danna-dos-Santos, A. The use of intermuscular coherence analysis as a novel approach to detect age-related changes on postural muscle synergy. Neurosci. Lett. 656, 108–113 (2017).
Saffer, M., Kiemel, T. & Jeka, J. Coherence analysis of muscle activity during quiet stance. Exp. brain Res. 185, 215–226 (2008).
Sozzi, S., Honeine, J. L., Do, M. C. & Schieppati, M. Leg muscle activity during tandem stance and the control of body balance in the frontal plane. Clin. Neurophysiol. 124, 1175–1186 (2013).
Dideriksen, J. L. et al. Coherence of the Surface EMG and Common Synaptic Input to Motor Neurons. Frontiers in Human Neuroscience 12, 207 (2018).
Boonstra, T. W. et al. Fatigue-related changes in motor-unit synchronization of quadriceps muscles within and across legs. J. Electromyogr. Kinesiol. 18, 717–731 (2008).
McAuley, J. H. & Marsden, C. D. Physiological and pathological tremors and rhythmic central motor control. Brain 123, 1545–1567 (2000).
Watanabe, T., Saito, K., Ishida, K., Tanabe, S. & Nojima, I. Age-Related Declines in the Ability to Modulate Common Input to Bilateral and Unilateral Plantar Flexors During Forward Postural Lean. Frontiers in Human Neuroscience 12, 254 (2018).
Papegaaij, S., Baudry, S., Négyesi, J., Taube, W. & Hortobágyi, T. Intracortical inhibition in the soleus muscle is reduced during the control of upright standing in both young and old adults. Eur. J. Appl. Physiol. 116, 959–967 (2016).
Nandi, T. et al. In Standing, Corticospinal Excitability Is Proportional to COP Velocity Whereas M1 Excitability Is Participant-Specific. Front. Hum. Neurosci. 12 (2018).
Slobounov, S., Hallett, M., Cao, C. & Newell, K. Modulation of cortical activity as a result of voluntary postural sway direction: an EEG study. Neurosci. Lett. 442, 309–13 (2008).
Fetz, E. E. & Cheney, P. D. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J. Neurophysiol. 44, 751–772 (1980).
Cheney, P. D., Kasser, R. & Holsapple, J. Reciprocal effect of single corticomotoneuronal cells on wrist extensor and flexor muscle activity in the primate. Brain Res. 247, 164–168 (1982).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of the study and writing of the manuscript. T.N. performed the experiments. T.N. T.H. and C.L. carried out the analyses.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nandi, T., Hortobágyi, T., van Keeken, H.G. et al. Standing task difficulty related increase in agonist-agonist and agonist-antagonist common inputs are driven by corticospinal and subcortical inputs respectively. Sci Rep 9, 2439 (2019). https://doi.org/10.1038/s41598-019-39197-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39197-z
This article is cited by
-
Upper and lower limb tremor in Charcot–Marie–Tooth neuropathy type 1A and the implications for standing balance
Journal of Neurology (2024)
-
Common synaptic inputs and persistent inward currents of vastus lateralis motor units are reduced in older male adults
GeroScience (2024)
-
Sensorimotor recalibration of postural control strategies occurs after whole body vibration
Scientific Reports (2023)
-
Effects of global postural alignment on posture-stabilizing synergy and intermuscular coherence in bipedal standing
Experimental Brain Research (2022)
-
Modulation of intracortical inhibition and excitation in agonist and antagonist muscles following acute strength training
European Journal of Applied Physiology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.