Abstract
Background:
Pre-clinical studies have demonstrated synergistic anti-tumour effects of chemotherapy (CT) and zoledronic acid (ZOL). Within the AZURE trial, designed to determine whether the addition of ZOL to neoadjuvant therapy improves disease outcomes, a subgroup received neoadjuvant CT. We report a retrospective evaluation comparing pathological response in the primary tumour between treatment groups.
Methods:
In total, 205 patients received neoadjuvant CT±ZOL (CT+ZOL, n=102; CT, n=103). The primary end point was pathologically assessed residual invasive tumour size (RITS) at surgery. Secondary end points were pathological complete response (pCR) rate and axillary nodal involvement. Following review of surgical pathology reports (n=195), outcome differences between groups were assessed adjusting for potential response modifiers.
Results:
Baseline characteristics and CT treatments were similar. In multivariate analysis, allowing for biological and clinical factors known to influence tumour response, the adjusted mean RITS in CT and CT+ZOL groups were 27.4 and 15.5 mm, respectively, giving a difference in means of 12 mm (95% confidence interval: 3.5–20.4 mm; P=0.006). The pCR rate was 6.9% in the CT group and 11.7% in the CT+ZOL group (P=0.146). There was no difference in axillary nodal involvement (P=0.6315).
Conclusion:
These data suggest a possible direct anti-tumour effect of ZOL in combination with CT, warranting formal evaluation in prospective studies.
Similar content being viewed by others
Background
Zoledronic acid (ZOL), a nitrogen-containing bisphosphonate (N-BP), is firmly established in the management of metastatic bone disease. It inhibits farnesyl diphosphate synthase within the mevalonate pathway and, through this mechanism, is a potent inhibitor of osteoclast-mediated bone resorption. In addition, there are pre-clinical data indicating that farnesyl diphosphate synthase inhibition by ZOL has both direct and indirect anti-tumour effects in breast cancer (Winter et al, 2008). Furthermore, synergistic anti-tumour effects with chemotherapy (CT) drugs commonly used in the treatment of breast cancer have been demonstrated. Recently, it has been reported that sequential treatment with doxorubicin followed by ZOL at clinically relevant doses elicited substantial anti-tumour effects in in vivo mouse models of subcutaneous breast tumours (Ottewell et al, 2008). The clinical evidence of an anti-tumour effect of ZOL, however, is uncertain, although recent data suggest that the addition of ZOL to endocrine treatments improves disease outcomes in pre-menopausal women with early breast cancer (Gnant et al, 2009).
Inconclusive results from adjuvant trials of the oral bisphosphonate, clodronate, in the 1990s formed the rationale for adjuvant trials of the more potent ZOL. It was anticipated that ZOL might have more definite beneficial anti-tumour effects. This could occur ‘indirectly’ through the inhibition of bone resorption and consequent reduction in bone-derived factors that disrupt the inter-relationships between cancer, bone and haematopoetic stem cell populations, thereby creating a less favourable microenvironment for the survival of metastatic tumour cells. Alternatively, or perhaps in addition, ‘direct’ effects such as induction of tumour cell apoptosis, reduction in proliferation rates and tumour angiogenesis, as well as potential synergistic effects with anti-cancer therapies, might be clinically important (Coleman, 2007).
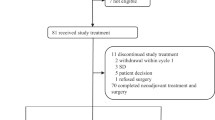
The AZURE trial (ISRCTN 79831382; BIG 01/04) is an academic study run by the National Institute for Health Research National Cancer Research Network (NIHR NCRN) in the United Kingdom with input from international collaborative groups. The study randomised 3360 women with axillary node-positive stage II/III breast cancers to determine whether the addition of ZOL to systemic therapy improves disease-related outcomes (Figure 1). Analysis and reporting of the AZURE trial awaits the occurrence of a pre-specified number of disease-free survival events. However, within this large study, ∼6% of patients received neoadjuvant CT (Figure 1). In light of the pre-clinical data and emerging clinical evidence of a potential anti-tumour effect of ZOL, we have conducted an exploratory retrospective evaluation of this subgroup. The objective was to determine whether the addition of ZOL to primary systemic neoadjuvant CT had any demonstrable influence on pathological response in the surgical resection specimen compared with the effects of neoadjuvant CT alone.
Patients and methods
Patient population
Of the 3360 patients recruited, the treating physicians elected to treat 205 patients with neoadjuvant CT. Patients receiving neoadjuvant CT on the AZURE trial were required to have either stage T3 or T4 disease, or biopsy-proven lymph node involvement (N1), and be scheduled to proceed to definitive surgery and/or radical radiotherapy with curative intent within 6 months of starting therapy.
Using a minimisation technique to randomise patients, the two groups were well balanced for T-stage, lymph node involvement, oestrogen receptor (ER) status, neoadjuvant systemic treatment plan and CT type (anthracycline/taxane), menopausal status, the use of statins (which also act on the mevalonate pathway) and treatment centre.
Baseline tumour size per se was not prospectively collected in AZURE. Furthermore, inherent problems exist related to inter-observer variability in the clinical assessment of breast tumour size and discrepancies between physical examination, mammographic and ultrasound estimates of size. We therefore instead incorporated tumour (T) stage, determined by clinical examination, into the statistical analysis, as this was a prospectively collected tumour characteristic. However, we also sought information from centres on baseline tumour measurements by clinical, ultrasound and/or mammographic assessment to enable sensitivity analyses.
Details of surgery and pathological response
Histopathologically assessed residual invasive tumour size (RITS) is reported on breast cancer pathology reports as part of the minimum data set, and the largest dimension of dominant invasive tumour focus is routinely recorded (NHS Breast Cancer Screening Programme (NHSBSP) and The Royal College of Pathologists, 2005). These data and the number of positive axillary lymph nodes at the time of definitive surgery were prospectively specified to be collected in AZURE, enabling the primary and secondary end points of this analysis to be determined.
The conventional end point of neoadjuvant CT studies in breast cancer is pathological complete response (pCR), representing the most robust surrogate marker of longer-term outcome (Fisher et al, 1997, 1998; van der Hage et al, 2001; Heys et al, 2002; Bear et al, 2006; Mieog et al, 2007). However, the definition of this has not been applied in a consistent and standardised manner throughout clinical trials (Kuroi et al, 2005). As AZURE was not primarily a neoadjuvant study, data regarding attainment of pCR were not prospectively collected. However, following a blinded central review of pathology reports, pCR was considered to have been achieved if the pathology report specifically indicated an absence of any residual invasive tumour within the breast and axilla, as per the recommended optimum definition (Gralow et al, 2008).
Determining RITS and nodal status
In cases in which there was no residual invasive tumour, ‘0 mm’ was recorded. In cases in which multifocal pathology was present (n=25), the largest dimension of residual invasive tumour focus was recorded. Occasionally, if a measurement of the largest focus was not given, but the overall size including invasive tumour islands was reported, this was recorded.
For the assessment of axillary nodal disease after CT, the number of positive axillary nodes (and total number of nodes resected) was recorded. No distinction between axillary nodal micrometastases (>0.2–<2 mm) and overt metastases (⩾2 mm) was made, with both considered as evidence of nodal metastasis.
Treatment
Case report forms were also evaluated to review details of neoadjuvant systemic treatments received. Duration of treatment was defined as the time from the first CT to surgery in days. Patients received neoadjuvant CT as per local guidelines. In light of the pre-clinical data, it was mandated that ZOL should be given after CT. Patients received ZOL as a 4 mg intravenous dose, given in 100 ml 0.9% normal saline over 15 min.
Statistical methodology
The primary end point for this study was RITS (mm) in the surgical resection specimen. Secondary end points included the number of positive axillary nodes (categorised as 0, 1–3, 4+ positive nodes) and pCR (%), as previously defined. Only pre-defined end points were subjected to statistical testing using a 5% (two-sided) significance level. All other data were summarised descriptively. As this was an exploratory evaluation, patients were analysed on a per protocol basis, with one patient randomised to CT+ZOL but who never started study treatment included in the CT control arm.
For the primary end point, the outcome difference between the two groups was compared using linear regression, adjusting for the following potential prognostic factors: tumour stage (T2, T3 or T4, at the time of randomisation), ER status, PgR status, menopausal status, CT type (anthracycline, taxane) and treatment duration. Patients with missing data for PgR status and treatment duration (n=8) were included as ‘unknown’ PgR status and ‘6 months’, respectively, representing the maximum time between the commencement of neoadjuvant therapy and definitive surgery allowed by the study protocol. A sensitivity analysis was also performed that adjusted for clinical baseline tumour size, instead of tumour stage, and the other factors as above.
Axillary nodal status was compared between the two treatment groups using ordered logistic regression to adjust for the factors as described above. The difference between treatment groups in the proportion of patients with pCR was compared using logistic regression, adjusting for the previously described factors. Missing data for PgR status and treatment duration (n=8) were substituted as above.
HER2/neu status was not routinely assessed at the time of trial recruitment. Furthermore, very few patients received trastuzumab in the neoadjuvant treatment period (n=6). Tumour grade was not included in multivariate analysis, as grade, in most UK centres, is not routinely assessed on diagnostic core specimens because of concerns regarding possible tumour heterogeneity and sampling error. It is also difficult to evaluate post-treatment grade reliably because of CT-induced cytological and histological changes (Kurosumi, 2006).
Role of the funding source
The trial design was reviewed and approved by Cancer Research UK's Clinical Trials Awards and Advisory Committee for access to NIHR NCRN resources. The research costs were supported by a grant from Novartis to the University of Sheffield, the sponsor of the study. The funders had no part in data collection and analysis, interpretation of data or writing the paper. The corresponding author had full access to the data and final responsibility for the decision to submit for publication.
Results
Of the 205 patients who received neoadjuvant CT, 103 patients were randomised to CT alone and 102 to CT+ZOL. Of these, 195 (95.1%) surgical histopathology forms were available for central review, blinded to treatment allocation, and were included in these exploratory analyses; 94 received CT+ZOL and 101 received CT alone. Ten patients were excluded because of missing pathology reports (n=8), no surgery (n=1) or patient death before surgery (n=1).
Clinicopathological baseline characteristics are shown in Table 1. Age and menopausal status of patients in both groups were similar, although there were 10 more pre-menopausal patients in the CT-alone group. The majority of patients presented with T3 or T4 tumours and most had unknown lymph node involvement before the commencement of CT. Table 1 also shows median and interquartile range (IQR) for baseline tumour size by clinical, ultrasound and/or mammographic assessment where available. The frequency of ER-negative tumours, an important predictor of CT response (MacGrogan et al, 1996; Kaufmann et al, 2007), was similar in the groups. In approximately one-third of patients, PgR status was unknown (either missing or not performed in some recruiting centres). This trial began before the routine use of trastuzumab in adjuvant therapy and HER2 status was unknown in 32.3% of patients.
Details of systemic treatment received are shown in Table 2. Treatment duration and the number of CT cycles were comparable in both groups. Dose intensities were similar between the two treatment groups (data not shown).
Residual invasive tumour size
Residual invasive tumour size could be determined in 182 patients (93 CT alone and 89 CT+ZOL). In the remaining 13 patients, 1 patient developed progressive disease during neoadjuvant CT and had palliative surgery almost 1 year later. For the remaining 12 patients (n=8 CT, n=4 CT+Z), the pathology reports gave no specific measurement of residual microscopic invasive tumour size describing ‘widespread tumour emboli’ or ‘occasional residual focus of infiltrating carcinoma’. Putting a measurement on these cases was not considered valid.
Median RITS was 30 mm (IQR 7–60 mm) in the CT-alone group and 21 mm (7–38 mm) in the CT+ZOL treatment group. In multivariate analysis (Table 3), the adjusted mean RITS in the CT-alone group was 27.4 and 15.5 mm in the CT+ZOL group, giving a statistically significant difference in means of 12 mm (95% CI 3.5–20.4 mm, P=0.0059). In a sensitivity analysis replacing T-stage with clinical tumour size at baseline, RITS remained smaller in the CT+ZOL group by 9.9 mm (95% confidence interval (CI) 0.2–19.7 mm; P=0.0465, n=137).
Multivariate analysis (Table 3) also revealed that treatment duration (P=0.0011) and taxane-based CT (P=0.0204) were significantly associated with RITS, indicating a smaller residual tumour size with increasing treatment duration and an increased residual tumour size with the use of taxane CT. This may, however, simply reflect an increased use of taxanes in patients responding poorly to the early cycles of anthracycline CT, as 31 patients planned at the time of randomisation to receive anthracyclines alone were subsequently switched to a taxane.
Secondary end points
Axillary nodal status
Information on the pathological status of axillary lymph nodes was available from 194 (99.5%) pathology reports. The median number of positive axillary lymph nodes was 3 (IQR 0–6) in the CT group and 2 (IQR 0–6) in the CT+ZOL group. There was no significant difference in axillary nodal involvement, categorised by 0 (30.7% CT alone vs 28.7% CT+ZOL), 1–3 (23.8 vs 29.8%) or ⩾4 (45.5 vs 40.4%) positive nodes, between the treatment groups in multivariate analysis (P=0.6315). The only factors that were significantly associated with a lower number of involved nodes were increasing treatment duration (P=0.0102) and ER-negative status (P=0.0129).
pCR
In total, 24 (12.3%) patients had no residual invasive disease in the breast, 10 (9.9%) patients in the CT-alone group and 14 (14.9%) patients in the CT+ZOL group. In all, 18 (9.2%) patients achieved pCR, defined as ‘the absence of residual invasive tumour within both breast and axilla’, 7 (6.9%) patients in the CT-alone group and 11 (11.7%) in the CT+ZOL group. Of the patients achieving pCR, two in the CT+ZOL and none in the CT groups had DCIS present in the absence of any remaining invasive disease. In multivariate analysis (n=195) adjusting for potential prognostic factors in addition to neoadjuvant treatment group, a trend for patients receiving CT+ZOL to have increased odds of achieving pCR (odds ratio=2.2, 95% CI 0.8–6.3, P=0.1457) was seen. Not surprisingly, given the low number achieving pCR, no statistically significant predictive factors for pCR were identified.
Mastectomy rate
Breast conservation surgery is often the primary aim in patients being treated with neoadjuvant CT, and therefore mastectomy rate is an important outcome parameter. Initial (as a representation of intent of surgery) type of surgery was recorded in all patients. Altogether, 80 (79.2%) patients in the CT-alone group had a mastectomy compared with 66 (70.2%) in the CT+ZOL treatment group.
Safety
The combination of CT+ZOL in the neoadjuvant treatment setting was well tolerated, with no increase in serious adverse event (SAE) reporting. There were 63 SAEs reported within the median neoadjuvant treatment period (147 days (IQR 129–184)): 36 events in the CT-alone group – 16 (44.4%) neutropenic sepsis, 7 (19.4%) neutropenia, 5 gastrointestinal, 3 pyrexia, 1 ‘other’ infection, 1 rash, 1 pleural effusion, 1 flu-like symptoms and 1 other symptom – and 27 in the CT+ZOL group – 14 (51.9%) neutropenic sepsis, 3 (11.1%) neutropenia, 3 (11.1%) ‘other’ infection, 5 gastrointestinal, 1 anaphylactic reaction and 1 cardiovascular or loss of consciousness.
Discussion
These retrospective exploratory data intriguingly suggest that a bisphosphonate may have effects on tumours outside bone. An improved pathological response in patients treated with CT+ZOL compared with CT alone was observed and suggests a possible direct anti-tumour effect of ZOL in combination with neoadjuvant CT.
There are some limitations to the interpretation of these data. Firstly, there was no central pathological review of the operative specimens and therefore probable inter-pathologist variation existed with respect to specimen processing and pathological evaluation. Secondly, assessment of RITS is somewhat imprecise, as asymmetry of residual disease and hypocellularity are not usually quantified during pathology reporting (Symmans et al, 2007). In addition, missing data may negatively affect the quality of a retrospective study and, as exploratory end points, these analyses are likely to be underpowered.
In this study, we have included T-stage to reflect the baseline extent of primary breast cancer in the main analysis. Baseline clinical measurements of tumour size were missing in 26% of patients. In a sensitivity analysis, the replacement of T-stage with clinical estimates of initial tumour size into the multivariate analysis did not materially affect the differences in RITS seen between treatments, with the beneficial effect of ZOL persisting.
The extent of residual tumour following CT has been well established as an intermediate surrogate end point for longer-term outcome such as relapse and survival (Carey et al, 2005). However, no standard validated internationally accepted classification exists for the reporting of pathological response to neoadjuvant CT. To date, neoadjuvant studies have used pCR as a validated intermediate surrogate marker of survival, despite it being a less common phenomenon. Therefore, for the majority of patients, ‘residual disease’ represents a spectrum ranging from those patients achieving ‘near’ pCR to those with resistant disease, potentially associated with widely differing prognoses.
Analysis of RITS potentially allows an exploratory evaluation of therapeutic effect in those patients achieving less than pCR. Furthermore, RITS after CT is of potential clinical relevance. In the largest published single-institution cohort from the MD Anderson Cancer Center (n=338), Chen et al (2004) published data on 5-year locoregional recurrence (LRR) rates following neoadjuvant CT and breast-conserving surgery. Pathologically assessed residual primary tumour >2 cm after neoadjuvant CT and breast-conserving surgery significantly predicted for LRR in multivariate analysis (hazard ratio 3.2; P=0.006, along with initial N2/N3 disease, residual multifocal pattern of disease and lymphovascular space invasion).
Although not widely validated, published studies have shown that pathological staging, including an evaluation of residual tumour size after neoadjuvant CT, may be useful in predicting longer-term outcome. In 2003, a revised AJCC TNM breast cancer classification was implemented (Greene et al, 2002), designating the letter ‘y’ to represent pathological stage of residual tumour after neoadjuvant therapy. Carey et al (2005) reported that higher pathological stage in the surgical resection specimen using this revised classification system was significantly associated with lower rates of distant disease-free survival and overall survival in stage II/III breast cancer patients treated with neoadjuvant CT (n=132, median follow-up 5 years). In addition, the Clinical-Pathologic Scoring (CPS) system has very recently been described to predict 5-year outcomes of breast cancer patients treated with neoadjuvant CT (Jeruss et al, 2008). This model incorporates clinical TNM stage at presentation and post-neoadjuvant CT pathological TNM stage, assigning points to each presenting clinical substage and post-treatment pathological substage, with a 5-year distant metastasis-free survival of 97% for a CPS score of 0 and 46% for a CPS score of 4.
In this analysis, we have reported a trend for a higher proportion of patients treated with CT+ZOL (11.7%) achieving pCR compared with CT alone (6.9%). It is not surprising that this is not statistically significant because of the small numbers of patients involved. Secondly, these figures require a degree of cautious interpretation, as they represent overall low rates of pCR, given that pCR rates of up to 30% have been reported in randomised studies of neoadjuvant CT regimens (Buzdar, 2007). However, it must be noted that many of the previously published studies included patients with T1–T2 stage disease, whereas in this cohort 98% of patients presented with T3–T4 disease.
The presence of nodal disease after neoadjuvant CT is associated with a poorer prognosis (Kuerer et al, 1999; Rouzier et al, 2002). We did not show any significant difference in post-treatment nodal status between the two treatment groups. This may not be surprising given that axillary lymph node metastases, considered as disseminated tumour cells, are selectively more resistant than primary tumour cells (von Minckwitz et al, 2008). However, of interest, the proportion of patients in the CT+ZOL group achieving pCR (including negative nodal status) was higher than in the CT-alone group, potentially reflecting increased chemosensitivity of nodal disease with complete pathological response in the breast, as has been previously described (Kuerer et al, 1999).
These data reported here follow shortly after the most promising clinical data to date regarding a potential anti-tumour effect of ZOL in early breast cancer from the ABCSG-12 study report (Gnant et al, 2009). In this trial, 1803 pre-menopausal women with endocrine-sensitive breast cancer were randomised to receive endocrine treatment (gonadotrophin-releasing hormone analogue and tamoxifen or anastrozole) with or without ZOL (4 mg intravenous infusion) 6 monthly for 3 years. With a median follow-up of 48 months, the addition of ZOL to endocrine therapy was associated with a 36% improvement in disease-free survival compared with endocrine treatment alone (hazard ratio=0.64 95% CI 0.46, 0.91, P=0.01). Furthermore, the benefits of ZOL were not confined to the bone, with a striking reduction in locoregional and distant extraosseus recurrence events as well. Despite a relatively low number of events, this is further evidence that ZOL may exhibit anti-tumour activity in patients with early breast cancer.
In the clinical setting, there are no fully published data regarding the potential anti-tumour effect of ZOL in combination with CT. However, there are pre-clinical data from several different in vitro and in vivo model systems of the beneficial anti-tumour effects of combination therapy with anti-cancer agents and ZOL (Santini et al, 2006). More specifically to breast cancer, beneficial effects of the use of combination treatments including ZOL and cytotoxic therapies have been shown in in vivo model systems of breast cancer, most of which included a high degree of tumour-induced bone disease. However, Ottewell et al (2008) recently reported that sequential treatment with clinically relevant doses of doxorubicin, followed 24 h later by ZOL, inhibited subcutaneous breast tumour growth in a mouse model of breast cancer soft tissue disease in the absence of tumour bone disease. The two drugs alone or in the reverse sequence had little or no effect on tumour growth. Simultaneous administration of doxorubicin and ZOL also reduced tumour growth somewhat, but only when the two drugs were given in the sequence of doxorubicin followed by ZOL was complete and durable inhibition of tumour growth achieved. The reduction in subcutaneous tumour growth was accompanied by increased tumour cell apoptosis, reduction in tumour cell proliferation and tumour angiogenesis. The molecular mechanisms behind this sequence-dependent synergy remain to be established, but giving sequenced treatment elicited changes in the expression of a number of genes and proteins associated with cell-cycle progression and apoptosis not occurring with single-agent doxorubicin or ZOL (Ottewell et al, 2010). These data therefore suggest that low serum concentrations of ZOL, in combination with cytotoxic drugs, are sufficient to exert anti-tumour effects in peripheral tissues and lead to the hypothesis that there may be beneficial anti-tumour effects in patients with early breast cancer.
In summary, these are the first clinical data suggesting that the addition of ZOL to neoadjuvant CT may improve pathological response in breast cancer, and give further support to other emerging data that ZOL may exhibit anti-tumour activity in combination with anti-cancer therapies. Formal evaluation in prospective neoadjuvant randomised trials incorporating detailed assessment of biomarkers is recommended.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bear HD, Anderson S, Smith RE, Geyer Jr CE, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS, Paik S, Soran A, Wickerham DL, Wolmark N (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 24: 2019–2027
Buzdar AU (2007) Preoperative chemotherapy treatment of breast cancer – a review. Cancer 110: 2394–2407
Carey LA, Metzger R, Dees EC, Collichio F, Sartor CI, Ollila DW, Klauber-DeMore N, Halle J, Sawyer L, Moore DT, Graham ML (2005) American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst 97: 1137–1142
Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, Strom EA, McNeese MD, Kuerer HM, Ross MI, Singletary SE, Ames FC, Feig BW, Sahin AA, Perkins GH, Schechter NR, Hortobagyi GN, Buchholz TA (2004) Breast conservation after neoadjuvant chemotherapy: the MD Anderson Cancer Center experience. J Clin Oncol 22: 2303–2312
Coleman R (2007) Potential use of bisphosphonates in the prevention of metastases in early-stage breast cancer. Clin Breast Cancer 7 (Suppl 1): S29–S35
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz Jr AB, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15: 2483–2493
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz Jr AB, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16: 2672–2685
Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rucklinger E, Greil R, Marth C (2009) Endocrine therapy plus zoledronic acid in premenopausal breast cancer. New Engl J Med 360: 679–691
Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26: 814–819
Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow MH (2002) American Joint Committee on Cancer Staging Manual, 6th edn. Springer-Verlag: New York
Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, Smith I, Walker LG, Eremin O (2002) Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer 3 (Suppl 2): S69–S74
Jeruss JS, Mittendorf EA, Tucker SL, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, Cormier JN, Buzdar AU, Hortobagyi GN, Hunt KK (2008) Staging of breast cancer in the neoadjuvant setting. Cancer Res 68: 6477–6481
Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, Colleoni M, Denkert C, Eiermann W, Jackesz R, Makris A, Miller W, Pierga JY, Semiglazov V, Schneeweiss A, Souchon R, Stearns V, Untch M, Loibl S (2007) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 18: 1927–1934
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17: 460–469
Kuroi K, Toi M, Tsuda H, Kurosumi M, Akiyama F (2005) Unargued issues on the pathological assessment of response in primary systemic therapy for breast cancer. Biomed Pharmacother 59 (Suppl 2): S387–S392
Kurosumi M (2006) Significance and problems in evaluations of pathological responses to neoadjuvant therapy for breast cancer. Breast Cancer (Tokyo, Japan) 13: 254–259
MacGrogan G, Mauriac L, Durand M, Bonichon F, Trojani M, de Mascarel I, Coindre JM (1996) Primary chemotherapy in breast invasive carcinoma: predictive value of the immunohistochemical detection of hormonal receptors, p53, c-erbB-2, MiB1, pS2 and GST pi. Br J Cancer 74: 1458–1465
Mieog JS, van der Hage JA, van de Velde CJ (2007) Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 94: 1189–1200
NHS Breast Cancer Screening Programme (NHSBSP) and The Royal College of Pathologists (2005) NHSBSP Guidelines for Pathology Reporting in Breast Disease Publication No. 58: 1–134
Ottewell PD, Lefley DV, Cross SS, Evans CA, Coleman RE, Holen I (2010) Sustained inhibition of tumor growth and prolonged survival following sequential administration of doxorubicin and zoledronic acid in a breast cancer model. Int J Cancer 126 (2): 522–532
Ottewell PD, Monkkonen H, Jones M, Lefley DV, Coleman RE, Holen I (2008) Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst 100: 1167–1178
Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, Vielh P, Bourstyn E (2002) Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol 20: 1304–1310
Santini D, Caraglia M, Vincenzi B, Holen I, Scarpa S, Budillon A, Tonini G (2006) Mechanisms of disease: preclinical reports of antineoplastic synergistic action of bisphosphonates. Nat Clin Pract Oncol 3: 325–338
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25: 4414–4422
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19: 4224–4237
von Minckwitz G, Sinn HP, Raab G, Loibl S, Blohmer JU, Eidtmann H, Hilfrich J, Merkle E, Jackisch C, Costa SD, Caputo A, Kaufmann M (2008) Clinical response after two cycles compared to HER2, Ki-67, p53, and bcl-2 in independently predicting a pathological complete response after preoperative chemotherapy in patients with operable carcinoma of the breast. Breast Cancer Res 10: R30
Winter MC, Holen I, Coleman RE (2008) Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev 34: 453–475
Acknowledgements
We thank Dr Patricia Verghani, Consultant Histopathologist, Royal Hallamshire Hospital, Sheffield, UK.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
RE Coleman has received honoraria and consulting fees from Novartis, Amgen, Roche, and Pfizer and has previously given expert testimony on behalf of Novartis. D Cameron received honoraria from Novartis and Roche. D Dodwell received adhoc consultancy/ad boards and lecture payments from Roche, Astra Zeneca and Novartis. R Burkinshaw is in receipt of research funding from Novartis Pharmaceuticals. All other authors have no conflict of interest.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Coleman, R., Winter, M., Cameron, D. et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer 102, 1099–1105 (2010). https://doi.org/10.1038/sj.bjc.6605604
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605604
Keywords
This article is cited by
-
Differences between zoledronic acid and denosumab for breast cancer treatment
Journal of Bone and Mineral Metabolism (2023)
-
Novel approaches to target the microenvironment of bone metastasis
Nature Reviews Clinical Oncology (2021)
-
Long-term outcomes of prolonged bisphosphonates more than 2 years in bone metastatic breast cancer: risk vs benefit
Irish Journal of Medical Science (1971 -) (2020)
-
Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid
Scientific Reports (2019)
-
Addition of zoledronic acid to neoadjuvant chemotherapy is not beneficial in patients with HER2-negative stage II/III breast cancer: 5-year survival analysis of the NEOZOTAC trial (BOOG 2010-01)
Breast Cancer Research (2019)