Abstract
Every year more than 500,000 deaths are attributed to trauma worldwide and severe hemorrhage is present in most of them. Transfused platelets have been shown to improve survival in trauma patients, although its mechanism is only partially known. Platelet derived-extracellular vesicles (PEVs) are small vesicles released from platelets upon activation and/or mechanical stimulation and many of the benefits attributed to platelets could be mediated through PEVs. Based on the available literature, we hypothesized that transfusion of human PEVs would promote hemostasis, reduce blood loss and attenuate the progression to hemorrhagic shock following severe trauma. In this study, platelet units from four different donors were centrifuged to separate platelets and PEVs. The pellets were washed to obtain plasma-free platelets to use in the rodent model. The supernatant was subjected to tangential flow filtration for isolation and purification of PEVs. PEVs were assessed by total count and particle size distribution by Nanoparticle Tracking Analysis (NTA) and characterized for cells of origin and expression of EV specific-surface and cytosolic markers by flow cytometry. The coagulation profile from PEVs was assessed by calibrated automated thrombography (CAT) and thromboelastography (TEG). A rat model of uncontrolled hemorrhage was used to compare the therapeutic effects of 8.7 × 108 fresh platelets (FPLT group, n = 8), 7.8 × 109 PEVs (PEV group, n = 8) or Vehicle (Control, n = 16) following severe trauma. The obtained pool of PEVs from 4 donors had a mean size of 101 ± 47 nm and expressed the platelet-specific surface marker CD41 and the EV specific markers CD9, CD61, CD63, CD81 and HSP90. All PEV isolates demonstrated a dose-dependent increase in the rate and amount of thrombin generated and overall clot strength. In vivo experiments demonstrated a 24% reduction in abdominal blood loss following liver trauma in the PEVs group when compared with the control group (9.9 ± 0.4 vs. 7.5 ± 0.5 mL, p < 0.001>). The PEV group also exhibited improved outcomes in blood pressure, lactate level, base excess and plasma protein concentration compared to the Control group. Fresh platelets failed to improve these endpoints when compared to Controls. Altogether, these results indicate that human PEVs provide pro-hemostatic support following uncontrolled bleeding. As an additional therapeutic effect, PEVs improve the outcome following severe trauma by maintaining hemodynamic stability and attenuating the development of ischemia, base deficit, and cardiovascular shock.
Similar content being viewed by others
Introduction
Traumatic injuries remain a major cause of death worldwide1. Uncontrolled bleeding due to trauma and resulting hemorrhagic shock contribute to more than 50,000 potentially preventable deaths per year in the United Sates, and more than 500,000 worldwide with mortality remaining around 14% in the most advanced trauma centers2,3. Contributing to uncontrolled bleeding is an intrinsic dysregulation of the blood coagulation system known as trauma-induced coagulopathy (TIC). TIC is associated with poorer outcomes including increased mortality4. Apart from rapid control of the source of bleeding, the primary treatment of uncontrolled bleeding and hemorrhagic shock is fluid resuscitation using non-blood or blood components to achieve hemostasis5. Although no large-scale clinical studies exist to either support or refute the use of non-blood components for fluid resuscitation, previous studies indicated that aggressive volume replacement, especially due to a large amount of non-blood isotonic fluid, may have negative effects in the setting of uncontrolled bleeding6.
Use of blood components is another resuscitative strategy. This strategy recommends transfusion of whole blood, red blood cells (RBCs), plasma and/or platelets (PLTs). The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) study, a multicenter clinical trial, reported that higher plasma and platelet ratios early in resuscitation were associated with an improvement in hemostasis and a decrease in hemorrhage-associated mortality in adult trauma patients7. Furthermore, transfusion of PLTs alone has been found to ameliorate TIC8,9 and improve survival of trauma patients10,11,12.
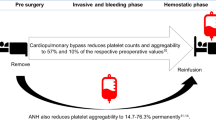
While the use of PLTs in uncontrolled bleeding raised high expectations in clinical settings, the use of platelet-derived extracellular vesicles (PEVs), a heterogeneous pool of vesicles released from PLTs, may have many advantages over the cell-based approach13,14. Short shelf life, reduction in cell function over time, and in some cases, occurrence of transfusion-related acute lung injury are some of the major limitations in the use of PLTs15,16,17,18. PEVs share many functional features with PLTs such as high procoagulant capacity19, but could provide an alternative to PLTs for transfusion given their stability following freeze-thaw cycles20. Such characteristics of PEVs effectively remove the logistical barriers of shelf-life limitations, storage, transportation and use in austere environments14. Furthermore, our group demonstrated that patients with TIC exhibited a reduction in PEVs levels19, indicating the importance of their hemostatic role following injury.
In the present study, we designed a series of in vitro and in vivo experiments to evaluate the procoagulant effects of PEVs and their ability to treat TIC and improve the outcome of trauma patients. We hypothesized that treatment with human PEVs promote hemostasis, reduce blood loss and attenuate the progression to hemorrhagic shock following severe trauma.
Materials and Methods
Preparation of fresh platelets (FPLTs)
Four PLTs units were purchased from the Gulf Coast Regional Blood Center (Houston, Texas). In brief, PLTs were prepared through centrifugation and filtration followed by resuspension in plasma, the preparation is known as platelet-rich plasma method21. For our experiments fresh platelets (FPLTs) were used 2 to 5 days after collection. On the day of the experiment, FPLTs were centrifuged and washed 3 times (931 RCF for 20 min at room temperature) in Calcium-free phosphate buffered saline (PBS) containing 0.02 U/ml apyrase and 1.0 µM prostacyclin (PGI2) to inhibit PLT-PLT interactions22. FPLTs were counted using an automated blood cell counter (Hemavet 950FS, DrewScientific, Waterbury, CT, USA) on the same day for in vivo experiments. The supernatant collected from the first centrifugation was stored at −20 °C for isolation of PEVs. Human platelets were used to increase the translational significance of the study.
Isolation of PEVs by sequential filtration
The supernatant collected from each PLT unit was thawed and processed to isolate the extracellular vesicles (EVs) using sequential filtration method as previously reported23 and in agreement with the recent recommendations by the International Society of Extracellular Vesicles24. In brief, the PLTs supernatant was passed through a 0.2 μm membrane to remove any floating cell debris. The supernatant was then loaded into the Millipore LabScale tangential flow filtration (TFF) system equipped with a Biomax 500 kDa Pellicon filter (Millipore, Billerica, MA). Three volume exchanges were performed with 500 mL calcium-free PBS and a target feed pressure below 20 pounds per square inch (psi) and retentate pressure below 10 psi. A final volume reduction step was then performed, with PEVs recovered in a final volume of approximately 10 ml of PBS. The procedure was performed at room temperature and the resultant PEVs concentrate was stored at −20 °C until the day of the experiment.
Particle size distribution and quantification of PEVs
To determine the particle size distribution and the number of the PEVs, nanoparticle tracking analysis was carried out using Nanoparticle Tracking Analysis (NTA) (NanoSight; alpha nanotech, Raleigh, NC) on samples diluted with PBS25. The system focuses a laser beam through a suspension of the particles of interest. These are visualized by light scattering using a conventional optical microscope perpendicularly aligned to the beam axis, which collects light scattered from every particle in the field of view. Three separate 30 second video recordings of all events were collected for further analysis by the nanoparticle tracking analysis software. The Brownian motion of each particle is tracked between frames to calculate its size using the Stokes-Einstein equation.
Flow cytometric characterization of PEVs
PEVs were analyzed for the phenotypic expression of cell surface markers specific for cell of origin (CD41) and the cell membrane tetraspanins (CD9, CD61, CD63 and CD81) and cytosolic protein HSP90 specific for EVs (all antibodies were purchased from Biolegend, San Diego, CA) using flow cytometry. To remove residual particles and precipitates, CD9, CD41, CD61, CD63 and CD81 antibodies were filtered using the Ultrafree®-MC/Durapore®-PVDF centrifugal filter tubes (Millipore, Hayward, CA). Antibody filtrate was used for staining the PEVs. Subsequently, the PEVs were incubated with antibodies at 4 °C for 30 minutes. Stained PEVs were washed with 200 μL of PBS by transferring them to a 0.22 μm Ultrafree-MC centrifugal filter tubes and were spun at 800 × g for 5 minutes. Biocytex Megamix Plus-SSC reference beads of sizes ranging from 0.16 μm, 0.2 μm, 0.24 μm and 0.5 μm were used to determine the relative sizes of PEVs (Thermo Fisher Scientific, Waltham, MA) (Fig. S1). Stained PEVs and reference beads were run on a LSR II benchtop flow cytometer (BD Biosciences, San Jose, CA) and the data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Measurement of PEV protein concentration
The protein concentration was measured using a commercially available kit (Bradford assay, Pierce Biochemicals). For the in vitro coagulation assays, all 4 samples were normalized to a concentration of 0.1 g/ml. Calcium-free PBS was used as vehicle in both in vivo and in vitro experiments. The ratio of EVs to protein (particle per mL/mg of protein per ml), as a purity assessment, was calculated as shown in previous validation studies25.
Calibrated automated thrombography (CAT) assay
Adapted from previous studies26,27,28, we used CAT assay to measure the overall potential of PEVs to generate thrombin using a calibrated automated thrombogran (CAT; Thrombinoscope, Maastricht, Netherlands). In brief, 10 µl of PEVs, tissue factor (TF) or vehicle under normalized protein concentration were mixed with 70 µl (1:8 ratio) of rat platelet poor plasma. Then, 20 µl of phospholipid 20 µM (MP-Reagent; Thrombinoscope, Maastricht, Netherlands) were added to maximize the sensitivity to TF. Finally, 20 µl of Calcium Chloride (FluCa; Thrombinoscope, Maastricht, Netherlands) were added to initiate the reaction. The variables analyzed were lag time (min), endogenous thrombin potential (ETP, nM/min), peak height (peak, nM), time to peak (ttpeak, min) and rate calculated as peak/ttpeak - lag time (nM/min). The variables were calculated by Thrombinoscope software (Fluoroskan Ascent, Thrombinoscope 5.0, Diagnostica Stago, Parsippany, NJ).
Viscoelastic hemostatic assay
The characteristics of the clot were assessed by thromboelastography (TEG) (TEG 5000 Thromboelastograph Analyzer, Haemoscope Corporation, Niles, IL) according to the manufacturer’s recommendations. The assay was performed using citrated rat plasma (thawed) and citrated rat whole blood (fresh).
Plasma: 875 µl of rat platelet poor plasma was mixed with 125 µl of PEVs, TF, or Vehicle (1:8 ratio) followed by exposure to CaCl2 and kaolin to start the coagulation reaction.
Whole blood: By measuring the hematocrit we adjusted the proportions of blood and PEVs sample to keep the same proportions (1:8 ratio) used with plasma samples. A volume of 927 µl of fresh blood was mixed with 72.5 µl of PEVs or vehicle followed by exposure to CaCl2 and kaolin to start the coagulation reaction. The variables analyzed were the α-angle (degrees) and the maximum amplitude (MA, mm). As suggested29, changes in TEG were compared to the parameters obtained from the same investigated animals and not from human standards.
Rat model of uncontrolled bleeding
The experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Houston. Animals were handled and maintained in accordance with the Guide for the Care and Use of Laboratory Animals. Following at least 3 days of acclimatization and ad libitum access to water and food, 32 male Sprague-Daley rats (340.5 ± 20 grams body weight) were anesthetized and surgically prepared as previously described30. In brief, under anesthesia, using 1–3% isoflurane titrated to allow systemic anesthesia under spontaneous breathing and buprenorphine for additional pain control, the animals were surgically prepared to cannulate the femoral artery and the jugular vein. A 3 French polyurethane catheter (Norfolk Access, Skokie, Illinois) was washed with heparinized saline (15 U/ml) to avoid intra-catheter blood clotting. Following the surgical procedure and without interrupting anesthesia, isoflurane was titrated to achieve a mean arterial pressure (MAP) between 90 and 110 mmHg, allowing collection of arterial blood for baseline assessment. Following the collection of baseline readings, a midline laparotomy was performed and approximately 50% of the middle hepatic lobe was excised and PEVs, FPLTs or Vehicle were infused after 60 seconds. The degree of injury was monitored by collecting the weight of the excised liver and measuring the bleeding surface area. Abdominal bleeding after liver injury was quantified with pre-weighed gauze pads measured every 15 minutes during 60 minutes. At 60 minutes post-injury, another arterial blood sample was collected for biochemical analysis followed by euthanasia.
Grouping and experimental design
The animals were divided in three experimental groups: (i) control group (n = 16; received 5 ml of PBS in the jugular vein catheter); (ii) FPLT group (n = 8; received 8.7 × 108 FPLTs resuspended in 3 ml of PBS + 2 ml of PBS to flush the line); and (iii) PEV group (n = 8; received 7.8 × 109 PEVs resuspended in 3 ml of PBS + 2 ml of PBS to flush the line). The experiments were done in pairs on the same day and were randomly assigned to either of the experimental groups. Each treatment animal was paired with a control animal. FPLT group was treated with washed platelets from one of the donors shown in Table 1A. The concentration of PEVs used was adapted from the maximal effective dose utilized in the calibrated automated thrombography assay reported here and the concentration of FPLT was adapted from a previous study22. PEVs group was treated with a pool of the 4 donors normalized to a protein concentration of 1.0 × 105 µg/ml. Treatments were given intravenous via the jugular vein catheter 60 seconds after the excision of the liver.
Blood physiological and biochemical assessment
Blood pressure was measured using a hemodynamic monitor (Power Lab, AD Instruments, Colorado Springs, CO) connected to the femoral artery. Blood levels of oxygen (O2), carbon dioxide (CO2), bicarbonate (HCO3), base excess (BE) and pH were quantified by sampling and measuring 200 μl of arterial blood with a blood gas analyzer (Stat Profile Critical Care Xpress, NOVA Biomedical, Waltham, WA) and lactate using a handheld testing system (Stat Strip Xpress Lactate Hospital Meter, NOVA Biomedical, Waltham, WA). The manual microhematocrit method was used to determine the hematocrit (Hct) and a refractometer for the plasma protein concentration.
Organs wet to dry (W/D) ratio
To determine water content in heart, lung, kidney, liver and intestine, samples of fresh organs were collected and weighed immediately after euthanasia. The organs were then placed in a 60 °C vacuum oven and weighed again 72 hours later after water evaporation.
Statistical analysis
The results were analyzed using GraphPad Prism 8 (GraphPad Prism version 8.1, GraphPad Software, La Jolla, CA). Outcome data collected at a single time point was compared using One-way analysis of variance (ANOVA) or Kruskal-Wallis test when normality was not met. Data collected from multiple time points were analyzed using two-way analysis of variance (2-way ANOVA) with Tukey post hoc test to compare the differences among groups at each time point. All values in tables are expressed as mean ± standard error of the mean (SEM). Differences were considered significant when the p-value was equal or smaller than 0.05.
Results
Characterization of PEVs
The PEVs obtained from four different donors showed a similar profile based on size distribution and surface markers. The age and gender of the donors as well as the platelet counts from each unit are presented in Table 1A. The protein concentration, EV count and size assessment (mean and mode) were determined and are presented in Table 1A and Fig. 1. It was observed that all the PEVs obtained from four different donors expressed platelet-specific marker CD41 (Figs. S2 and S3). Additionally, they also expressed tetraspanin proteins CD9, CD61, CD63, CD81 and cytosolic protein HSP90 that are known to be present on EVs (Figs. S2 and S3). Variation in the expression of EV-specific tetraspanin proteins in our study in comparison to the other studies might be attributed to the different technical procedures followed to isolate EVs23,31,32. Likewise, the use to 0.2 µm filter to remove cell derbies, for the pure and highly efficient isolation of EVs from a platelet unit exhibited a highly pure homogeneous EV population. Although, the final isolated EV preparation may not represent the entire EV population from a platelet unit.
Procoagulant effect PEVs in vitro
We tested the procoagulant capabilities of each of the isolated PEVs samples both separately and as a pool using two different methods (Fig. 2 and Table 2). Thrombin generation assay in rat plasma shows that, compared to vehicle, PEVs isolated from each of the 4 donors exhibited a dose-response effect increasing the rate of the reaction as shown by reductions in lagtime and ttPeak and increased velocity. TF, used as positive control, exhibited a strong effect on lagtime and ttPeak similar to the highest dose of PEVs, but only a mild effect on velocity. Likewise, PEVs exhibited a dose-dependent effect on Peak and ETP, suggesting a contribution to the overall amount of thrombin generated. (Fig. 2). Thromboelastography analysis was performed in rat plasma and whole blood using the highest dose PEVs (3.2 × 108 EVs) shown in Fig. 2 and the same proportions of plasma (1:8 ratio). In whole blood, PEVs treatment demonstrated an increase in MA compared to Vehicle and an increase in the α-angle parameter. In plasma PEVs treatment also demonstrated an increase in MA compared to Vehicle but no difference was observed in the α-angle (Table 2). The changes in MA and the α-angle indicates that the overall strength of the clot generated is greater in the presence of PEVs.
Dose-response procoagulant effect of PEVs. Multiple dilutions of PEVs isolates from 4 different donors alone and combined were exposed to rat fresh frozen plasma. Generation of thrombin was assessed by CAT. Rh-TF and PBS were used as positive and negative control respectively. ETP = endogenous thrombin potential, ttPeak = time to peak, TF = tissue factor.
Blood and hemodynamic parameters from in vivo model of uncontrolled bleeding
The body weight and blood biochemical parameters at baseline (hematocrit, plasma, lactate, BE, CO2, O2 and pH) were comparable among the Vehicle, FPLT and PEVs groups (Table 3A). The degree of the injury was similar in all groups, as shown by the weight of the liver excised, the bleeding surface and the MAP 60 seconds after liver excision (Table 3B). Assessment of peritoneal bleed during the first 30 minutes postinjury demonstrated an accumulation of 8.1 mL of blood on average in the control group and 7.6 mL in the FPLTs group vs. a significant reduction to 6.6 mL in accumulated blood in the PEVs group. The difference in bleeding between control and PEVs groups was the greatest at 60 minutes postinjury (9.9 and 7.5 mL, respectively) (Fig. 3A). Likewise, mean MAP was similar among groups at baseline (Table 3A) and dropped to 64, 70, 68, and 67.1 mmHg at 15, 30, 45 and 60 min postinjury in the control group compared to 88.5, 93.6, 92, and 90.9 mmHg in the PEVs indicating a significant improvement with PEVs therapy. In contrast, FPLT infusion failed to improve the MAP vs. Vehicle (Fig. 3B). Arterial blood sampling collected 60 minutes postinjury demonstrated that the Htc, O2, CO2 and pH had a similar change in all groups (Table 4). However, the total plasma protein dropped to 3 g/dL in the Vehicle and FPLTs groups compared to a 4 g/dL in the PEVs group suggesting a significant improvement in oncotic pressure. Lactate and BE were clearly increased in Vehicle (5 mmol/L and −2.9 mmol/L, respectively) and FPLTs groups (3 mmol/L and −5.6 mmol/L) compared to maintained levels in the PEVs group (1 mmol/L and 3.5 mmol/L) indicating a mitigated metabolic decompensation in this group. Lung, heart, kidney and liver water content, assessed by WD ratio, was similar among the treatment groups and untreated controls (Table 4).
Treatment effect on hemodynamics and hemostasis. (A) Bleed volume continuously collected from the peritoneal cavity following liver excision. (B) MAP measured from a femoral artery catheter showing a rise in blood pressure in PEVs treated group. Vehicle, n = 16; fresh platelets (FPLTs), n = 8; platelet extracellular vesicles (PEVs), n = 8. Box plots Showing the median ± 99 percentile and compared by 2-way ANOVA, *p < 0.05 vs. Control. ***p < 0.001 vs. Control.
Discussion
In the present study, we showed that a well-characterized EV preparation isolated from platelet units have a potent procoagulant effect demonstrated by both in vivo and in vitro models. Coagulation assays demonstrated that our preparation has a consistent effect, as shown by the similar effects from each of the 4 isolates on both thrombin generation and TEG assays. An important aspect from the results is the comparison between PEVs and TF treatments since a number of studies have suggested that the procoagulant features of PEVs are primarily result from their high TF and phosphatidylserine (PS)33,34. This concept is in agreement with our observations given that PEVs dose-dependently increased both the rate and overall amount of thrombin generation. Furthermore, viscoelastic assay demonstrates that in whole blood, PEVs increased the fibrin polymerization rate (α-angle) and the strength of the clot (MA), likely as a result from the interaction with the platelets present in blood. However, the experiment in platelet-free plasma also showed that the strength of the clot was superior in the presence of PEVs suggesting a significant interaction with other components of the clot. A potential explanation for this effect could be the expression of specific integrins in PEVs capable of binding fibrinogen and promoting clot formation35, or by a change in fibrin clot structure triggered by the increase in thrombin concentration36.
We used the most efficacious dose of PEVs shown by in vitro assays to test the therapeutic effect of such treatment on an in vivo uncontrolled bleeding model. The experiment demonstrated that PEVs therapy provided an effective hemostatic effect as compared to vehicle-treated animals and superior to FPLTs treated animals. Although a number of publications have suggested that EVs could be employed as a procoagulant drug37, the present study is the first to demonstrate the concept in a clinically relevant animal model.
Another important finding from the animal experiments was that PEVs treatment markedly improved the blood pressure, the levels of total protein and lactate in plasma, and the base deficit. The evidence provided cannot explain the order of such outcomes, though, we speculate that, as shown by the MAP and plasma protein concentration, the hydrostatic and oncotic pressure were maintained in the PEVs group as a result of the preserved blood volume. Consequently, perfusion improved in the PEVs group compared to FPLT and Vehicle counterparts, attenuating ischemia and reducing the generation of lactate and preventing the development of metabolic acidosis and cardiovascular shock. The group treated with FPLTs failed to improve the hemodynamics, plasma protein concentration, lactate levels and the base deficit, and although transfused PLTs have shown to improve the outcome in trauma patients12, resuscitation with platelets alone in the absence of plasma likely reduces the effectiveness. Likewise, our PEVs preparation is noticeably superior to platelet transfusion from a storage perspective, since platelets can only be used within 5 days post-withdraw and cannot be frozen.
These results are in agreement with previous results from our group demonstrating the decrease in PEVs associated with both TIC19 and with a decrease in survival of trauma patients38 and with Windelov et al. showing that trauma hypocoagulable patients have lower levels of PEVs39. Remarkably, Windelov et al. found as well that lactate levels and injury severity were inversely correlated with the concentration of PEVs in trauma patients, suggesting the potential role of PEVs to mitigate trauma severity39. Moreover, acidosis prevents the release of EVs from platelets, therefore in the present study PEVs administration could be compensating for the loss of endogenous EVs40.
Although in our study we did not evaluate the direct effect of PEVs on the endothelium, Miyazawa et al. demonstrated that PEVs protects the microvasculature from permeability disrupting factors41. Likewise, Mause et al. demonstrated that PEVs promote vascular integrity as well as endothelial recovery42; therefore, the attenuated decreased in plasma protein concentration shown by the animal experiments is likely a consequence of a PEVs-induced anti-leak effect. Severe sepsis shares a number of features with trauma, and high levels of PEVs have shown to be associated with improved outcomes in septic patients, specifically with lower incidence of multi-organ failure43.
To acquire a deep understanding of hemodynamic physiology it is necessary to investigate plasma proteins, the blood cells and the endothelium. In this regard, an important limitation from the in vitro coagulation studies is that, our assays did not account for interactions with the endothelium, although the animal experiment partially addressed this limitation. On a different context, a limitation from in vivo experiments was the fact that we did not collect blood from the animals in shorter time points, as this approach would have allowed us to explore the dynamics of the biomarkers measured and it would give us the possibility to measure the immediate coagulation effect caused by the treatment. However, this would have affected the assessment of uncontrolled bleeding and the parallel hemodynamic compensatory response.
Conclusion
The present findings indicate that the therapeutic benefits of platelets during hemorrhage is partly due to the effects of platelet-derived EVs. Here we employed a PEVs preparation showing an efficacious hemostatic effect and the advantage of frozen storage. Furthermore, the results indicate that as an additional therapeutic effect, PEVs improve the outcome following severe trauma by maintaining hemodynamic stability and mitigating the development of ischemia and metabolic acidosis. The study is the first to demonstrate evidence to use PEVs as an alternative therapy providing hemostasis for trauma patients. Before these promising findings can be translated into clinical practice, further research is needed to expand the characterization of the PEVs isolates with a particular emphasis to elucidate donor-to-donor variability within a large selection of donors.
References
Kauvar, D. S. & Wade, C. E. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care 9(Suppl 5), S1–9, https://doi.org/10.1186/cc3779 (2005).
Drake, S. A. et al. Establishing a Regional Trauma Preventable/Potentially Preventable Death Rate. Annals of surgery, https://doi.org/10.1097/SLA.0000000000002999 (2018).
Christensen, M. C. et al. Global differences in causes, management, and survival after severe trauma: the recombinant activated factor VII phase 3 trauma trial. The Journal of trauma 69, 344–352, https://doi.org/10.1097/TA.0b013e3181e74c69 (2010).
Chang, R., Cardenas, J. C., Wade, C. E. & Holcomb, J. B. Advances in the understanding of trauma-induced coagulopathy. Blood 128, 1043–1049, https://doi.org/10.1182/blood-2016-01-636423 (2016).
Kaur, P., Basu, S., Kaur, G. & Kaur, R. Transfusion protocol in trauma. Journal of emergencies, trauma, and shock 4, 103–108, https://doi.org/10.4103/0974-2700.76844 (2011).
Ertmer, C., Kampmeier, T., Rehberg, S. & Lange, M. Fluid resuscitation in multiple trauma patients. Current opinion in anaesthesiology 24, 202–208, https://doi.org/10.1097/ACO.0b013e3283445326 (2011).
Holcomb, J. B. et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 313, 471–482, https://doi.org/10.1001/jama.2015.12 (2015).
Spinella, P. C. et al. Whole blood for hemostatic resuscitation of major bleeding. Transfusion 56(Suppl 2), S190–202, https://doi.org/10.1111/trf.13491 (2016).
Cardenas, J. C., Wade, C. E. & Holcomb, J. B. Mechanisms of trauma-induced coagulopathy. Current opinion in hematology 21, 404–409, https://doi.org/10.1097/MOH.0000000000000063 (2014).
Gunter, O. L. Jr. et al. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. The Journal of trauma 65, 527–534, https://doi.org/10.1097/TA.0b013e3181826ddf (2008).
Holcomb, J. B. et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Annals of surgery 248, 447–458, https://doi.org/10.1097/SLA.0b013e318185a9ad (2008).
Cardenas, J. C. et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv 2, 1696–1704, https://doi.org/10.1182/bloodadvances.2018017699 (2018).
Curvers, J. et al. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion 44, 49–58 (2004).
Lopez, E., Srivastava, A. K., Pati, S., Holcomb, J. B. & Wade, C. E. Platelet-Derived Microvesicles: A Potential Therapy for Trauma-Induced Coagulopathy. Shock 49, 243–248, https://doi.org/10.1097/SHK.0000000000000974 (2018).
Etchill, E. W. et al. Platelet Transfusion in Critical Care and Surgery: Evidence-Based Review of Contemporary Practice and Future Directions. Shock 47, 537–549, https://doi.org/10.1097/SHK.0000000000000794 (2017).
Pidcoke, H. F. et al. Refrigerated platelets for the treatment of acute bleeding: a review of the literature and reexamination of current standards. Shock 41(Suppl 1), 51–53, https://doi.org/10.1097/SHK.0000000000000078 (2014).
Goldman, M. et al. Proceedings of a consensus conference: towards an understanding of TRALI. Transfusion medicine reviews 19, 2–31 (2005).
Katus, M. C., Szczepiorkowski, Z. M., Dumont, L. J. & Dunbar, N. M. Safety of platelet transfusion: past, present and future. Vox Sang 107, 103–113, https://doi.org/10.1111/vox.12146 (2014).
Matijevic, N. et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thrombosis research 134, 652–658, https://doi.org/10.1016/j.thromres.2014.07.023 (2014).
Frank, J. et al. Extracellular vesicles protect glucuronidase model enzymes during freeze-drying. Sci Rep 8, 12377, https://doi.org/10.1038/s41598-018-30786-y (2018).
Van der Meer, P. F. Platelet concentrates, from whole blood or collected by apheresis? Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis 48, 129–131, https://doi.org/10.1016/j.transci.2013.02.004 (2013).
Torres Filho, I. P. et al. Refrigerated platelets stored in whole blood up to 5 days adhere to thrombi formed during hemorrhagic hypotension in rats. J Thromb Haemost 15, 163–175, https://doi.org/10.1111/jth.13556 (2017).
Harting, M. T. et al. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 36, 79–90, https://doi.org/10.1002/stem.2730 (2018).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of extracellular vesicles 7, 1535750, https://doi.org/10.1080/20013078.2018.1535750 (2018).
Webber, J. & Clayton, A. How pure are your vesicles? Journal of extracellular vesicles 2, https://doi.org/10.3402/jev.v2i0.19861 (2013).
Tian, Y. et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 125, 2151–2159, https://doi.org/10.1182/blood-2014-09-598805 (2015).
Huby Mdel, P. et al. Establishment of methods for performing thrombelastography and calibrated automated thrombography in rats. Shock 42, 27–30, https://doi.org/10.1097/SHK.0000000000000163 (2014).
Cardenas, J. C. et al. Overexpression of the cell cycle inhibitor p16INK4a promotes a prothrombotic phenotype following vascular injury in mice. Arteriosclerosis, thrombosis, and vascular biology 31, 827–833, https://doi.org/10.1161/ATVBAHA.110.221721 (2011).
Wohlauer, M. V. et al. A standardized technique for performing thromboelastography in rodents. Shock 36, 524–526, https://doi.org/10.1097/SHK.0b013e31822dc518 (2011).
Pawelczyk, N. S. et al. Association of hemodilution and blood pressure in uncontrolled bleeding. The Journal of surgical research 184, 959–965, https://doi.org/10.1016/j.jss.2013.03.072 (2013).
Willms, E., Cabanas, C., Mager, I., Wood, M. J. A. & Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol 9, 738, https://doi.org/10.3389/fimmu.2018.00738 (2018).
Li, P., Kaslan, M., Lee, S. H., Yao, J. & Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 7, 789–804, https://doi.org/10.7150/thno.18133 (2017).
Owens, A. P. III & Mackman, N. Microparticles in hemostasis and thrombosis. Circ Res 108, 1284–1297, https://doi.org/10.1161/CIRCRESAHA.110.233056 (2011).
Sinauridze, E. I. et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thrombosis and Haemostasis 97, 425–432, https://doi.org/10.1160/th06-06-0313 (2007).
Merten, M., Pakala, R., Thiagarajan, P. & Benedict, C. R. Platelet Microparticles Promote Platelet Interaction With Subendothelial Matrix in a Glycoprotein IIb/IIIa Dependent Mechanism. Circulation 99, 2577–2582, https://doi.org/10.1161/01.cir.99.19.2577 (1999).
Campbell, R. A., Overmyer, K. A., Bagnell, C. R. & Wolberg, A. S. Cellular procoagulant activity dictates clot structure and stability as a function of distance from the cell surface. Arteriosclerosis, thrombosis, and vascular biology 28, 2247–2254, https://doi.org/10.1161/ATVBAHA.108.176008 (2008).
Chao, F. C. et al. Infusible platelet membrane microvesicles: a potential transfusion substitute for platelets. Transfusion 36, 536–542 (1996).
Matijevic, N. et al. Microvesicle phenotypes are associated with transfusion requirements and mortality in subjects with severe injuries. Journal of extracellular vesicles 4, 29338, https://doi.org/10.3402/jev.v4.29338 (2015).
Windelov, N. A. et al. Low level of procoagulant platelet microparticles is associated with impaired coagulation and transfusion requirements in trauma patients. J Trauma Acute Care Surg 77, 692–700, https://doi.org/10.1097/TA.0000000000000437 (2014).
Etulain, J. et al. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb Haemost 107, 99–110, https://doi.org/10.1160/TH11-06-0443 (2012).
Miyazawa, B. et al. Regulation of Endothelial Cell Permeability by Platelet-Derived Extracellular Vesicles. J Trauma Acute Care Surg (In press), https://doi.org/10.1097/TA.0000000000002230 (2019).
Mause, S. F. et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation 122, 495–506, https://doi.org/10.1161/circulationaha.109.909473 (2010).
Soriano, A. O. et al. Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Critical Care Medicine 33, 2540–2546, https://doi.org/10.1097/01.Ccm.0000186414.86162.03 (2005).
Acknowledgements
This study was supported in part by James H. “Red” Duke, Jr. M.D. endowment and the William Stamps Farish fund.
Author information
Authors and Affiliations
Contributions
Conception and design: E.L., A.K.S., C.E.W. In vivo experiments: E.L., J.B., Y.W. Biochemical and in vitro assays: E.L., A.K.S., J.B., Y.W., P.P.T., B.M. Analysis and interpretation: E.L., A.K.S., J.B., Y.W., J.C.C., P.P.T., B.M., E.G., J.B.H., S.P., C.E.W. Drafting the manuscript: E.L., A.K.S. Critical revision of the manuscript: E.L., A.K.S., J.C.C., J.B.H., S.P., C.E.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lopez, E., Srivastava, A.K., Burchfield, J. et al. Platelet-derived- Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Sci Rep 9, 17676 (2019). https://doi.org/10.1038/s41598-019-53724-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53724-y
This article is cited by
-
Transforming research to improve therapies for trauma in the twenty-first century
Critical Care (2024)
-
Exosome-mediated repair of spinal cord injury: a promising therapeutic strategy
Stem Cell Research & Therapy (2024)
-
Platelet-derived extracellular vesicles promote endothelial dysfunction in sepsis by enhancing neutrophil extracellular traps
BMC Immunology (2023)
-
Lyophilized apoptotic vesicle-encapsulated adhesive hydrogel sponge as a rapid hemostat for traumatic hemorrhage in coagulopathy
Journal of Nanobiotechnology (2023)
-
Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery
Journal of Biomedical Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.