Abstract
Study design:
Experimental Study.
Objectives:
The aim of this study was to investigate the neuroprotective effects of iloprost and piracetam on spinal cord ischemia/reperfusion (I/R) injury in the rabbit.
Settings:
The Experimental Research Center of Selcuk University, Konya, Turkey.
Methods:
A total of 24 rabbits were divided into four groups of six rabbits each, as follows: group 1 (n=6) sham, laparotomy only; group 2 (n=6) I/R; group 3 (n=6) I/R+iloprost; and group 4 (n=6) I/R+piracetam. I/R was established in groups 2, 3 and 4. Subsequently, they were followed up neurologically for 24 h until the rabbits were killed; biochemical and histopathological examinations of samples from the spinal cord were carried out.
Results:
Neurological examination results were significantly better in the iloprost and piracetam groups compared with the I/R group (P<0.05). Neuroprotection was achieved with iloprost and piracetam by suppressing malondialdehyde (P<0.05), increasing glutathione peroxidase activity (P<0.05) and decreasing the xanthine oxidase level. In histopathological assessment, iloprost and piracetam groups were statistically different from the I/R group in terms of the number of apoptotic neurons in gray matter and white matter, as well as in terms of degenerated neurons and glial cells (P<0.05). No statistical difference was determined between the four groups in the number of degenerated glial cells (P>0.05).
Conclusion:
This study has shown that iloprost and piracetam have neuroprotective effects in I/R injury both neurologically and histopathologically because of inhibition of lipid peroxidation.
Similar content being viewed by others
Introduction
Paraplegia, which stems from spinal cord ischemia, can be seen after surgical procedures related to the thoracic and thoracoabdominal aorta.1, 2 The incidence of neurological injury occurring as a result of ischemia/reperfusion (I/R) injury to the spinal cord ranges between 4 and 33%.1, 2, 3 Various methods have been proposed to protect the spinal cord from this complication.4, 5 However, paraplegia seems to be a durable complication,6 and the mechanism of this complication is not yet fully understood.7, 8, 9
Iloprost is a prostacyclin analog. It has some well-known antivasospastic, antiischemic and neuroprotective effects.10, 11 Recent studies indicate that neutrophils, which are active in tissue injury in I/R models, have a crucial role. It is thought that iloprost's healing effect is a result of its inhibition of neutrophil accumulation.10, 11
Piracetam, a nootropic agent, is known to exert its effect by activating and protecting neuronal cell functions. The exact mechanism of piracetam's neuroprotective effects is still unclear. Some experimental data point to the facilitating role of piracetam in cholinergic synaptic transmission.12
In this study, we observe and compare the effects of iloprost and piracetam on histopathological and biochemical alterations, and also on neurological functions, in a spinal cord I/R rabbit model. The neuroprotective effects of iloprost on an experimental traumatic injury of the spinal cord was studied previously,7 but the effects of iloprost and pirecetam on spinal cord I/R injury were first investigated in this study.
Materials and methods
This study was conducted after receiving endorsement from the ethics board of the Experimental Medicine Research and Application Center (27 January 2009, issue number 2009-2). The study included 24 New Zealand male rabbits with an average weight range of 2.15–2.8 kg. The animals were kept at 21 °C and fed with standard feed. The experimental animals were anesthetized by intramuscular administration of ketamine (70 mg kg−1; Ketalar, Parke-Davis Eczacıbaşı, Istanbul, Turkey) and xylazine (5 mg kg−1; Rompun, Bayer, Istanbul, Turkey) in combination. The animals did not require a mechanical ventilator. An intravenous catheter was placed in the ear vein of the animals and preoperative cefazolin 10 mg kg−1 (Cefamezin, Eczacıbaşı, Istanbul, Turkey) was administered as a single dose. As maintenance, 0.9% NaCl (20 ml h−1) was given throughout the experiment.
The study groups were formed as follows:
-
Group 1: sham (n=6); laparotomy only.
-
Group 2: I/R (n=6); ischemia was applied for 20 min, followed by reperfusion for 24 h.
-
Group 3: I/R+iloprost (n=6); iloprost (25 ng kg−1 min−1; Ilomedin, Bayer Schering Pharma AG, Berlimed SA, Spain) was administered at the beginning of the I/R period and intravenous infusion was initiated. The infusion was continued for 1 h and reperfusion was applied.Group 4: I/R+piracetam (n=6); 250 mg kg−1 of piracetam (Nootropil, UCB Pharma, Istanbul, Turkey) was administered intraperitoneally at the beginning of the I/R period and then reperfusion was performed as in the other groups. Piracetam was continued intraperitoneally at the same dose every 6 h.
The effective dosage of iloprost7 and piracetam13 was decided according to previous studies.
Ischemia/reperfusion procedure
The spinal cord ischemia model was established as described by Zivin and DeGirolami.8, 9 In short, a ‘Bulldog’ 1 cm below the thoracoabdominal aorta and renal artery was clamped with an atraumatic vascular clamp. Heparin (100 U kg−1) was injected before clamping. The clamp was localized close to the L3 vertebra. The animals were subjected to ischemia for 20 min and then to a reperfusion period and blood flow was achieved. Animals in the sham (control) group underwent laparotomy only, without the application of the clamp. Anatomical structures were closed gently and the animals were returned to their cages.
After the procedure, the experimental animals were followed up neurologically, and motor inefficiency and healing rates were noted. At the end of the experimental period, the animals were killed by administering an overdose of an anesthetic agent, and spinal cord tissue samples between L1 and L3 were taken for biochemical and histopathological evaluation.
Neurological assessment
The neurological situation was assessed using the Tarlov scoring system. The animals were followed up for 24 h after the I/R period and neurological assessment was carried out at the first, eighth and twenty-fourth hour. Neurological scores were assessed as follows: 0: paraplegic, 1: severe paraplegic, 2: some functional movement, 3: ataxic dysconjugate movement, 4: minimal ataxia and 5: normal function.
Biochemical assessment
Subjects and sample preparation
For homogenization of tissues, 0.2 mM Tris-HCl buffer with pH=7.5 was prepared. This buffer is used in methods in which nitrous oxide (NO), glutathione peroxidase (GSH-Px), xanthine oxidase (XO) and malondialdehyde (MDA) are used. Hexadecyl trimethyl ammonium bromide (0.5%) was used in myeloperoxidase (MPO) determination. The tissue in the glass tubes was homogenized at 16 000 r.p.m. min−1 by ultrasonic homogenizer. After this assay, the homogenate was centrifuged at 3220 r.p.m. for 30 min (at 6 °C). This supernatant was used for GSH-Px, XO and protein assays. For tissue MPO determination, tissues were homogenized in 0.5% hexadecyl trimethyl ammonium bromide at 3220 r.p.m. for 45 min (at 4 °C). MPO and protein assays were determined using this supernatant.
Measurement of MDA
MDA levels, as an index of lipid peroxidation, were determined by thiobarbituric acid reaction according to Uchiyama and Mihara.14 The principle of the method depends on measurement of the pink color produced by the interaction of barbituric acid with MDA, elaborated as a result of lipid peroxidation. The colored reaction 1,1,3,3-tetraethoxypropane was used as the primary standard.
Measurement of GSH-Px levels
The levels of GSH-Px were determined using an indirect method (Sigma, St Louis, MO, USA, product code: CGP). The principle of the method is based on the oxidation of glutathione to oxidized glutathione catalyzed by GSH-Px, which is then coupled to the recycling of oxidized glutathione back to glutathione using glutathione reductase and NADPH (β-nicotinamide adenine dinucleotide phosphate). The decrease in NADPH absorbance measured at 340 nm during the oxidation of NADPH to NADP is indicative of GSH-Px activity, as GSH-Px is the rate-limiting factor of the coupled reactions.
Measurement of XO activity
XO activity is measured by the principle of the formation of uric acid from xanthine. The addition of 100% trichloroacetic acid finalizes the reaction and formation of uric acid ends. The absorbance of uric acid is determined at 293 nm using a spectrophotometer and the amount of uric acid produced in 30 min is calculated. The enzyme activity is given as U per mg protein.
Measurement of nitrite and nitrate
The levels of nitrite and nitrate were measured using a photometric end point determination method (Roche Diagnostic GmbH, Mannheim, Germany, catalog no: 1 756 281). The principle of nitrate is reduced to nitrite by reduced NADPH in the presence of the enzyme nitrate reductase. The nitrite formed reacts with sulfanilamide and N-(1-naphtyl)-ethylenediamine dihydrochloride to result in a red–violet diazo dye. The diazo dye is measured on the basis of its absorbance in the visible range (550 nm).
Measurement of MPO activity
MPO activity was determined by a modification of O-dianisidine. The assay mixture, in a cuvette of 1 cm path length, contained 0.2 ml of 0.1 mol l−1 phosphate buffer (pH 7.1), 0.09 ml of H2O2 (3% v/v), 0.1 ml of 0.02 mol l−1 O-dianisidine (freshly prepared in methanol) and 0.1 ml sample in a final volume of 2 ml. The sample was added last and the change in absorbance at 410 nm was observed for 30 min. All measurements were carried out in duplicate. One unit of MPO is defined as that giving an increase in absorbance of 0.001 per min and specific activity is given as units per mg of protein.
Histopathological study
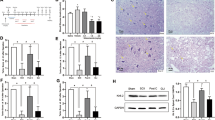
Tissue samples were fixed in 10% paraformaldehyde and prepared with autotechnicon and then embedded in paraffin. Slices (5 mm) were obtained with a microtome and stained with hematoxylin and eosin. Hematoxylin- and eosin-stained specimens were examined under a Nikon Eclipse E400 light microscope (Nikon Instruments Inc, Melville, NY, USA) (Figure 1).
Histopathological evaluation of degenerated glial and neuronal cells in the L2 segment of experimental I/R spinal cord. Blue arrows indicate degenerated glial cells and black arrows indicate degenerated neuronal cells (hematoxylin and eosin, scale=0.05 mm). A full color version of this figure is available at the Spinal Cord online.
For detecting apoptosis in tissues, a TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)-based apoptosis kit (Fragel DNA fragmentation kit, Calbiochem, Darmstadt, Germany) was used. In this method, 4-micron-thick sections cut from paraffin blocks were first deparaffinized and rehydrated. They were then permeabilized with proteinase K and endogenous peroxidase inactivated by 10% H2O2. For DNA labeling, Tdt labeling reaction mix and Tdt enzyme mixture were used. For detection of reaction, DAB solution in H2O2/urea mixture was used. Methyl green (3%) was used for counter staining. Samples were then dehydrated and mounted with xylene. Terminal deoxynucleotidyl transferase dUTP nick end labeling-positive brown-stained cells were accepted as apoptotic in concordance with the positive control supplied by the manufacturer (Figure 2).
Statistical analysis
Statistical analysis by means of computer-assisted data analysis was performed with software (SPSS 13.0) for windows. All values were presented as mean±s.e.m.
The differences between groups in biochemical data were analyzed by one-way analysis of variance tests and Tukey HSD. The differences between groups in neurological examination scores and histophatological results were analyzed by the Kruskal–Wallis H-test and the Mann–Whitney U-test using Bonferroni's correction. P-values <0.05 were accepted as significant.
Results
Neurological assessment
For each group, neurological examinations were performed at the first, eighth and twenty-fourth hour (Table 1).
We determined significant differences between the I/R group and the other groups. At the firsthour, significant differences were determined between group 1 and groups 2 and 4; between group 2 and groups 1, 3 and 4; between group 3 and groups 1 and 2; and between group 4 and groups 1 and 2 (P<0.05 for all). No significant difference was found between groups 3 and 4 (P>0.05). Statistically similar findings were also seen at the eighth and twenty-fourth hour (Figures 3, 4, 5).
Neurologically, there was a significant difference between groups 2 (I/R), 3 (iloprost) and 4 (piracetam; P<0.05). However, no significant difference was found between the two treatment (iloprost and piracetam) groups (P>0.05).
Biochemical assessment
As shown in Table 2, when the I/R group and controls were compared, there was a significant increase in MDA levels in the I/R group (P<0.05). On the other hand, MDA formation was partially prevented in iloprost- and piracetam-treated groups. The MDA levels in the piracetam-treated group were closer to those of the sham group. A greater decrease in MDA levels was observed in the group treated with iloprost, and MDA levels decreased more dramatically compared with those in the I/R group (P<0.05). Nevertheless, no significant difference in MDA levels was determined between the iloprost and piracetam groups (P >0.05). The MDA levels in the two treatment groups were significantly lower than those of the I/R group (P<0.05).
GSH-Px activity was similar in sham and I/R groups. GSH-Px activity increased significantly in the iloprost and piracetam groups compared with the I/R group (P<0.05), but no significant difference was determined between the iloprost and piracetam groups (P>0.05).
XO activity was significantly higher (P<0.05) in the I/R group when compared with controls and with the groups treated with iloprost or piracetam. Treatment with iloprost and piracetam suppressed XO activity. No significant difference was determined in XO activity between the iloprost and piracetam groups (P>0.05).
There was no statistically significant difference between sham and I/R groups in terms of tissue nitrate levels (P>0.05). However, NO levels in the iloprost and piracetam groups were lower compared with the sham group, but the decrease was significant only in the iloprost group (P<0.05). When compared with the I/R group, no significant difference was found between iloprost and piracetam groups (P>0.05).
No significant difference was determined in tissue MPO levels between the four groups (P>0.05).
Histopathological assessment
In terms of apoptosis in the gray matter, the I/R group had higher apoptosis values than the other three groups and the difference was statistically significant (P<0.05). There was no statistical significance between groups 1 and 3 or between groups 1 and 4 in terms of apoptosis in the gray matter of the spinal cord (P>0.05).
When apoptosis in the white matter of the spinal cord was examined, the highest apoptosis value was in the I/R group. In iloprost and piracetam groups, the number of apoptotic cells was significantly lower compared with the I/R group (P>0.05). Although this value was higher than that in the control group, the difference was not statistically significant (P>0.05).
The lowest number of neurons was in the I/R group. Neurons were highest in the iloprost group and the difference was significantly meaningful (P<0.05). The number of neurons in the piracetam group was statistically close to that of the control group (P>0.05), and it was statistically significantly higher when compared with the I/R and iloprost groups (P<0.05).
The number of degenerated neurons was highest in the I/R group and it was lower in the iloprost, piracetam and control groups, and the difference was statistically significant (P<0.05). Although the number was higher in the iloprost and piracetam groups than in controls, the difference was not statistically different (P>0.05).
The lowest number of glial cells was seen in the I/R group, and the difference between the I/R and the other three groups was statistically significant (P<0.05).
In terms of degenerated glial cells, although there was no significant difference between the four groups (P>0.05), the highest number was seen in the I/R group.
A summary of findings on histopathological examinations can be seen in Table 3.
Discussion
Paraplegia can be seen after thoracoabdominal vascular surgery. As a result of the clamping of thoracic aorta, the decrease in spinal blood flow affects experimental animals and humans significantly.15 The acute inflammatory responses lead to the production of reactive oxygen types and result in lipid peroxidation.16
Free radical and reactive oxygen species (ROS) have a role in the etiology and progression of many diseases and in aging.16 Oxidative stress is a result of an imbalance between ROS, endogenous antioxidant defense mechanisms and repair capacity. The removal of the aortic clamp in thoracic aorta surgery results in the excessive production of ROS and oxidative injury in DNA and proteins, with cell membrane peroxidation in spinal neurons. The effects of ROS on membrane lipids are realized at various metabolic levels. Lipids are destroyed with lipid peroxidation, the ultra-structure of neural membranes is degenerated and critical functions of membrane connective enzymes are inhibited.16
In this study, iloprost and piracetam decreased the depletion in GSH-Px activity and deactivated free radicals formed in the spinal cord by I/R. At the same time, the increase in NO and MDA concentrations in iloprost and piracetam rabbit tissue was prevented. In this study, lipid peroxidation is monitored with MDA measurement. MDA free-radical injury to the cell membrane is seen after lipid peroxidation. In the I/R group, a significant increase in MDA concentrations was observed in the spinal cord of rabbits. Iloprost and piracetam decreased MDA concentrations significantly. This is probably because of their capacity to eliminate ROS. Iloprost and piracetam protect the spinal cord by preventing lipid peroxidation. With its protective effect on GSH-Px, they decrease free radicals and prevent oxidants. Our study shows that NO increased significantly in the I/R group and that iloprost and piracetam decreased this increase. In oxidative stress, free radicals produce NO. MPO is an enzyme settled in leukocytes. Tissue MPO levels increased because of leukocyte infiltration in the I/R group. However, iloprost and piracetam prevented this increase, and in the I/R group, neutrophil and mononuclear phagocytes were infiltrated in the spinal cord. On the basis of our findings, we suggest that iloprost and piracetam can be extraordinary agents in the protection against oxidative stress in thoracic aorta surgery.
This study has some limitations. The number of rabbits in each group and the time period for neurological assessment may be augmented and dose-dependent results may be investigated. Further studies based on our findings may be more helpful for investigating these promising medications for I/R injury of the spinal cord.
References
Kiziltepe U, Turan NN, Han U, Ulus AT, Akar F . Resveratrol, a red wine polyphenol, protects spinal cord from ischemia-reperfusion injury. J Vasc Surg 2004; 40: 138–145.
Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS . Protective effect of melatonin on experimental spinal cord ischemia. Spinal Cord 2003; 41: 533–538.
Svensson LG, Rickards E, Coull A, Rogers G, Fimmel CJ, Hinder RA . Relationship of spinal cord blood flow to vascular anatomy during thoracic aortic cross-clamping and shunting. J Thorac Cardiovasc Surg 1986; 91: 71–78.
Tabayashi K, Niibori K, Konno H, Mohri H . Protection from postischemic spinal cord injury by perfusion cooling of the epidural space. Ann Thorac Surg 1993; 56: 494–498.
Zvara DA . Thoracoabdominal aneurysm surgery and the risk of paraplegia: contemporary practice and future directions. J Extra Corpor Technol 2002; 34: 11–17.
Kirsh JR, Helfaer MA, Lange DC, Traystma RJ . Evidence for free radical mechanism of brain injury resulting from ischemia/reperfusion-induced events. J Neurotrauma 1992; 9: 157–163.
Attar A, Tuna H, Sargon MF, Yuceer N, Turker RK, Egemen N . Early protective effects of iloprost after experimental spinal cord injury. Neurol Res 1998; 4: 353–359.
Zivin JA, DeGirolami U . Spinal cord infarction: a highly reproducible stroke model. Stroke 1980; 11: 200–202.
DeGirolami U, Zivin JA . Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol 1982; 41: 129–149.
Dosluoglu HH, Aktan AO, Yegen C, Okboy N, Yalcm AS, Yahn R et al. The cytoprotective effects of verapamil and iloprost (ZK 36374) on ischemia/reperfusion injury of kidneys. Transpl Int 1993; 3: 138–142.
Takamatsu H, Tsukada H, Watanabe Y, Cui Y, Kataoka Y, Hosoya T et al. Specific ligand for a central type prostacyclin receptor attenuates neuronal damage in a rat model of focal cerebral ischemia. Brain Res 2002; 2: 176–182.
Geber J, Cop J, Cvitanović B, Anić T, Bjegović M . The effect of piracetam on the recurrent inhibition of motor neurones. Neurologija 1990; 39: 163–168.
Keil U, Scherping I, Hauptmann S, Schuessel K, Eckert A, Müller WE . Piracetam improves mitochondrial dysfunction following oxidative stress. Br J Pharmacol 2006; 147: 199–208.
Uchiyama M, Mihara M . Determination of malondialdehyde precursors in tissues by thiobarbituric acid test. Anal Biochem 1978; 86: 279–286.
Kalkan E, Cicek O, Unlu A, Abusoglu S, Kalkan SS, Avunduk MC et al. The effects of prophylactic zinc and melatonin application on experimental spinal cord ischemia–reperfusion injury in rabbits: experimental study. Spinal Cord 2007; 45: 722–730.
Chan PH . Role of oxidants in ischemic brain damage. Stroke 1996; 27: 1124–1129.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kalkan, E., Keskin, F., Kaya, B. et al. Effects of iloprost and piracetam in spinal cord ischemia–reperfusion injury in the rabbit. Spinal Cord 49, 81–86 (2011). https://doi.org/10.1038/sc.2010.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.76
Keywords
This article is cited by
-
Neuroprotective effect of piracetam-loaded magnetic chitosan nanoparticles against thiacloprid-induced neurotoxicity in albino rats
Inflammopharmacology (2023)
-
Piracetam Attenuates LPS-Induced Neuroinflammation and Cognitive Impairment in Rats
Cellular and Molecular Neurobiology (2017)
-
Studies on protection against ischemia reperfusion injury after SCI
Spinal Cord (2016)
-
Effect of piracetam, vincamine, vinpocetine, and donepezil on oxidative stress and neurodegeneration induced by aluminum chloride in rats
Comparative Clinical Pathology (2016)
-
Piracetam Ameliorated Oxygen and Glucose Deprivation-Induced Injury in Rat Cortical Neurons Via Inhibition of Oxidative Stress, Excitatory Amino Acids Release and P53/Bax
Cellular and Molecular Neurobiology (2014)