Abstract
To test the hypothesis that plasminogen activator inhibitor-1 (PAI-1) may serve as a candidate marker for the malignancy of colorectal cancer (CRC), we performed a quantitative RT–PCR for PAI-1 gene and evaluated the possible relationship between PAI-1 gene expression levels and clinicopathological findings in CRC. A significant increase in PAI-1 expression scores was observed in lymph node metastasis-positive CRCs (2.19±0.43) compared to negative ones (0.35±0.42) (P=0.0037) as well as in distant metastasis-positive CRCs (3.50±1.18) compared to negative ones (0.99±0.30). The PAI-1 expression score markedly increased with the tumour stage (P=0.0063; ANOVA test). Moreover, multivariate analysis revealed the PAI-1 expression score to be a strong and independent prognostic factor for CRC (P=0.0432). These results suggested that PAI-1 might serve as a new parameter for the prediction of prognoses in CRC.
Similar content being viewed by others
Main
There is now good evidence that a series of genetic alterations in both dominant oncogenes and tumour suppressor genes are involved in the pathogenesis of human colorectal cancer (CRC). Activation of oncogenes such as the ras gene, and inactivation of tumour suppressor genes such as the APC and p53 genes, have been identified in CRC (Bos et al, 1987; Baker et al, 1990; Nishisho et al, 1991). In addition, we found that several other genes are related to the pathogenesis of this disease (Hibi et al, 1996, 1997, 2002, 2004; Yamazaki et al, 2002). An investigation of genetic changes is important to clarify the tumorigenic pathway of CRC (Vogelstein et al, 1988).
Plasminogen activator inhibitor-1 (PAI-1), a 45-kDa serine proteinase inhibitor with a reactive peptide bond, Arg345–Met346, is a multifaceted proteolytic inhibitor that not only functions as a fibrinolytic inhibitor, but also plays an important role in signal transduction, cell adherence, and cell migration. There is clinical evidence implicating PAI-1 as a key factor in tumour invasion and metastasis (Potempa et al, 1994; Lijnen, 2005). Moreover, PAI-1 has been linked to a poor prognosis in several cancers (Nekarda et al, 1994; Cantero et al, 1997; Chambers et al, 1998; Foekens et al, 2000; Konecny et al, 2001). Previously, we also demonstrated that PAI-1 expression was significantly correlated with a poor prognosis in esophageal squamous carcinoma (Sakakibara et al, 2004). These results prompted us to examine the PAI-1 expression level in other cancers, especially in CRC.
To test the hypothesis that PAI-1 may serve as a candidate marker for the malignancy of CRC, we performed a quantitative reverse transcription-PCR (RT–PCR) and evaluated the relationship between the PAI-1 gene expression levels and clinicopathological findings in CRC.
Materials and methods
Patients and tissue specimens
The study group consisted of 55 CRC patients (mean age 64.5 years; range 41–85 years), who underwent surgical operations at the Gastroenterological Surgery of the Nagoya University Graduate School of Medicine from 1994 to 2002. Written informed consent, as indicated by the institutional review board, was obtained from all patients. All patients were followed at our hospital with periodical examinations. The median follow-up was 33 months. In total, 41, 4, and 10 patients received R0, R1, and R2 resection, respectively. Although no patients received neoadjuvant therapy, 25 patients received adjuvant therapy. The distribution of these patients was not related to PAI-1 expression scores. A total of 17 patients have been lost to follow-up until now. All tumours and corresponding normal tissues were collected at the surgical resection and stored at −80°C. They were graded according to their tumour-node-metastasis (TNM) stage as follows: 12 had stage I disease; 12 had stage II; 23 had stage III; and eight had stage IV. The patients were classified into two groups according to age, sex, histology, tumour size, tumour site, depth of tumour invasion, lymph node metastasis, perineal dissemination, carcinoembryonic antigen (CEA), and TNM stage.
RNA preparation and reverse transcription
Total RNA was extracted from CRC and corresponding normal tissues with guanidium thiocyanate as described previously (Hibi et al, 1996). The amount of RNA was measured spectrophotometrically by absorbance at 260 nm. First-strand cDNA was generated from RNA as described previously (Hibi et al, 1991).
Quantitative RT–PCR
Quantitative RT–PCR was performed in an ABI sequence detection system 7000 using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Thermocycling was carried out at a final volume of 50 μl containing 2.0 μl of the cDNA sample, 1.0 μl each of the PAI-1 primers (forward and reverse), and 25 μl of Mix SYBR Green I/Enzyme (including Taq DNA polymerase, reaction buffer, and deoxynucleotide triphosphate mixture). The PAI-1 primers for quantitative PCR were described previously (Castello et al, 2002). PCR amplification consisted of 50 cycles (95°C for 15 s, 60°C for 60 s, and 72°C for 18 s) after an initial denaturation step (95°C for 10 min). To correct for differences in both quality and quantity among samples, GAPDH was used as an internal control. GAPDH primers were purchased from Applied Biosystems. Plasminogen activator inhibitor-1 and GAPDH mRNA variability were determined from triplicate samples, the quantity of which was in error by less than 10%. We applied an average quantity of triplicated samples. The targets were obtained from the same mRNA preparations.
PAI-1 expression score
We calculated the relative amounts of CRC (T) and corresponding normal tissue (N) mRNA that we had normalised to an internal control GAPDH mRNA. Next, we applied the logarithmic scale as described previously (Pasco et al, 2001; Wang et al, 2003; Sakakibara et al, 2004). We then defined the PAI-1 expression score as follows:

Statistical and multivariate analyses
Data were expressed as means±s.e. Differences between the means of analysed variables observed were calculated by the Student’s t-test. The significance in correlations between tumour stages and variables was determined by one-way ANOVA. P<0.05 (two-tailed) was considered significant. Survival rates were calculated by the Kaplan–Meier method for analysis of censored data. To compare the prognostic significance of the PAI-1 expression score with that of other parameters, multivariate analysis was performed. Variables were eliminated from the model one by one in a backward fashion and re-included only if the P-value was less than 0.05.
Results
We analysed PAI-1 expression levels in 55 CRC samples using a quantitative RT–PCR. The mRNA concentrations were determined after an extensive optimisation of PCR conditions, including MgCl2 concentrations, reaction temperature, and cycling times. This provided us with a highly sensitive, specific, and reproducible real-time RT–PCR for specific detection of these mRNAs.
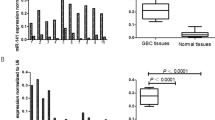
To determine the role of PAI-1 expression in CRC, we examined the correlation of CRC expression scores with the clinicopathological features. Figure 1 shows the distribution of the PAI-1 expression scores (the average was 1.35±0.33). Figure 2 shows the differences in PAI-1 expression scores according to lymph node metastasis. A significant increase in PAI-1 expression scores was observed in lymph node metastasis-positive CRCs (2.19±0.43) compared to negative ones (0.35±0.42) (P=0.0037). Figure 3 shows the differences in PAI-1 expression scores according to distant metastasis. A significant increase in PAI-1 expression scores was observed in distant metastasis-positive CRCs (3.50±1.18) compared to negative ones (0.99±0.30). These results are summarised in Table 1. As shown in Figure 4, the PAI-1 expression score was significantly increased with the tumour stage (stage I=0.01±0.63, stage II=0.66±0.61, stage III=1.67±0.36, stage IV=3.50±1.18) (P=0.0063; ANOVA test). We then examined the cumulative survival of patient groups according to their PAI-1 expression scores (more or less than 2). Interestingly, the high PAI-1 expression-score group showed significantly poorer survival rates than the low PAI-1 expression-score group (Figure 5, P<0.0001).
Difference in PAI-1 expression scores according to lymph node metastasis. Upper and lower limits of boxes, and line across boxes, indicate the 75th and 25th percentiles, and the median, respectively. Upper and lower horizontal bars indicate maximal and minimal scores, respectively. Outliers are illustrated as circles. Plasminogen activator inhibitor-1 expression scores were 2.19±0.43 in metastasis-positive CRCs and 0.35±0.42 in metastasis-negative ones (P=0.0037; Student's t-test).
To confirm the prognostic significance of the PAI-1 expression score, other clinicopathological variables that might affect survival were further analysed by Cox regression analysis. In univariate analysis, the depth of tumour invasion (P=0.0154), lymph node involvement (P<0.0001), distant metastasis (P<0.0001), and PAI-1 expression score (P<0.0001) were significantly correlated with survival (Table 2). To determine the independent value and the relative risk (RR) of these prognostic factors, multivariate analysis was performed. Two prognostic factors were found to be independent values: lymph node metastasis (P=0.0267) and PAI-1 expression score (P=0.0432). Taken together, these findings showed that the PAI-1 expression score constituted a strong and independent prognostic factor for CRC.
Discussion
The plasminogen activation system plays a role in cancer progression, presumably via extracellular matrix degradation and tumour migration (Pedersen et al, 1994). It is generally believed that serine protease, a urokinase-type plasminogen activator (uPA), initiates a proteinase cascade at the cell surface and promotes tumour invasion and angiogenesis. Urokinase-type plasminogen activator is frequently overexpressed in several cancers and is a strong prognostic indicator for decreased patient survival rates (Umeda et al, 1997; Duffy et al, 1999). Plasminogen activator inhibitor-1, the protease inhibitor, is mainly synthesised in vascular endothelial cells and regulates fibrinolytic activity in the vasculature by controlling uPA activity. Recently, the involvement of PAI-1 in tumour growth was suggested because of its high expression levels in tumour extracts. At first, PAI-1 was expected to inhibit tumour progression by inhibiting uPA activity on the tumour cell surface. However, prognostic studies have indicated that PAI-1 is also a clinical marker for a poor prognosis in a variety of human cancers, suggesting that it plays an important role in promoting tumour progression and invasion (Grondahl-Hansen et al, 1993; Cho et al, 1997; Knoop et al, 1998). No clear explanation has yet been found for this apparent paradox. Although the exact tumour biological functions of PAI-1 remain uncertain, it is expressed in multiple cell types and has multiple molecular interactions. This discrepancy could be due to a difference in tumour histology, or it may merely reflect the biological tumour features of different types of cancer.
Tumour growth and metastasis are angiogenesis-dependent. A tumour must continuously stimulate the growth of new capillary blood vessels to promote its growth. Furthermore, angiogenesis is required for tumour cells to enter the circulation and metastasise to distant sites, such as liver, lung, or bone. Tumour cells simultaneously secrete proteases (uPA) and their inhibitors (PAI-1), and the balance between the two precisely regulates the level of extracellular proteolysis, thus either promoting or suppressing angiogenesis (Folkman et al, 2001). It is likely that excess PAI-1 decreases cell adhesion to the extracellular matrix by interfering with uPAR binding to vitronectin, thus facilitating cell invasion and migration (Abe et al, 1999). Other reports have also indicated that excess PAI-1 plays an important role in tumour growth and metastasis by stimulating angiogenesis (McMahon et al, 2001; Bajou, 2002; Devy et al, 2002). Recently, it has been reported that deficient PAI-1 expression in host mice prevented local invasion and tumour vascularisation (Bajou et al, 1998). When this PAI-1 deficiency was circumvented by an intravenous injection of a replication-defective adenoviral vector expressing human PAI-1, the invasion and its associated angiogenesis resumed. This experimental evidence demonstrated that host-produced PAI-1 is essential for cancer cell invasion and angiogenesis (Gutierrez et al, 2000; Bajou et al, 2004).
In this study, we demonstrated for the first time that PAI-1 expression increased with the CRC stage and was associated with a poor prognosis. In univariate analysis, the PAI-1 expression score was significantly correlated with survival of CRC. Moreover, in multivariate analysis, the PAI-1 expression score was a strong and independent prognostic factor in CRC, second to the lymph node involvement. These results suggested that PAI-1 might serve as a new parameter for the prognostic prediction of CRC.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abe J, Urano T, Konno H, Erhan Y, Tanaka T, Nishino N, Takada A, Nakamura S (1999) Larger and more invasive colorectal carcinoma contains larger amounts of plasminogen activator inhibitor type 1 and its relative ratio over urokinase receptor correlates well with tumor size. Cancer 86: 2602–2611
Bajou K (2002) Role of PAI-1 plasminogen activator inhibitor in tumor invasion and angiogenesis]. Bull Mem Acad Roy Med Belg 157: 313–318
Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, Carmeliet P, Foidart JM, Noel A (2004) Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene 23: 6986–6990
Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM (1998) Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 4: 923–928
Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B (1990) Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249: 912–915
Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B (1987) Prevalence of ras gene mutations in human colorectal cancers. Nature 327: 293–297
Cantero D, Friess H, Deflorin J, Zimmermann A, Brundler MA, Riesle E, Korc M, Buchler MW (1997) Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br J Cancer 75: 388–395
Castello R, Estelles A, Vazquez C, Falco C, Espana F, Almenar SM, Fuster C, Aznar J (2002) Quantitative real-time reverse transcription-PCR assay for urokinase plasminogen activator, plasminogen activator inhibitor type 1, and tissue metalloproteinase inhibitor type 1 gene expressions in primary breast cancer. Clin Chem 48: 1288–1295
Chambers SK, Ivins CM, Carcangiu ML (1998) Plasminogen activator inhibitor-1 is an independent poor prognostic factor for survival in advanced stage epithelial ovarian cancer patients. Int J Cancer 79: 449–454
Cho JY, Chung HC, Noh SH, Roh JK, Min JS, Kim BS (1997) High level of urokinase-type plasminogen activator is a new prognostic marker in patients with gastric carcinoma. Cancer 79: 878–883
Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson V, Gerard RD, Gils A, Carmeliet G, Carmeliet P, Declerck PJ, Noel A, Foidart JM (2002) The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J 16: 147–154
Duffy MJ, Maguire TM, McDermott EW, O’Higgins N (1999) Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J Surg Oncol 71: 130–135
Foekens JA, Peters HA, Look MP, Portengen H, Schmitt M, Kramer MD, Brunner N, Janicke F, Meijer-van Gelder ME, Henzen-Logmans SC, van Putten WL, Klijn JG (2000) The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res 60: 636–643
Folkman J, Browder T, Palmblad J (2001) Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost 86: 23–33
Grondahl-Hansen J, Christensen IJ, Rosenquist C, Brunner N, Mouridsen HT, Dano K, Blichert-Toft M (1993) High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res 53: 2513–2521
Gutierrez LS, Schulman A, Brito-Robinson T, Noria F, Ploplis VA, Castellino FJ (2000) Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res 60: 5839–5847
Hibi K, Nakamura H, Hirai A, Fujikake Y, Kasai Y, Akiyama S, Ito K, Takagi H (1996) Loss of H19 imprinting in esophageal cancer. Cancer Res 56: 480–482
Hibi K, Nakayama H, Kodera Y, Ito K, Akiyama S, Nakao A (2004) CDH13 promoter region is specifically methylated in poorly differentiated colorectal cancer. Br J Cancer 90: 1030–1033
Hibi K, Nakayama H, Koike M, Kasai Y, Ito K, Akiyama S, Nakao A (2002) Colorectal cancers with both p16 and p14 methylation show invasive characteristics. Jpn J Cancer Res 93: 883–887
Hibi K, Taguchi M, Nakamura H, Hirai A, Fujikake Y, Matsui T, Kasai Y, Akiyama S, Ito K, Takagi H (1997) Alternative splicing of the FHIT gene in colorectal cancers. Jpn J Cancer Res 88: 385–388
Hibi K, Takahashi T, Sekido Y, Ueda R, Hida T, Ariyoshi Y, Takagi H (1991) Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer. Oncogene 6: 2291–2296
Knoop A, Andreasen PA, Andersen JA, Hansen S, Laenkholm AV, Simonsen AC, Andersen J, Overgaard J, Rose C (1998) Prognostic significance of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 in primary breast cancer. Br J Cancer 77: 932–940
Konecny G, Untch M, Pihan A, Kimmig R, Gropp M, Stieber P, Hepp H, Slamon D, Pegram M (2001) Association of urokinase-type plasminogen activator and its inhibitor with disease progression and prognosis in ovarian cancer. Clin Cancer Res 7: 1743–1749
Lijnen HR (2005) Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost 3: 35–45
McMahon GA, Petitclerc E, Stefansson S, Smith E, Wong MK, Westrick RJ, Ginsburg D, Brooks PC, Lawrence DA (2001) Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J Biol Chem 276: 33964–33968
Nekarda H, Schmitt M, Ulm K, Wenninger A, Vogelsang H, Becker K, Roder JD, Fink U, Siewert JR (1994) Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res 54: 2900–2907
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P (1991) Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253: 665–669
Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, Nicholson GC (2001) Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab 86: 1884–1887
Pedersen H, Brunner N, Francis D, Osterlind K, Ronne E, Hansen HH, Dano K, Grondahl-Hansen J (1994) Prognostic impact of urokinase, urokinase receptor, and type 1 plasminogen activator inhibitor in squamous and large cell lung cancer tissue. Cancer Res 54: 4671–4675
Potempa J, Korzus E, Travis J (1994) The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem 269: 15957–15960
Sakakibara T, Hibi K, Kodera Y, Ito K, Akiyama S, Nakao A (2004) Plasminogen activator inhibitor-1 as a potential marker for the malignancy of esophageal squamous cell carcinoma. Clin Cancer Res 10: 1375–1378
Umeda T, Eguchi Y, Okino K, Kodama M, Hattori T (1997) Cellular localization of urokinase-type plasminogen activator, its inhibitors, and their mRNAs in breast cancer tissues. J Pathol 183: 388–397
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319: 525–532
Wang EJ, Kripke DF, Stein MT, Parry BL (2003) Measurement of illumination exposure in postpartum women. BMC Psychiatry 3: 5
Yamazaki T, Hibi K, Takase T, Tezel E, Nakayama H, Kasai Y, Ito K, Akiyama S, Nagasaka T, Nakao A (2002) PGP9.5 as a marker for invasive colorectal cancer. Clin Cancer Res 8: 192–195
Acknowledgements
We thank M Taguchi for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sakakibara, T., Hibi, K., Koike, M. et al. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of colorectal cancer. Br J Cancer 93, 799–803 (2005). https://doi.org/10.1038/sj.bjc.6602743
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602743
Keywords
This article is cited by
-
ACT001, a novel PAI-1 inhibitor, exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma
Cell Death & Disease (2019)
-
In vitro evaluation of anticancer properties of exopolysaccharides from Lactobacillus acidophilus in colon cancer cell lines
In Vitro Cellular & Developmental Biology - Animal (2016)
-
Elevated Tumor Expression of PAI-1 and SNAI2 in Obese Esophageal Adenocarcinoma Patients and Impact on Prognosis
Clinical and Translational Gastroenterology (2012)
-
ARNTL2 and SERPINE1: potential biomarkers for tumor aggressiveness in colorectal cancer
Journal of Cancer Research and Clinical Oncology (2012)
-
Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities
Cancer and Metastasis Reviews (2012)