Abstract
Retrospective multicentre study aiming at analysing the etiology, characteristics and outcome of bloodstream infections (BSI) in people living with HIV (PLWHIV) in an era of modern antiretroviral therapy. Between 2008 and 2015, 79 PLWHIV had at least 1 BSI, for a total of 119 pathogens isolated. Patients were mainly male (72.1%), previous intravenous drug users (55.7%), co-infected with HCV or HBV (58.2%) and in CDC stage C (60.8%). Gram-positive (G+) pathogens caused 44.5% of BSI, followed by Gram-negative (G−), 40.3%, fungi, 10.9%, and mycobacteria, 4.2%. Candida spp. and coagulase-negative staphylococci were the most frequent pathogens found in nosocomial BSI (17% each), while E.coli was prevalent in community-acquired BSI (25%). At the last available follow-up, (mean 3.2 ± 2.7 years) the overall crude mortality was 40.5%. Factors associated with mortality in the final multivariate analysis were older age, (p = 0.02; HR 3.8, 95%CI 1.2–11.7) CDC stage C (p = 0.02; HR 3.3, 95%CI 1.2–9.1), malignancies, (p = 0.004; HR 3.2, 95%CI 1.4–7.0) and end stage liver disease (p = 0.006; HR 3.4, 95%CI 1.4–8.0). In conclusion, the study found high mortality following BSI in PLWHIV. Older age, neoplastic comorbidities, end stage liver disease and advanced HIV stage were the main factors correlated to mortality.
Similar content being viewed by others
Introduction
Due to the availability of modern combined antiretroviral therapy (cART), patients living with HIV (PLWHIV) have experienced a reduction in overall mortality and incidence of AIDS-defining conditions1,2. The natural history of HIV infection has changed, evolving from a disease with a bad short-term prognosis to a long-term chronic infection, forcing physicians to focus on new clinical problems, such as aging, kidney disease, osteoporosis, diabetes, cardiovascular diseases and the effects of long-term drug exposure. Indeed, late complications in otherwise responding HIV-infected patients exist and include, among others, neoplastic diseases requiring chemotherapy, putting these patients at risk of opportunistic infections similar to those encountered in the non-HIV positive population with the same cancer. In the meantime, in some countries, including Italy, up to 29% of the patients arrive late at a HIV diagnosis, with CD4+ T-cell (CD4+) count <200/mmc or with an AIDS-defining condition (infection and/or cancer) already present at the moment of diagnosis3. In this patient population, the immunological recovery can be challenging4,5, and they may require long hospitalization for the combined treatment of the HIV infection, AIDS-related infections and sometimes neoplastic diseases6. At this point, they are at risk of developing classic healthcare-associated infections, such as bacteremia, which further complicate an already difficult situation. Indeed, non-AIDS defining bacterial infections are among the main causes of hospital admissions nowadays7,8,9,10.
The aim of the present study is to analyze the etiology, clinical characteristics and outcome of bloodstream infections (BSI) in PLWHIV in an era of cART in a country with a high rate of late-presenters.
Results
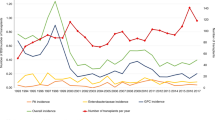
Throughout the study period, 2379 PLWHIV were followed in the 2 participating centers with 2281 hospitalizations. A total of 79/2379 (3.3%) patients had at least 1 BSI, for a total of 104 episodes (67, 64.4%, community acquired and 37, 35.5%, hospital-acquired, Fig. 1) and 119 isolated pathogens. BSI accounted for or complicated a total of 93 of 2281 hospitalizations (4.0%), with a median duration of hospital stay of 17 (10–36) and 59 (31.5–100.5) days in community-acquired and nosocomial episodes, respectively (p < 0.0001).
Number of community acquired and nosocomial episodes of bloodstream infections (BSI) and CD4+ T-lymphocytes (CD4) count in the whole study population (a) and in patients newly diagnosed with HIV (b) in the years of the study. P values in the graph are referred to the CD4 trend across the study period.
The majority of PLWHIV with BSI were male (72.1%), previous intravenous drug users (55.7%), co-infected with HCV or HBV (58.2%) and in CDC stage C (60.8%). General characteristics of the study population are given in Table 1. The mean follow-up period after the first BSI episode was 3.2 ± 2.7 years.
The median CD4+ count at first BSI was 175 (88–331.5) cells/μl as compared to 420 (252–610) in the general population of PLWHIV observed at the first visit to the 2 outpatient clinics during the study period (p < 0.0001). Among patients with at least 1 BSI, the proportion of late-presenters was 24.0% (19 of 79) as opposed to 10.4% (239 of 2304) in the group of patients without any BSI (p < 0.0001).
A total of 14 patients developed more than 1 BSI episode. They had similar characteristics as compared with patients with a single episode (i.e., age, sex, CDC stage, frequency of HBV and HCV co-infection and of late presentation, CD4+ count and nadir at the time of the first BSI).
Among the 119 isolated pathogens, there were 53 (44.5%) Gram-positive (G+) cocci, 48 (40.3%) Gram-negative (G−) rods, 13 (10.9%) fungi (9 candidemia and 4 cryptococcal infections) and 5 (4.2%) mycobacteria (2 M. tuberculosis complex and 3 M. avium complex). Candidemia was caused by C. albicans in 6/9 cases, C. glabrata, C. tropicalis and C. parapsilosis in 1/6 cases each. The median CD4+ T-cell count (cells/μl) was 210 (102–299.5) in patients who developed a BSI caused by G−, 132 (60–250) in G+, 77 (11.5–120) in mycobacteria and 62 (35–215) in fungal BSI, with a significant decreasing trend in the different groups of etiologies (p = 0.011). Parallelly, the frequency of late presenting in each group of etiologies was also different, higher in mycobacterial (80.0%) than in fungal (53.8%), G+ (24.5%) and G− (14.6) BSI, p < 0.0001.
In Table 2, all pathogens are classified, whether isolated in nosocomial or community-acquired episodes. As expected, Candida spp. and coagulase-negative staphylococci (CONs) were more often nosocomial in origin (8 of 9 each), while E.coli was prevalent in community-acquired BSI (18 episodes, 24.6%). With respect to community-acquired episodes, nosocomial BSI were more often observed in patients on antiretroviral treatment and with BSI caused by Candida spp. On the other side, hospitalization for an acute infectious disease or febrile syndrome was protective towards nosocomial episodes, compared to other causes of hospitalization (area under the curve of the model, AUC = 0.855, Table 3).
In terms of antimicrobial susceptibility, no G− rod was resistant to carbapenems, while 8 out of 37 Enterobacteriaceae and 0 of 9 Pseudomonas spp. were ESBL-producers. Among G+ cocci, 7 out of 9 CONs (7/7 nosocomial) and 8 of 14 S. aureus strains (3/8 nosocomial) were resistant to methicillin, while 9 of 11 enterococci were resistant to ampicillin. All but one G+ (i.e., one nosocomial-acquired E. faecium) were susceptible to vancomycin. Two of 9 candidemias were due to fluconazole resistant strains (one C. albicans and one C. glabrata).
The observational analysis showed that the crude mortality after the first BSI episode in the 79 patients was 6.3%; 8.9%, 21.5% and 27.8% at 2, 4, 24 and 48 weeks, while the overall mortality at the last available follow up was 40.5%, (32/79 patients), with a median time between infection and death of 231 days (48–438). Four factors were associated with mortality at multivariable analysis: age >43 years (p = 0.02; HR 3.8, 95%CI 1.2–11.7), CDC stage C (p = 0.02; HR 3.3, 95%CI 1.2–9.1), malignancies (p = 0.004; HR 3.2, 95%CI 1.4–7.0), and end stage liver disease (p = 0.006; HR 3.4, 95%CI 1.4–8.0) (Table 4).
Discussion
In the present study, we analyzed the etiology and outcome of BSI in PLWHIV in the years 2008 to 2015 at two Infectious Disease Centers in Northern Italy. Despite the fact that the study has been conducted in the context of a modern cART era, only 66% of the patients were actually on antiretroviral treatment, and a high number of late presenters (24%) and patients in advanced stage of disease (61% in CDC stage C) were still present in our series. The patients who developed BSI had lower CD4+ counts and higher frequency of late presenters when compared to the global population of PLWHIV without BSI, confirming the importance of immune recovery for also preventing bacterial infections other than those commonly codified as AIDS-defining11. Indeed, we found a quite high crude mortality rate in the first weeks after BSI, with a continuous increasing trend in the months following the episode, arriving to 40% after a median time of 231 days. This high mortality rate likely reflects the severity of the underlying diseases of patients who develop BSI and the high frequency of comorbidities in PLWHIV, possibly favored by a somewhat inadequate immunological reconstitution and by chronic immune activation12. This is shown by the fact that patients with BSI had lower CD4+ counts than the general population of PLWHIV and that, among them, mortality was higher in those with older age, more advanced CDC stage and comorbidities such as malignancies or end stage liver disease. Surprisingly, we did not find a reduced HR for mortality in patients on cART, whether they had HIV-RNA < 200 copies/ml or not. However, cART is expected to diminish the risk of comorbidities found to be associated with higher mortality in our series, such as neoplastic diseases or AIDS-defining illnesses13, so that it is possible that the lack of association between cART use and reduced mortality might be simply due to the small sample size.
On the other hand, the high frequency of nosocomial episodes found suggests how BSI can also complicate the clinical course in otherwise responding HIV-infected patients, as these episodes were not linked to the CDC stage of disease but on the contrary more frequent in patients taking antiretroviral treatment who were mainly hospitalized for non-infectious illnesses.
The use of a modern cART might however have played a role in the general study population, as we found that the epidemiology of pathogens causing BSI changed with respect to previous data14,15,16,17,18,19,20,21,22,23,24. In fact, we found a high prevalence of G− as the cause of community-acquired BSI, with E. coli as the main causative agent overall. Even if an increasing prevalence of this pathogen had already been signaled in PLWHIV15, in our knowledge, it was the principal etiology of all BSI only in one previous study24,25. This change may be linked to easier and earlier access to cART in comparison with the past, with consequent decreasing frequency of “AIDS-defining BSI” and increase of other common causes of BSI, shared with HIV-negative patients26.
Less data is available on the etiology of hospital-acquired BSI in PLWHIV14,16,27,28,29. Candidemia had previously been reported as an emergent cause of BSI in PLWHIV25, but its burden seemed still higher in our work, where it was the first cause of nosocomial BSI and significantly correlated with the nosocomial occurrence of the infection. A possible interpretation is that the better survival of PLWHIV is burdened by longer and more frequent hospitalizations, and this constitutes a well-known risk factor for the development of candidemia30. Indeed, nosocomial BSI in PLWHIV has increased in last years14,31 and constitutes about 35% of the episodes in our work.
Finally, we also assessed the resistance profile of principal G−, G+ and Candida spp. isolated from blood cultures. Despite the fact that resistance to carbapenems is a major concern in Italy, even with increasing rates in last years32, it did not have a major impact in PLWHIV during the study period, as no G− harbored carbapenem-resistant rods. Moreover, the majority of Enterobacteriaceae were susceptible to third generation cephalosporins, also if some ESBL producer strains were found. Numbers are too low to draw any conclusions, but on the basis of the descriptive analysis of our population, we can only say that the rising resistance reported in other contexts33 seem not to spare PLWHIV. The same is true also for G+. In fact, we found a quite high proportion of community acquired methicillin resistant S. aureus, in accordance with the literature data34, while among CONs methicillin resistance was confined to nosocomial episodes. A single vancomycin resistant E. faecium was isolated, while all other G+ conserved vancomycin susceptibility.
The study has several limitations. A major limit is that we did not have a control group, so we could not demonstrate that the mortality we found, although unusually high, was significantly different from the mortality in PLWHIV without BSI with similar clinical conditions. Moreover, the retrospective design did not permit the analysis of all underlying conditions and concomitant therapies that may have influenced both the primary and secondary outcomes of the study. For the same reason, we cannot exclude an underestimation of the real number of BSI, as it is possible that in some cases either the blood cultures were not promptly collected or early antimicrobial treatment gave negative results before hospital admission or blood collection. In addition, changes in infection-control practices or in the distribution of pathogens over time in the participating centers may have played a role in the epidemiologic framework described in the article that appeared different from those previously described in other similar studies13,14,15,16,17,18,19,20,21,22,23. Finally, the relatively low incidence of BSI in PLWHIV did not permit the analysis of a large amount of data, and fortuity may have played a role in the definition of some variables or in the lack of significance of others.
With these limitations, our study highlights new emerging features of BSI in PLWHIV in the recent cART era. BSI share the etiologies of both community-acquired and nosocomial episodes with HIV-negative patients but are still marked by high mortality, correlated not only to an advanced HIV stage but also to other major comorbidities. In the future, we expect that the scenario will change again as in the present study the history of intravenous drug use, now a declining risk factor, and HCV co-infection, now present only in a minority of new HIV diagnosis, are still highly represented35,36. Moreover, there are still numerous patients with low CD4+ counts and with advanced diseases. Both hopefully will reduce in the next years due to new and earlier cART strategies. Multicenter and prospective studies in the future might better define BSI epidemiological changes, risk factors and outcomes in the continually evolving population of PLWHIV.
Methods
This is a retrospective study conducted in the period 2008–2015 at two Infectious Diseases Units in the city of Genoa (Italy, Liguria Region), the Ospedale Policlinico San Martino and the Ente Ospedaliero Ospedali Galliera. The 2 units follow slightly more than 2000 HIV-positive patients in a Region with an estimated overall number of about 3000 HIV-infected patients in treatment or follow up.
Data about all HIV-infected patients >18 years followed at the two centers were retrieved from the Database of the Liguria HIV Network (RLH-DB): (www.reteligureHIV.it). The Liguria HIV Network is a locally developed online platform that supports the direct connection between medical and laboratory records of HIV-infected patients, allowing the automatic and prospective transfer of anonymized data37,38. Each patient has an identification code, which is registered in RLH-DB at the engagement (first ambulatory visit for outpatients or first day of hospital stay for inpatients). Safety and precision are granted by the approved use of hospital anonymized codes. The use of RLH-DB was approved by the Ligurian Ethical Committee. At the moment of registration in RHL-DB, patients sign an informed consent form in which they declare if they agree to use their clinical data, in anonymous form, for scientific purposes. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and in accordance with Italian national laws.
All patients registered in RLH-DB who gave written consent to study participation were considered eligible. The BSI episodes were extrapolated by revision of all positive blood cultures registered in the online platform. The number of hospitalizations in PLWHIV was obtained by reviewing discharge diagnostic codes of all patients hospitalized during the study period, on the basis of the International Classification of Diseases (ICD-9).
BSI was defined as at least one positive blood culture for fungi or bacteria in the setting of a compatible clinical disease. For BSI due to the same pathogen (microbial or fungal) at least 30 days between subsequent episodes were required to define separate BSI episodes. For BSI due to mycobacteria, multiple positive blood cultures, in the same patient, were considered as one single episode. For blood cultures positive for more than one pathogen within 48 hours, a single, polimicrobial, episode was considered. For common skin contaminants, like coagulase-negative staphylococci, Corynebacterium, Peptostreptococcus, Bacillus and Propionibacterium species, at least 2 positive blood cultures were required. For cryptococcosis only cases with positive blood cultures were included. For each patient the following information were collected at baseline (at the time of RLH-DB registration) or at time of BSI. Baseline information included gender, HBV co-infection (defined by HBsAg positivity), HCV co-infection (defined by qualitative HCV-RNA positivity), CDC stage (www.CDC.org), CD4+ count nadir. Information at time of each BSI included age, CD4+ count, detectable or undetectable (<200 copies/ml) plasma HIV-RNA, site of acquisition (nosocomial if first positive blood culture after at least 48 hours of admission, community-acquired if positive blood culture within the first 48 hours of hospitalization or in case of mycobacterial etiology), etiology of BSI, susceptibility pattern of the isolated pathogen (for Candida spp. only fluconazole susceptibility). Isolates were identified with the Vitek 2 system (bioMérieux, Marcy l’Etoile, France) and/or by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry (MALDI Biotyper, Bruker Daltonics, Leipzig, Germany, or VitekMS, bioMérieux). The in vitro susceptibility of the isolates was assessed with the Vitek 2 system (bioMérieux). Results were interpreted in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints. Major comorbidities and primary causes of hospitalizations were extrapolated by the RLH-DB data and the discharge diagnostic codes ICD-9 at each hospitalization. Patients who had CD4+ count <350 cells/µl at the moment of the diagnosis of HIV infection were defined as late presenters39. Number of BSI per patient was also collected, as well as the total number of hospitalizations in the overall cohort and the number of hospitalizations due to or complicated by a BSI. The outcome (survival) was evaluated at 2, 4, 24, 48 weeks after the episode and thereafter till the last available follow-up. The computerized systems for patient registration used by the participating centers, allow information on the deaths on the entire nation that are notified and updated in real time thanks to connection with the national registry office.
Statistical analysis
Quantitative data are presented as medians (1st and 3rd quartile) or means (±standard deviation) and categorical data as absolute numbers and percentages. Quantitative data were compared by Mann-Whitney U test, or, when more than two groups were compared, by Kruskal-Wallis test. Categorical variables were compared by chi-square test. Cox regression model was used to assess the impact of the above mentioned variables on survival. For the analysis of survival only the etiology of the last episode was considered. The impact of the same variables on site of BSI acquisition (community versus hospital acquired) was investigated using a generalized linear model, in which BSI episodes within the same subject were assumed as correlated to each other. Age was dichotomized according to the best threshold obtained from the receiver operating characteristic (ROC) curve analysis40. Only factors significantly associated with the outcome at univariate analysis were included in a multivariate model with a stepwise procedure. P-values < 0.05 were considered statistically significant. All analyses were carried out using the SAS software version 9.3 (Institute Inc., Cary, NC, USA).
Data Availability
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
References
Smith, C. J. et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 384, 241–248 (2014).
Søgaard, O. S. et al. Severe bacterial non-aids infections in HIV-positive persons: incidence rates and risk factors. J Infect 66, 439–446 (2013).
Girardi, E. et al. Delayed presentation and late testing for HIV: demographic and behavioral risk factors in a multicenter study in Italy. J Acquir Immune Defic Syndr. 36, 951–9 (2004).
Kaufmann, G. R. et al. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS. 16, 359–67 (2002).
Moore, R. D. & Keruly, J. C. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 44, 441–6 (2007).
Mussini, C. et al. Patients presenting with AIDS in the HAART era: a collaborative cohort analysis. AIDS. 22, 2461–9 (2008).
Bonnet, F. et al. Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine cohort, 2000–2004. HIV Med. 8, 547–554 (2007).
Hessamfar, M. et al. Severe Morbidity According to Sex in the Era of Combined Antiretroviral Therapy: The ANRS CO3 Aquitaine Cohort. PLoS One. 9, e102671 (2014).
Ferry, T. et al. Uncontrolled viral replication as a risk factor for non-AIDS severe clinical events in HIV-infected patients on long-term antiretroviral therapy: APROCO/COPILOTE (ANRS CO8) cohort study. J Acquir Immune Defic Syndr. 51, 407–415 (2009).
Ford, N. et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2, e438–44 (2015).
O’Connor, J. et al. Effect of immediate initiation of antiretroviral therapy on risk of severe bacterial infections in HIV-positive people with CD4 cell counts of more than 500 cells per μL: secondary outcome results from a randomised controlled trial. Lancet HIV. 4, e105–e112 (2017).
Duffau, P. et al. Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS. 29, 2099–108 (2015).
Molina, J. M. et al. Which HIV-infected adults with high CD4 T-cell counts benefit most from immediate initiation of antiretroviral therapy? A post-hoc subgroup analysis of the START trial. Lancet HIV. 5, e172–e180 (2018).
Afessa, B., Morales, I. & Weaver, B. Bacteremia in hospitalized patients with human immunodeficiency virus: A prospective, cohort study. BMC Infect Dis. 1, 13 (2001).
Archibald, L. K. et al. A hospital-based prevalence survey of bloodstream infections in febrile patients in Malawi: implications for diagnosis and therapy. J Infect Dis. 181, 1414–20 (2000).
Ortega, M. et al. Bloodstream infections among human immunodeficiency virus-infected adult patients: epidemiology and risk factors for mortality. Eur J Clin Microbiol Infect Dis. 27, 969–76 (2008).
Archibald, L. K., den Dulk, M. O., Pallangyo, K. J. & Reller, L. B. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis. 26, 290–6 (1998).
Bell, M. et al. Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. Int J Infect Dis. 5, 63–9 (2001).
Crump, J. A. et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 52, 341–8 (2011).
Mootsikapun, P. Bacteremia in adult patients with acquired immunodeficiency syndrome in the northeast of Thailand. Int J Infect Dis. 11, 226–31 (2007).
Edge, M. D. & Rimland, D. Community-acquired bacteremia in HIV-positive patients: protective benefit of co-trimoxazole. AIDS. 10, 1635–9 (1996).
Pedro-Botet, M. L. et al. Changes in bloodstream infections in HIV-positive patients in a university hospital in Spain (1995–1997). Int J Infect Dis. 6, 17–22 (2002).
Tumbarello, M. et al. HIV-associated bacteremia: how it has changed in the highly active antiretroviral therapy (HAART) era. J Acquir Immune Defic Syndr. 23, 145–51 (2000).
Phe, T. et al. Does HIV status affect the aetiology, bacterial resistance patterns and recommended empiric antibiotic treatment in adult patients with bloodstream infection in Cambodia? Trop Med Int Health. 18, 485–94 (2013).
Taramasso, L., Tatarelli, P. & Di Biagio, A. Bloodstream infections in HIV-infected patients. Virulence. 7, 320–8 (2016).
Mehl, A. et al. Trends in antimicrobial resistance and empiric antibiotic therapy of bloodstream infections at a general hospital in Mid-Norway: a prospective observational study. BMC Infect Dis. 17, 116 (2017).
Petrosillo, N. et al. Nosocomial bloodstream infections among human immunodeficiency virus-infected patients: incidence and risk factors. Clin Infect Dis. 34, 677–85 (2002).
Stroud, L. et al. Nosocomial infections in HIV-infected patients: preliminary results from a multicenter surveillance system (1989–1995). Infect Control Hosp Epidemiol. 18, 479–85 (1997).
Mitha, M., Furuya, E. Y. & Larson, E. Risk of healthcare associated infections in HIV positive patients. J Infect Prev. 15, 214–220 (2014).
Bassetti, M. et al. Incidence, risk factors, and predictors of outcome of candidemia. Survey in 2 Italian university hospitals. Diagn Microbiol Infect Dis. 58, 325–31 (2007).
Petrosillo, N. et al. Nosocomial infections in HIV infected patients. Gruppo HIV e Infezioni Ospedaliere. AIDS. 13, 599–605 (1999).
Alicino, C. et al. Trends in the annual incidence of carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a 8-year retrospective study in a large teaching hospital in northern Italy. BMC Infect Dis. 15, 415 (2015).
Boucher, H. W. et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48, 1–12 (2009).
Popovich, K. J., Weinstein, R. A. & Hota, B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 46, 787–94 (2008).
Istituto superiore di Sanità. Aggiornamento delle nuove diagnosi di infezione da HIV e dei casi di AIDS in italia al 31 dicembre 2015. In: Supplemento del Notiziario dell’Istituto Superiore di Sanità, Volume 29 - Number 9, Supplement 1, 2016, http://www.iss.it/binary/ccoa/cont/dic_2015.pdf. Accessed 01 Aug 2017.
Platt, L. et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 16, 797–808 (2016).
Fraccaro, P. et al. The ligurian human immunodeficiency virus clinical network: a web tool to manage patients with human immunodeficiency virus in primary care and multicenter clinical trials. Med 2 0. 2, e5 (2013).
Gazzata, R., Giannini, B. & Giacomini, M. A SOA-Based Platform to Support Clinical Data Sharing. Journal of Healthcare Engineering, https://doi.org/10.1155/2017/2190679 (2017).
Antinori, A. et al. Late presentation of HIV infection: a consensus definition. HIV Med. 12, 61–4 (2011).
Metz, C. E. Basic principles of ROC analysis. Semin Nucl Med. 8, 283–98 (1978).
Author information
Authors and Affiliations
Contributions
L.T. and A.D.B. ideated and wrote the final version of the study; F.L., A.M. and G.C. built and organized the final database and managed data entry in the online platform; F.B. performed all the statistical analyses; B.G. and M.G. built the online platform and performed data extraction and elaboration; G.C. and C.V. critically reviewed the study design and the final version of the paper; A.D.B., C.V. and G.C. coordinated the work across sites; L.T., F.L., A.M., G.C. and A.D.B. checked the accuracy of data. All the authors reviewed the scientific contents of the work and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taramasso, L., Liggieri, F., Cenderello, G. et al. Bloodstream infections in patients living with HIV in the modern cART era. Sci Rep 9, 5418 (2019). https://doi.org/10.1038/s41598-019-41829-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41829-3
This article is cited by
-
Evaluation of Different Blood Culture Bottles for the Diagnosis of Bloodstream Infections in Patients with HIV
Infectious Diseases and Therapy (2023)
-
Blood-based inflammation biomarkers of neurocognitive impairment in people living with HIV
Journal of NeuroVirology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.