Abstract
Objectives:
This review analyzed efficacy, tolerability and safety of oral antimuscarinic (AM) drugs in adults suffering from neurogenic detrusor overactivity (NDO).
Methods:
A comprehensive search of major literature bases was conducted to identify all references.
Results:
Thirty studies, thereof 16 randomized controlled trials (RCT), enrolling 1479 patients were identified and included in the review. Results were grouped in dose-finding, placebo- and active-controlled, flexible dose and combined high-dose AM drugs, and various studies. Key urodynamic outcome parameters, such as maximum detrusor pressure and maximum cystometric bladder capacity, demonstrated the efficacy of AM in NDO, following 2–3 weeks of treatment. Contrary to idiopathic detrusor overactivity (IDO), no placebo effects manifested. Other important parameters, such as impact on the upper urinary tract function and morphology, issues of continence, post-void residual urine, catheterisation, urinary tract infections and quality of life, were investigated to a limited extent only. Incidence rates of adverse events were comparable for NDO and IDO. Most of the studies, especially RCT, were undertaken with oxybutynin immediate release (IR), trospium chloride IR, propiverine IR and propiverine extended release. In NDO, these drugs are best investigated.
Conclusions:
AM drugs are effective in NDO, they normalize the intravesical pressure and increase cystometric bladder capacity. However, other important parameters are not adequately investigated so far and should be recognized in future studies.
Similar content being viewed by others
Introduction
Recently published reviews on antimuscarinic (AM) drugs focus either on idiopathic detrusor overactivity (IDO)/overactive bladder (OAB),1 or on bladder outlet obstruction and lower urinary tract (LUT) symptoms.2, 3 Studies on neurogenic detrusor overactivity (NDO) were not included in these reviews, although AM alone or in combination with intermittent catheterisation (IC) are the mainstay for NDO therapy: ‘AM drugs are the first-line choice for treating neurogenic LUT (NLUTD). They are the most useful medication available for NLUTD and provide an established approach to managing NDO (level of evidence 1a)’.4
The aim of AM treatment in NDO is different from IDO, as detrusor pressure during filling and voiding is crucial. Lowering or normalizing the detrusor pressure with AM is an important goal, demanding urodynamic work-up and follow-up. Consequently, AM ‘prevent renal and bladder damage and potentially improve long-term outcomes (LE 1a)’.4 This is in contrast to IDO/OAB, in which improvement of the key symptoms, urgency with or without incontinence, frequency and nocturia, is intended.
In the only meta-analysis focussing on NDO, Madhuvrata et al.5 restricted their approach to 16 randomized controlled trials (RCT), however, with a rather statistical than clinical approach and inclusion criteria deviant from our review. The review did not address issues such as bladder compliance, duration of detrusor contractions, IC, and function and morphology of the upper urinary tract.
This review aims at analyzing the efficacy, tolerability and safety of AM in adults suffering from NDO. Although only the RCTs in this review give the core information according to evidence-based criteria, all publications on this topic with respect to the criteria given below are included, thus providing comprehensive information, also unmasking deficits and unmet clinical needs, which should be addressed in future studies.
Materials and methods
Search strategy
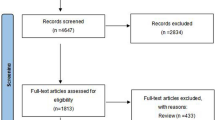
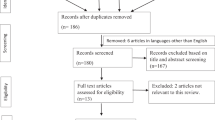
The review is based on a comprehensive search of all major literature bases (MEDLINE, EMBASE) and abstract books from annual meetings of the American Association of Urology, International Continence Society, European Association of Urology and International Medical Society of Paraplegia from January 1986 to February 2012 (Figure 1: body of evidence). The following search terms were used: AM drugs, darifenacin, fesoterodine, imidafenacin, oxybutynin, propiverine, solifenacin, tolterodine, trospium chloride, propantheline, NDO, detrusor hyperreflexia, adults and human. There were no restrictions on the inclusion of publications by language. Publications in languages other than English were included, if an abstract in English was available. Inclusion criteria comprised patients with proven NDO and an age of ⩾18 years. Case studies and studies enrolling ‘mixed’ patient populations of IDO and NDO were not included.
There were nine AM included in this review: darifenacin, fesoterodine, imidafenacin, oxybutynin, propiverine, solifenacin, tolterodine, trospium chloride and propantheline for historic reasons. AM were administered at various doses, dose intervals and by various immediate (IR) and extended release (ER) formulations. Our review is restricted to orally administered AM. Intravenous,6 transdermal,7 and intravesical, alone or combined with oral8 formulations, are either clinically not relevant (intravenous) or data were too scarce.
Key urodynamic parameters, such as maximum detrusor pressure and maximum cystometric bladder capacity, and clinical parameters, such as the achievement of continence, the need for catheterisation, autonomic dysreflexia, and impact on upper urinary tract function and morphology, urinary tract infections (UTI) and quality of life (QoL) were reviewed. Typical AM adverse events (AE), dry mouth rates and discontinuation rates served as tolerability and safety outcome parameters.
Statistical analysis
Owing to the limited number of studies in the different categories, statistical analyses were considered as not feasible.
Results
Study characteristics
The studies were grouped as dose-finding (Table 1a), placebo-controlled (Table 1b), active-controlled (Table 1c), flexible dose and combined high-dose AM (Table 1d), and various, and classified according to their LE.9 Studies investigating flexible and combined high-dose AM share the common characteristic that applied doses are not in conformity with doses recommended in the respective summary of product characteristics. Altogether, 30 studies, thereof 16 RCT, enrolling 1479 patients were analyzed. Study details, such as design, treatment duration, treatment groups, patient number, gender, age and diagnosis are given in the respective tables. Most patients suffered from suprasacral spinal lesions, mostly due to spinal cord injury (SCI). In none of the RCT, a majority of patients presenting with suprapontine NDO was enrolled, and studies enrolling both clinical entities did not analyze subpopulations separately.
Dose-finding studies
Three dose-finding studies (Table 1a), two of them placebo-controlled, were conducted with propiverine IR,10 tolterodine IR11 and trospium chloride IR.12
Placebo-controlled studies
The study characteristics of the five placebo-controlled proof of efficacy studies with oxybutynin IR,13 propiverine IR,14 trospium chloride IR,15 tolterodine IR16 and solifenacin17 are summarized in Table 1b.
Active-controlled studies
Six active-controlled studies (Table 1c) compared oxybutynin IR with propantheline IR,18 oxybutynin IR with trospium chloride IR,19 oxybutynin IR with propiverine IR,20 oxybutynin IR with tolterodine IR,16 propiverine IR with propiverine ER21 and solifenacin with oxybutynin.17
Flexible dose studies and combined high-dose AM
Six studies investigated, whether flexible dosing improved therapeutic response (Table 1d), two with oxybutynin ER,22, 23 one with trospium chloride IR,24 one with solifenacin,25 one compared oxybutynin IR with tolterodine IR,16 another tolterodine ER with trospium chloride IR.26 Two studies combined high-dose AM in patients nonresponsive to dosage-escalated monotherapy.27, 28
Various studies
Nine studies were grouped as various studies: one investigated short-term efficacy of trospium chloride IR,29 one the onset of efficacy of tolterodine IR30 and another tolterodine ER.31 Three studies, one with darifencin32 and two with solifenacin were open-label, noncomparative studies.33, 34 Three studies investigated long-term outcomes of propiverine IR,35 and fesoterodine,36 another open-label retrospective study compared tolterodine with oxybutynin.37 The studies of Tan and Toh,37 and Yamanishi et al.31 lack important study details.
Efficacy
Dose-finding studies
Based on the urodynamic results the dose-finding studies showed distinct dose–response relationships, reflected in a decrease of maximum detrusor pressure and paralleled by an increase of maximum cystometric capacity.10, 11, 12 These effects were superior to placebo, if a placebo arm was incorporated.11, 12
Placebo- and active-controlled studies
The results with respect to the key efficacy outcome parameters, maximum detrusor pressure and maximum cystometric bladder capacity, are summarized in Tables 2a and 2b: Comparing AM with placebo resulted in significant differences with negligible effects of placebo.13, 14, 15 Unequivocally, placebo- and active-controlled studies with reported urodynamic outcomes demonstrated a significant decrease of ∼30–40% in maximum detrusor pressure, paralleled by a significant increase of maximum cystometric bladder capacity of ∼30–40% for oxybutynin IR, propiverine IR, propiverine ER and trospium chloride IR.13, 14, 15, 19, 20, 21 The effects of solifenacin 10 mg were distinctly less pronounced.17 Moreover, equieffectivity of oxybuynin IR 5 mg t.i.d. and trospium chloride IR 20 mg b.i.d.,19 and oxybutynin IR 5 mg t.i.d. and propiverine IR 15 mg t.i.d.20 was shown. Propiverine IR 15 mg t.i.d. and propiverine ER 45 mg s.i.d. proved to be equieffective in regards to the primary efficacy outcome parameter reflex volume.21
Results on changes of bladder compliance and in duration of detrusor contraction14 were incompletely or not at all reported. This applies also to upper urinary tract function and morphology (Tables 2a and 2b).
Flexible dose studies and combined high-dose AM
All studies with flexible doses (Table 1d) ended up with higher doses compared with those in studies with fixed doses: Self-selected dosing of oxybutynin IR, up to 15 mg per day, and tolterodine IR, up to 12 mg per day, resulted in improved efficacy without compromising tolerability.16 In a dose titration study of oxybutynin ER, 74.4% of patients requested daily doses of at least 15 mg; doses up to 30 mg were effective and well tolerated.22 Doubling the recommended AM dosage in patients nonresponsive to recommended doses improved urodynamic parameters significantly.26 Moreover, the same authors showed that 85% of patients nonresponsive to dosage-escalated monotherapy could be treated successfully by combined high-dose AM.27
Various studies
Geirsson et al.30 investigated the onset of efficacy, following single doses of tolterodine IR, which manifested as early as 0.5 hours post dosing. Orally administered darifenacin was effective in an uncontrolled, open-label study in patients with NDO due to multiple sclerosis (MS).32 Two uncontrolled, open-label studies with limited number of patients were undertaken with solifenacin:33, 34 van Rey and Heesakkers34 showed clinical improvements for micturition frequency, pad use and voided volume in patients with MS; no urodynamic data were given.
Clinical, long-term and QoL outcomes
Frequency and mode of bladder emptying, for example, IC, as well as autonomic dysreflexia, were not addressed in a way, allowing sound conclusions. Moreover, only few of the placebo- and active-controlled studies investigated achievement of continence in detail20, 21 or to a limited extent:16, 18 Stöhrer et al.20 reported a decrease of 1.3 incontinence episodes following oxybutynin IR compared with a decrease of 1.6 episodes following propiverine IR; however, details on adjunct IC are not given. Effects of AM on UTI, upper urinary tract function and morphology are not reported.
Long-term efficacy of AM was demonstrated in three studies: Propiverine IR showed constant efficacy during a 1-year follow-up, the efficacy profile favored 45 mg compared with 30 mg.35 In a study with SCI patients comparing tolterodine and oxybutynin tolterodine demonstrated an increasing cystometric bladder capacity over a mean follow-up period of 3 years, whereas oxybutynin did not (no dosages given).37 Fesoterodine 4 mg showed significant improvements of micturition frequency and incontinence episodes in MS patients after a mean follow-up period of 22 months.36
QoL was investigated in three open-label studies: Some domains of the King's Health Questionnaire were significantly improved following tolterodine.31 With solifenacin, a disease-specific QoL outcome measure showed only modest improvements in one of the subscales, investigating patients with MS34 With 4 mg fesoterodine, a global improvement in QoL was observed in patients with MS already after 1 month, and was maintained during follow-up of 22 months.36
Tolerability and safety
All studies (Tables 3a and 3b), exempting one,15 showed either lower overall AE or dry mouth rates, or discontinuation rates for placebo compared with AM.13, 14, 16, 17 The most frequently reported AE was dry mouth, higher incidence rates are reported for oxybutynin IR compared with trospium chloride IR,19 tolterodine16 and propiverine.20 Higher doses of AM were not necessarily associated with higher rates of AE.22, 24, 27 The combination of two different AM, administered in higher-than-recommended dosages, revealed increased rates of AE, resulting in a drop-out rate of 15% because of typical AM AE. The three long-term studies35, 36, 37 reported AE rates of up to 13%,35 and drop-out rates of 27%, due to side effects or unsatisfactory outcomes.36
Discussion
The aim of treatment in NDO is to preserve upper urinary tract function and to manage incontinence, possibly to restore continence, by achieving a low detrusor pressure with adequate bladder capacity and regular bladder emptying, with tolerable or without post-void residual (PVR).4 In contrast to IDO/OAB, AM drugs decrease the maximum detrusor pressure in NDO38 up to ∼40 cm H2O, as was shown in SCI patients.13, 14, 15, 19, 20, 21 According to Mc Guire's et al.39 findings, this is the crucial detrusor leak point pressure in children with NLUTD due to myelomeningocele. However, there is no evidence for this threshold value in adults. The magnitude of pressure reduction is correlated with its baseline value. This may be the reason why in the study of Amarenco et al.17 with a mixed patient population of MS (N=95) and SCI (N=81), the decrease in detrusor pressure at leak was only 11.7 cm H2O.
Achieving a low intravesical pressure situation, associated with an increase in maximum cystometric bladder capacity, should improve incontinence, especially when combined with IC. However, the achievement of continence was reported in the RCTs not at all,13, 14, 15 only to a limited extent7, 16, 18 or rarely in detail.20, 21 Nevertheless, for patients on IC the mere reduction of incontinence episodes is not sufficient, they want to become continent in-between catheterisations without using condom catheters or pads. This issue should be addressed in more detail in future studies. However, it has to be considered that this outcome parameter is secondary to maximum detrusor pressure, the most crucial surrogate parameter, reflecting a successful or insufficient protection of the renal function.
In contrast to IDO/OAB, PVR urine increased in most studies significantly, and thus contributed positively to the clinical situation in patients on IC, because the increase of PVR reflects detrusor relaxation, thus enhancing the chance to become continent in-between catheterisations. The reasons for the increase of PVR are two-fold: A decrease of detrusor pressure and ongoing detrusor-sphincter-dyssynergia. None of the studies defined the type and the severity of detrusor-sphincter-dyssynergia.
Most patients with NDO require long-term or even life-long AM medication. However, study duration was limited to 2–3 weeks in most studies, long-term outcomes were only scarcely addressed. Therefore, the impact of lowering the detrusor pressure on the upper urinary tract could not be investigated:35, 36, 37 No information is given, whether an initially normal upper urinary tract remained stable or a pathological upper urinary tract recovered. Nevertheless, the proven normalization of pressure situation following short-term treatment presents a surrogate parameter, thus most probably indicative of normalization of the upper urinary tract as long-term outcome.
The results of dose-finding and flexible dose studies confirmed that the detrusor pressure is further decreased with higher dosages. Also self-selected dosing has shown that patients preferred higher doses than recommended. This is in accordance with the Guidelines of the European Association of Urology on NLUTD, stating that ‘neurogenic patients usually need a higher dose of AM agents than patients with IDO (LE 1b).’4
In regards to tolerability and safety, the study results demonstrate that oxybutynin IR has the highest dry mouth rate compared with all other AM (Tables 3a and 3b), which is in accordance with the results given in the meta-analyses by Chapple et al.1 and Kessler et al.40 in patients with IDO/OAB. Although many neurogenic patients may take AM life-long, only one of the studies41 addressed central nervous side effects explicitly, which are relevant also in this group of patients. Therefore, further studies, especially long-term studies, should incorporate this issue. This is especially important, because in the future more elderly, already cognitively impaired patients with NDO need AM treatment.
The fact that in flexible dose studies, AM despite higher doses, often two- or three-fold of the recommended doses, do not necessarily induce a higher overall rate of AE or dry mouth, is remarkable; a possible explanation could be the younger age of these predominantly SCI patients. The fact that the combination of two or even three AM in high doses showed an increased drop-out rate is possibly because of the fact that patients are unlikely to accept two or three drugs for the same disease at the same time.
Power considerations of study planning were not given or not taken into account. Also the statistical analysis is limited because of the small number of patients in multiple categories. Moreover, only six head-to-head-studies of the different AM drugs are available. Whether these issues may limit the validity of the conclusions is debatable. However, one has to bear in mind, that the relatively rare incidence rate of NDO compared with IDO/OAB precludes studies with comparable patient numbers. Moreover, the nonexistence of placebo effects in NDO studies may counteract the limited patient numbers.13, 14, 15
The evaluation of the studies revealed some deficits, as clinically important parameters were either not evaluated or not reported: the impact of AM on bladder compliance, which was either not assessed or the results given were difficult to interpret due to nonuniform methodology;42 the issue, whether AM shorten a prolonged detrusor contraction, the need for IC, exact data on the achievement of continence, for example, in-between IC, the impact of AM on UTI frequency and on QoL. This deficiency with respect to QoL may be because of the fact that many studies were conducted at a time when this issue was not yet a primary research focus. The obvious drawbacks with respect to clinical, long-term and QoL parameters should be considered in future studies. Nevertheless, AM still remain the first-line treatment for NDO, even in the era of botulinum neurotoxine type A. Moreover, the impact of different underlying disorders, the impact of bladder management strategies—AM may be dosed differently in patients voiding spontaneously compared with those performing IC—cannot be assessed due to missing data.
Conclusions
AM are effective in NDO with an acceptable tolerability profile. The placebo- and active-controlled RCTs as core studies document a decrease of maximum detrusor pressure, and an increase of maximum cystometric bladder capacity. This is endorsed by uncontrolled, open-label studies. Flexible dose studies indicate that higher doses than recommended may improve the efficacy. Oxybutynin, propiverine and trospium chloride were investigated extensively, whereas darifenacin, fesoterodine, solifenacin and tolterodine were investigated only to a limited extent. Our conclusions have to be restricted primarily to patients with spinal cord lesions, as patients with suprapontine detrusor overactivity (Morbus Parkinson, stroke, dementia and MS) are only included in two uncontrolled studies with limited patient numbers.
Drawbacks are the short duration in most of the studies, not allowing to document effects on upper urinary tract function and morphology. Moreover, important urodynamic findings (bladder compliance) as well as important clinical aspects were not evaluated or not adequately reported to allow conclusions. This should be taken into account in future study planning.
DATA ARCHIVING
There were no data to deposit.
References
Chapple C, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D . The effects of antimuscarinic tretaments in overactive bladder: An update of a systematic review and meta-analysis. Eur Urol 2008; 54: 543–562.
Novara G, Galfano A, Ficarra V, Artibani W . Anticholinergic drugs in patients with bladder outlet obstruction and lower urinary tract symptoms: a systematic review. Eur Urol 2006; 50: 675–683.
Kaplan SA, Roehrborn CG, Abrams P, Chapple CR, Bavendam T, Guan Z . Antimuscarinics for treatment of storage lower urinary tract symptoms in men: a systematic review. Int J Clin Pract 2011; 65: 487–507.
Stöhrer M, Blok B, Castro-Diaz D, Chartier-Kastler E, Del Popolo G, Kramer G et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol 2009; 56: 81–88.
Madhuvrata P, Hasafa Z, Singh M, Abdel-Fattah M . Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol 2012; 62: 816–830.
Bycroft J, Leaker B, Wood S, Knight S, Shah G, Craggs M . The effect of darifenacin on neurogenic detrusor overactivity in patients with spinal cord injury. Annual meeting of the International Continence Society, Florence, Italy, 2003. Neurourol Urodyn 2003; 22: A190.
Kennelly MJ, Lemack GE, Foote JE, Trop CS . Efficacy and safety of oxybutynin transdermal system in spinal cord injury patients with neurogenic detrusor overactivity and incontinence: an open-label, dose-titration study. Urology 2009; 74: 741–745.
Pannek J, Sommerfeld HJ, Bötel U, Senge T . Combined intravesical and oral oxybutynin chloride in adult patients with spinal cord injury. Urology 2000; 55: 358–362.
Oxford Centre for Evidence-based Medicine Levels of evidence (May 2011). Produced by Bob Philips, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin Dawessince November 1998.
Mazur D, Göcking K, Wehnert J, Schubert G, Herfurth G, Alken RG . Clinical and urodynamic effects of oral propiverine therapy in neurogenic urinary incontinence: a multicentre dose-optimizing study. Urologe A 1994; 33: 447–452.
Van Kerrebroeck P, Amarenco G, Thüroff J, Madersbacher HG, Lock MT, Messelink EJ et al. Dose-ranging study of tolterodine in patients with detrusor hyperreflexia. Neurourol Urodyn 1998; 17: 499–512.
Stöhrer M, Bersch U, Göcking K, Schurch B, Kramer G . Dose finding study for treatment of detrusor hyperreflexia by trospium chloride. Presented at: Annual Meeting of the International Medical Society of Paraplegia. Iguassu, Brazil 1998.
Stöhrer M, Frohneberg D, Loechner-Ernst D, Sauter P, Mandaika B . Oxybutynin in the treatment of detrusor hyperreflexia in patients with spinal cord injury: A multicentre randomised double-blind study. Presented at: Annual Meeting of the American Spinal Cord Injury Association. Orlando, USA 1990.
Stöhrer M, Madersbacher H, Richter R, Wehnert J, Dreikorn K . Efficacy and safety of propiverine in SCI-patients suffering from detrusor hyperreflexia—a double-blind, placebo-controlled clinical trial. Spinal Cord 1999; 37: 196–200.
Stöhrer M, Bauer P, Giannetti BM et al. Effect of trospium chloride on urodynamic parameters in patients with detrusor hyperreflexia due to spinal cord injuries. A multicentre placebo-controlled double-blind trial. Urol Int 1991; 47: 138–143.
Ethans KD, Nance PW, Bard RJ, Casey AR, Schryvers OI . Efficacy and safety of tolterodine in people with neurogenic detrusor overactivity. J Spinal Cord Med 2004; 27: 214–218.
Amarenco G, Sutory M, Fagertun H, Wright M, Compion G, DeRidder D . Solifenacin is effective and well tolerated in patients with neurogenic detrusor overactivity. Preliminary results from the SONIC urodynamic study. Eur Urol, Suppl 2012; 11: e467.
Gajewski JB, Awad SA . Oxybutynin versus propantheline in patients with multiple sclerosis and detrusor hyperreflexia. J Urol 1986; 135: 966–968.
Madersbacher H, Stöhrer M, Richter R, Burgdörfer H, Hachen HJ, Mürtz G . Trospium chloride versus oxybuynin: a randomized, double-blind, multicentre trial in the treatment of detrusor hyperreflexia. Brit J Urol 1995; 75: 452–456.
Stöhrer M, Mürtz G, Kramer G, Schnabel F, Arnold EP, Wyndaele JJ., Propiverine Study Group. Propiverine compared to oxybutynin in neurogenic detrusor overactivity—results of a randomized, double blind, multicenter clinical study. Eur Urol 2007; 51: 235–242.
Stöhrer M, Mürtz G, Kramer G, Warnack W, Primus G, Jinga V et al. Efficacy and tolerability of propiverine hydrochloride extended release compared to immediate release in patients with neurogenic detrusor overactivity. Spinal Cord 2013; 51: 209–213.
Bennett N, O’Leary M, Patel AS, Xavier M, Erickson JR, Chancellor MB . Can higher doses of oxybutynin improve efficacy in neurogenic bladder? J Urol 2004; 171: 749–751.
O‘Leary M, Erickson JR, Smith CP, McDermott C, Horton J, Chancellor MB . Effect of controlled-release oxybutynin on neurogenic bladder function in spinal cord injury. J Spinal Cord Med 2003; 26: 159–162.
Menarini M, Del Popolo G, Benedetto D, Haselmann J, Bödeker RH, Schwantes U et al. Trospium chloride in patients with neurogenic detrusor overactivity: Is dose titration of benefit to the patients? Int J Clin Pharmacol Ther 2006; 44: 623–632.
Persu C, Gaevlete PA . Double dose of solifenacin for the treatment of neurogenic detrusor overactivity. Eur Urol, Suppl 2012; 11: e469.
Horstmann M, Schaefer T, Aguilar Y, Stenzl A, Sievert KD . Neurogenic bladder treatment by doubling the recommended antimuscarinic dosage. Neurourol Urodyn 2006; 25: 441–445.
Amend B, Hennenlotter J, Schaefer T, Horstmann M, Stenzl A, Sievert KD . Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur Urol 2008; 53: 1021–1028.
Cameron AP, Clemens JQ, Latini JM, McGuire EJ . Combination drug therapy improves compliance of the neurogenic bladder. J Urol 2009; 182: 1062–1067.
Madersbacher M, Stöhrer M, Richter R, Giannetti BM, Mürtz G . High-dose administration of trospium chloride in the treatment of detrusor hyperreflexia. Urologe 1991; 30: 260–263.
Geirsson G, Schoefield D, Haughie S, Steihdotsdottir S, Hardardottir A, Glue P et al. Assessment of time to onset: the effect of single oral dose of tolterodine on neurogenic detrusor overactivity in spinal cord injury patients. Presented at: Annual Meeting of the International Continence Society 2006 (Abstract 309).
Yamanishi T, Mizuro T, Yoshida K, Uchiyama T, Sakakibara R . Efficacy of tolterodine ER for the treatment of neurogenic detrusor overactivity or low compliance bladder—assessment by urodynamic study. Presented at: Annual Meeting of the International Continence Society 2009 (Abstract 330).
Carl S, Laschke S . Darifenacin is also effective in neurogenic bladder dysfunction (multiple sclerosis). Urology 2006; 68 (Suppl): 250.
Spinelli M, Citari M, Zanollo L et al. Solifenacin in neurogenic overactive bladder. Eur Urol Suppl 2007; 6: 273.
van Rey F, Heesakkers J . Solifenacin in multiple sclerosis patients with overactive bladder: a prospective study. Adv Urol 2011; 2011: 834753.
Mazur D, Göcking K, Wehnert J, Dorschner W, Schubert G, HerFurth G et al. Verträglichkeit und Wirksamkeit einer Langzeittherapie mit Propiverinhydrochlorid bei neurogener Hatrninkontinenz. Eine multizentrische Studie. Kontinenz 1994; 2: 74–78.
Proietti S, Lepri E, Lepri L, Lolli C, Gubbiotti M, Giannantoni A . Efficacy and tolerability of fesoterodine fumarate in the treatment of overactive bladder symptoms in patients affected by multiple sclerosis: Long term follow up. Eur Urol, Suppl 2012; 11: e468.
Tan YK, Toh KL . Tolterodine improves the compliance and cystometric capacity of adult neurogenic bladders secondary to spinal cord injury. Presented at: Annual Meeting of the International Continence Society 2009 (Abstract 331).
Digesu GA, Khullar V, Cardozo L, Salvatore S . Overactive bladder symptoms: do we need urodynamics? Neurourol Urodyn 2003; 22: 105–108.
McGuire EJ, Woodside JR, Borden TA, Weiss RM . Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 1981; 126: 205–209.
Kessler TM, Bachmann LM, Minder C, Löhrer D, Umbehr M, Schünemann HJ et al. Adverse event assessment of antimuscarinics for treating overactive bladder: A network meta-analytic apoproach. PLoS One 2011; 6: e16718.
Uchiyama T, Sakakibara R, Liu Z et al. The effects of anticholinergic drugs on cognitive impairment, mental dysfunction and motor dysfunction in patients with neurological disease. 35thAnnual Meeting of the ICS, 28 August-2 September 2005 Montreal, Canada. Conference Proceedings.
Wyndaele JJ, Gammie A, Bruschini H, De Wachter S, Fry CH, Jabr RI et al. Bladder compliance what does it represent: Can we measure it, and is it clinically relevant? Neurourol Urodyn 2011; 30: 714–722.
Acknowledgements
We are grateful to Guus Kramer and Saladin Alloussi for valuable contributions to the manuscript, and to Florian Schirm, Datamap Freiburg, Germany, for advise given with respect to statistical issues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Helmut Madersbacher is a member of the advisory board of Allergan/Switzerland, Apogepha/Germany, Astellas/Austria, and a scientific advisor of Pohl-Boskamp/Germany. He received lecture honoraria from Allergan/Switzerland, Apogepha/Germany, Astellas/Austria, Dr Pfleger/Germany and Rottapharm-Madaus/Germany. Gerd Mürtz is a scientific advisor of Apogepha/Germany. Manfred Stöhrer received lecture honoraria from Allergan/Germany and Apogepha/Germany. He is a scientific advisor of Farco/Germany.
Additional information
Supplementary Information accompanies this paper on the Spinal Cord website
Supplementary information
Rights and permissions
About this article
Cite this article
Madersbacher, H., Mürtz, G. & Stöhrer, M. Neurogenic detrusor overactivity in adults: a review on efficacy, tolerability and safety of oral antimuscarinics. Spinal Cord 51, 432–441 (2013). https://doi.org/10.1038/sc.2013.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2013.19
Keywords
This article is cited by
-
Sex differences in urological management during spinal cord injury rehabilitation: results from a prospective multicenter longitudinal cohort study
Spinal Cord (2023)
-
Kurzfassung der S2k-Leitlinie medikamentöse Therapie der neurogenen Dysfunktion des unteren Harntraktes (NLUTD)
Die Urologie (2023)
-
Stem Cell Therapy in Spinal Cord Injury-Induced Neurogenic Lower Urinary Tract Dysfunction
Stem Cell Reviews and Reports (2023)
-
Non-pharmacological and drug treatment of autonomic dysfunction in multiple system atrophy: current status and future directions
Journal of Neurology (2023)
-
Efficacy, according to urodynamics, of OnabotulinumtoxinA compared with antimuscarinic drugs, for neurogenic detrusor overactivity: a systematic review and network meta-analysis
Scientific Reports (2022)