Abstract
Study design:
Retrospective chart review.
Objectives:
To investigate the role of plasma creatinine (p-creatinine) in monitoring renal deterioration in patients up to 50 years after spinal cord injury (SCI).
Setting:
The Clinic for Spinal Cord Injuries, Rigshospitalet, Denmark.
Methods:
A total of 119 patients with a traumatic SCI during the years 1944–1975 were included in the study. P-creatinine measurements, results from renography and glomerular filtration rate (GFR) measured with 51Cr-EDTA clearance were obtained from medical records and analyzed using a linear mixed model and linear regression analyses.
Results:
When compared with median p-creatinine level in the first 5-year period after injury, the level of p-creatinine was stable throughout the first 30 years and decreased significantly after the 30th until 45th year post injury. Only patients with a functional distribution outside the 30–70% limits on renography or a relative GFR ⩽51% of that expected had a significantly elevated level of p-creatinine. Significance was not found for patients with a distribution outside the 40–60% limits on renography or relative GFR ⩽75%. By comparing Cr-EDTA clearance and p-creatinine in terms of exceeding the upper reference level, p-creatinine revealed 17% sensitivity, 100% specificity, 100% positive predictive value and 73% negative predictive value as a diagnostic test for renal deterioration defined as GFR ⩽75%.
Conclusion:
P-creatinine decreases over time in patients with SCI with a level below the upper reference limit and is a poor detector of early renal deterioration in patients with SCI.
Similar content being viewed by others
Introduction
Individuals who have sustained a spinal cord injury (SCI) are at increased risk of developing renal insufficiency, because of neurogenic bladder dysfunction. For this reason, SCI patients are recommended to participate in a lifelong follow-up program of the renal function. Despite numerous attempts to evaluate the follow-up program, the best method to monitor the renal function in this patient group has not yet been established.1 A widely used method is the repeated measurement of plasma creatinine (p-creatinine),2 and equations like the Cockcroft and Gault or MDRD (modification of diet in renal disease) equations have been derived to estimate glomerular filtration rate (GFR) from p-creatinine, age, weight and gender.3, 4 P-creatinine is primarily derived from the metabolism of creatine and phosphocreatine in muscles.5 Patients with SCI often have a reduced muscle mass because of diffuse muscle atrophy and for this reason the level of p-creatinine is often decreased in such patients.2 The hypothesis that p-creatinine overestimates the renal function in this patient group has been demonstrated in several studies since the 1980s,6, 7, 8, 9 but despite this p-creatinine is still a commonly accepted method to monitor renal function in SCI patients. Recognizing this, we wanted to investigate if there was a trend in the level of p-creatinine over a 50-year follow-up period and how well p-creatinine correlated with renal deterioration. To our knowledge, only three studies have investigated the relationship between p-creatinine and renal function monitored by 51Cr-EDTA or 99mTc-DTPA clearance, which is considered the gold standard.2, 10, 11 Of these three studies, none of them investigated p-creatinine over time and two of them only comprised 31 and 36 patients, respectively.
Materials and methods
Study population
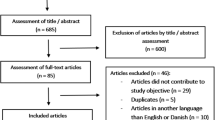
This retrospective study originally included 154 patients who were registered with a traumatic SCI between the years 1944 and 1975 and who initially were admitted to the Clinic of Spinal Cord Injuries in Hornbæk, Denmark.
Data regarding patient demographic, neurological level and completeness of injury according to Frankel et al.12 and the primary bladder emptying method13 were obtained from medical records, as well as all p-creatinine, renography and GFR measurements by using 51Cr-EDTA clearances. Of the 154 patients, there were 13 patients with missing medical records and 7 patients who had not sustained an actual traumatic SCI. Patients who were not followed up with minimum one renography or GFR (15 patients) were excluded from the study. Thus, 119 patients were included in the final analyses as shown in Figure 1.
None of the patients had diabetes at the time of SCI. There were six individuals with only one functional kidney: two of them because of a traumatic kidney damage in relation to the SCI and four because of a complete nephrectomy post injury.
Procedures
After the SCI, patients who accepted the follow-up program were offered a routine check about every second year lifelong at the Clinic for Spinal Cord Injuries. In the early years, follow-up consisted of p-creatinine measurement and X-ray of the abdomen or intravenous pyelography as a minimum, but since 1980 renography was performed on a regular basis and, when considered necessary, GFR was measured using plasma clearance determination of a radioactive tracer.
The majority of renographies were performed with 99m-Tc-labelled mercaptoacetyltriglycine three, but renography with dimercaptosuccinic acid, diethylentriamine pentaacetic acid (DTPA) and 131I-hippuran was also accepted in this study. In consensus with previous studies,14, 15 renography was considered moderately abnormal when there was a split renal function ⩽40% in one kidney and considered more severely abnormal when there was a split renal function ⩽30% in one kidney.
GFR was measured using a single injection technique with a radioactive isotope-marked tracer, in most cases with 51Cr-EDTA, and converted to an age and gender-adjusted relative GFR.16 Relative GFR ⩽75% was considered moderately abnormal and ⩽51% more severely abnormal. In this study, patients with at least one abnormal renography and/or GFR were categorized with moderate or more severe renal affection. Individuals with only one functional kidney since the time of injury and with no abnormal GFR measurements were classified as having normal renal function.
P-creatinine values were registered and if necessary converted from the unit mg dl−1 to μmol l−1 by dividing by 0.0113. When there was more than one p-creatinine measurement from the same year post injury, the highest value was registered. P-creatinine measurements (with a maximum of one value per year) were further assembled in the form of individual median p-creatinine values for the periods 0–5 years post injury (YPI), 6–10 YPI, and so on, and statistical analyses were performed on the logarithmical values of median 5-year p-creatinine levels. The upper reference level for a normal p-creatinine value was 105 μmol l−1 for males and 90 μmol l−1 for females, which is the official upper reference level since 2003 in our hospital and is the lowest upper reference level used in the follow-up period.
We also wanted to investigate how well the level of p-creatinine correlated with the renal function; thus, a linear regression analysis on relative GFR and p-creatinine measurements from the same year post injury was done. When a patient had more than one GFR and p-creatinine measurement from the same year, the lowest GFR and its corresponding p-creatinine level were selected. In order to get the best linear fit and to make the results comparable to previous studies, the analysis was performed on the reciprocal value, 1/p-creatinine. In addition, p-creatinine as a diagnostic test was evaluated by comparing p-creatinine measurements with GFR measurements.
All statistical analyses were performed using SPSS and Microsoft Excel 2011 software packages. P-values less than 0.05 were considered significant and P-values were calculated with Fisher’s exact test. To characterize the trend in p-creatinine over time, a linear mixed module with individual median 5-year p-creatinine values was used and illustrated as mean±normal range, which is a 95% prediction interval of the population. Correlation analysis was performed and Pearson’s correlation coefficient, r, and determination coefficient, r2, were calculated.
Results
Study population
Patient characteristics and primary bladder emptying methods are shown in Tables 1 and 2.
The primary bladder emptying methods have changed over time. Reflex triggering and bladder expression were the most frequently applied methods at the time of discharge after the initial post-injury rehabilitation, whereas clean intermittent catheterization and indwelling catheter (urethral or suprapubic) were the most frequently applied methods at the last follow-up.
P-creatinine over time
The average number of years with a p-creatinine measurement per patient was 17 years (±7.4), mean (±s.d.). Figure 2 shows no increase in the mean of 5-year median p-creatinine values over time for both males and females, and Table 3 confirms that the means of 5-year median p-creatinine values are stable in the first 30 years after SCI and show a significant decrease when comparing 0–5 YPI with 31–45 YPI. The last period of follow-up was 46–50 YPI, after which the means became unreliable because of small sample sizes. The means of p-creatinine never exceeded the upper reference level of p-creatinine (male 105 μmol l−1 and female 90 μmol l−1), and when looking at all the p-creatinine samples 73 patients (61%) never exceeded the upper reference limit, 24 patients (20%) exceeded the limit one time and 22 patients (19%) exceeded the limit more than one time. Six male patients experienced a p-creatinine level of 200 μmol l−1 or more: five of them with concomitant severe renal affection with functional distribution outside 30–70% on renography (four patients), r-GFR51 (one patient) and/or in dialysis treatment (two patients). The last patient did not have renal deterioration according to renography or GFR measurements and was hospitalized with encephalitis simultaneous with a p-creatinine level of 224 μmol l−1.
P-creatinine and renal function
In the linear mixed module analysis, we found no significant difference in the level of individual median p-creatinine in 5-year periods between patients with moderate renal affection and patients with normal renal function. Patients with more severe renal affection had a significantly higher level of individual 5-year median p-creatinine compared with patients with normal or moderate renal affection, with a difference of 12% (95% CI: 7–19%, P<0.0001). When comparing patients with all p-creatinine values within the normal reference interval with patients with one or more p-creatinine values exceeding the reference level, there was a significant difference with regard to severe renal affection (P0.04) but not with regard to moderate renal affection (P=0.13).
Figure 3 shows the relationship between relative GFR and p-creatinine, based on the results from 79 patients with a GFR and a p-creatinine measurement from the same YPI (38±8, means±s.d.). With renal deterioration defined by the gold standard Cr-EDTA clearance as r-GFR ⩽75%, the use of p-creatinine as a diagnostic test can be evaluated. We considered p-creatinine as a positive test for renal deterioration when exceeding the upper reference level. Of the 79 patients, there were 4 true positive, 20 false negative, 0 false positive and 55 true negative tests, revealing 17% sensitivity, 100% specificity, 100% positive predictive value and 73% negative predictive value of p-creatinine as a diagnostic test for renal deterioration.
Plots of r-GFR (% of normal reference value) versus p-creatinine for 79 spinal cord-injured patients: 69 male patients (♦) and 10 female patients ( × ) from the same year post injury: 38 (±8) mean (±s.d.). The upper reference level for P-creatinine is 105 μmol l−1 for males (horizontal solid line) and 90 μmol l−1 for females (horizontal dotted line). The lower reference level for relative GFR is 75% (vertical dotted line).
Figure 4 shows the relationship between relative GFR and 1/p-creatinine, and a significant linear relationship between GFR and 1/p-creatinine for the 69 male patients was found with Pearson’s coefficient r=0.448 and a determination coefficient r2=0.201 (P-value <0.0001). There was a non-significant relationship for the 10 female patients with r=0.275 and r2=0.075 (P-value=0.443).
Discussion
The aim of the present study was to investigate the trend of p-creatinine over time in patients with SCI and to examine if p-creatinine is useful in predicting renal deterioration. This study indicates that SCI patients have a steady level of p-creatinine during the first 30 years and a significant decrease after the 30th YPI. This finding is consistent with previous studies showing no increase in p-creatinine over time.7, 9, 14, 15, 17 The longest follow-up in previous studies was 18.7 YPI,14 and only one study with maximum 16 years follow-up found a significant decrease in p-creatinine over time.7 To our knowledge, our study has the longest follow-up period of 50 YPI.
In normal individuals, GFR declines with aging, with an average decrease of 10 ml min−1 per 1.73 m2 per decade after 30 years of age. Meanwhile, the total muscle mass declines with increasing age, and together these two changes result in a normal to slightly decreased p-creatinine concentration in older individuals.3 The fact that this study shows a steady level and later a significant decrease in p-creatinine over time could be a reflection of an increasing age in the patient population, perhaps accompanied by a more extensive loss of muscle mass over time in patients with a SCI compared with normal individuals. But, most importantly, no general increase in p-creatinine over time was found, despite the fact that SCI patients are at increased risk of developing renal insufficiency.2
The present study found that only patients with severe renal affection on renography (30–70% or more abnormal) or GFR measurement (relative GFR ⩽51%) would have a higher level of p-creatinine and p-creatinine values exceeding the upper reference limit, whereas this could not be detected in patients with less severe deterioration of renal function.
Other studies have investigated the correlation between 1/p-creatinine and GFR expressed with Pearson’s correlation coefficient (r). Thomassen et al.10 found a non-significant correlation between GFR measured with 51Cr-EDTA clearance and 1/s-creatinine in 31 SCI patients, with Pearson’s correlation coefficient r=0.26, and Jenkins et al.18 found a non-significant correlation between GFR measured with creatinine clearance and 1/s-creatinine for 20 male SCI patients, with r=0.29. The fact that our study included more SCI male patients (n=69) than the two mentioned above may explain the significant correlation found in our study for the male patients. In comparison we found a stronger correlation (r=0.448), but the correlation is not strong, as r is not close to 1, and only 20% of the variation in 1/p-creatinine can be explained by a change in GFR (r2=0.20), indicating that the results of p-creatinine measurements correspond poorly to the results of GFR measurements. A 17% sensitivity of p-creatinine as a diagnostic test in terms of p-creatinine values exceeding the upper reference level underlines the poor use of p-creatinine in diagnosing renal deterioration.
A few studies have described p-creatinine compared with renal function within the last 15 years,2, 10, 11, 17, 18, 19, 20 most of them using creatinine clearance as a measure of the renal function. Only three of the papers used the gold standards 51Cr-EDTA clearance10, 11 or 99mTc-DTPA clearance.2 They all concluded that s-creatinine underestimated the renal deterioration, except for one study, Sepahpanah et al.,17 which concluded that s-creatinine was better in monitoring renal function than creatinine clearance.
A newer marker, cystatin C, has shown great potential in detecting renal deterioration in patients with SCI.10, 11, 18 It is produced by most nucleated cells at a stable rate and is, most importantly, not affected by the level of muscle mass, gender or age,10 which makes it a promising test in this patient population and hence it should probably substitute p-creatinine in the future.
Limitations
An important limitation to our retrospective study is the risk of missing data. Some patients did not attend the annual evaluations in many years after the injury and were then found again as a result of the additional follow-up effort. In this study 12 patients (10%) were not followed with p-creatinine in the first 20 YPI.
A retrospective study like this one with the earliest SCI in 1944 and with investigations carried out in different hospitals may also have some limitations in terms of comparing the investigation results. The method of measuring p-creatinine has for instance changed throughout the years in Rigshospitalet, where the majority of the p-creatinine measurements have been performed. For most of the measurements the Jaffe method has been used, but after 2003 an enzymatic IDMS calibrated method, CREA plus Roche Modular Analytics with a lower reference level, was introduced. We have considered if this could result in an artificial decrease in the level of p-creatinine over time, but as the patient population had sustained the SCI over a 31-year period, the date of decreasing p-creatinine values was spread out over the interval 28–59 YPI, and therefore this should not particularly affect our conclusion. Additionally, we only used the lowest and most conservative upper reference limit introduced in 2003 in our analyses to make sure we did not overlook any increase in the p-creatinine values.
Estimated GFR values can be calculated from p-creatinine measurements from the patient’s weight, age and gender, using, for example, the MDRD equation, and the estimated GFR and measured GFR can be compared accurately, but there were no repeated measures of weight available in this study, and therefore we were unable to make GFR estimations.
In this study, the renal function was determined from the results of renography with regard to functional renal distribution and GFR measured with a radioactive marked tracer. The presence of one abnormal investigation categorized the patients in either moderate or more severe renal affection. This definition of renal deterioration has limitations though, as it does not include a potential improvement in later investigations.
Conclusion
The findings of this study support that p-creatinine is a poor detector of early renal deterioration, and until new tests like Cystatin C are further evaluated we recommend Cr-EDTA clearance as the gold standard in monitoring renal function in patients with SCI.
Data Archiving
There were no data to deposit.
References
Cameron AP, Rodriguez GM, Schomer KG . Systematic review of urological followup after spinal cord injury. J Urol 2012; 187: 391–397.
MacDiarmid SA, McIntyre WJ, Anthony A, Bailey RR, Turner JG, Arnold EP . Monitoring of renal function in patients with spinal cord injury. BJU Int 2000; 85: 1014–1018.
Perrone RD, Madias NE, Levey AS . Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 1992; 38: 1933–1953.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254.
Thomas L, Huber AR . Renal function—estimation of glomerular filtration rate. Clin Chem Lab Med 2006; 44: 1295–1302.
Sawyer WT, Hutchins K . Assessment and predictability of renal function in spinal cord injury patients. Urology 1982; 19: 377–380.
Mohler JL, Barton SD, Blouin RA, Cowen DL, Flanigan RC . The evaluation of creatinine clearance in spinal cord injury patients. J Urol 1986; 136: 366–369.
Mirahmadi MK, Byrne C, Barton C, Penera N, Gordon S, Vaziri ND . Prediction of creatinine clearance from serum creatinine in spinal cord injury patients. Paraplegia 1983; 21: 23–29.
Kuhlemeier KV, McEachran AB, Lloyd LK, Stover SL, Fine PR . Serum creatinine as an indicator of renal function after spinal cord injury. Arch Phys Med Rehabil 1984; 65: 694–697.
Thomassen SA, Johannesen IL, Erlandsen EJ, Abrahamsen J, Randers E . Serum cystatin C as a marker of the renal function in patients with spinal cord injury. Spinal cord 2002; 40: 524–528.
Erlandsen EJ, Hansen RM, Randers E, Petersen LE, Abrahamsen J, Johannesen IL . Estimating the glomerular filtration rate using serum cystatin C levels in patients with spinal cord injuries. Spinal cord 2012; 50: 778–783.
Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 1969; 7: 179–192.
Biering-Sorensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ . International lower urinary tract function basic spinal cord injury data set. Spinal cord 2008; 46: 325–330.
Weld KJ, Wall BM, Mangold TA, Steere EL, Dmochowski RR . Influences on renal function in chronic spinal cord injured patients. J Urol 2000; 164: 1490–1493.
Biering-Sorensen F, Nielans HM, Dorflinger T, Sorensen B . Urological situation five years after spinal cord injury. Scand J Urol Nephrol 1999; 33: 157–161.
Brochner-Mortensen J, Jensen S, Rodbro P . Delimitation of plasma creatinine concentration values for assessment of relative renal function in adult patients. Scand J Urol Nephrol 1977; 11: 257–262.
Sepahpanah F, Burns SP, McKnight B, Yang CC . Role of creatinine clearance as a screening test in persons with spinal cord injury. Arch Phys Med Rehabil 2006; 87: 524–528.
Jenkins MA, Brown DJ, Ierino FL, Ratnaike SI . Cystatin C for estimation of glomerular filtration rate in patients with spinal cord injury. Ann Clin Biochem 2003; 40: 364–368.
Lee JP, Dang AT . Evaluation of methods to estimate glomerular filtration rate versus actual drug clearance in patients with chronic spinal cord injury. Spinal Cord 2011; 49: 1158–1163.
Chikkalingaiah KB, Grant ND, Mangold TM, Cooke CR, Wall BM . Performance of simplified modification of diet in renal disease and Cockcroft-Gault equations in patients with chronic spinal cord injury and chronic kidney disease. Am J Med Sci 2010; 339: 108–116.
Acknowledgements
The study has been supported by grants from the Lundbeck foundation. We thank Julie Forman, Associate Professor from the Department of Biostatistics, University of Copenhagen, for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Elmelund, M., Oturai, P. & Biering-Sørensen, F. 50 years follow-up on plasma creatinine levels after spinal cord injury. Spinal Cord 52, 368–372 (2014). https://doi.org/10.1038/sc.2014.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.24
Keywords
This article is cited by
-
Urologic surveillance of persons with spinal cord injuries – a scoping review
Spinal Cord (2024)
-
Evaluating estimated glomerular filtration rates of creatinine and cystatin C for male patients with chronic spinal cord injury
Spinal Cord (2018)
-
Recommendations for urological follow-up of patients with neurogenic bladder secondary to spinal cord injury
International Urology and Nephrology (2018)
-
Surveillance and management of urologic complications after spinal cord injury
World Journal of Urology (2018)
-
Forty-five-year follow-up on the renal function after spinal cord injury
Spinal Cord (2016)