Abstract
Background:
The recent introduction of a dynamic nuclear polarisation technique has permitted noninvasive imaging of tumour cell metabolism in vivo following intravenous administration of 13C-labelled cell substrates.
Methods:
Changes in hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate metabolism were evaluated in both MDA-MB-231 cells and in implanted MDA-MB-231 tumours following doxorubicin treatment.
Results:
Treatment of MDA-MB-231 cells resulted in the induction of apoptosis, which was accompanied by a decrease in hyperpolarised 13C label flux between [1-13C]pyruvate and lactate, which was correlated with a decrease in the cellular NAD(H) coenzyme pool. There was also an increase in the rate of fumarate conversion to malate, which accompanied the onset of cellular necrosis. In vivo, the decrease in 13C label exchange between pyruvate and lactate and the increased flux between fumarate and malate, following drug treatment, were shown to occur in the absence of any detectable change in tumour size.
Conclusion:
We show here that the early responses of a human breast adenocarcinoma tumour model to drug treatment can be followed by administration of both hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate. These techniques could be used, therefore, in the clinic to detect the early responses of breast tumours to treatment.
Similar content being viewed by others
Main
There has been substantial growth and investment in drug discovery and development programmes in oncology in recent years, with the aim of producing therapeutics targeted at the spectrum of genetic abnormalities found in cancer (Hu et al, 2000; Semenza, 2003; Bryant et al, 2005). This rapid expansion has moved the field of clinical oncology closer to the realisation of personalised medicine, in which patients receive drugs, individually or in combination, that are tailored to their specific disease. The delivery of this approach should be facilitated by noninvasive imaging methods that allow an early assessment of treatment response, identifying unsuccessful treatments at an early stage and enabling the selection of more effective treatment (reviewed in Brindle (2008a)).
Currently, tumour treatment responses are assessed from reductions in tumour size (Eisenhauer et al, 2009). However, this approach lacks sensitivity and many weeks may elapse before a change in size is detected (Neves and Brindle, 2006; Weissleder and Pittet, 2008; Brindle, 2008a). In some cases, for example with cytostatic therapies, there may be no change in size despite a positive response to treatment (Brindle, 2008a). Measurements of tumour physiology or biochemistry, however, can give a much earlier indication of treatment response. Positron emission tomography (PET) measurements of the uptake of the glucose analogue [18F] 2-fluoro-2-deoxy-D-glucose (FDG) are being used increasingly in the clinic to stage disease and provide early evidence of treatment response (Eisenhauer et al, 2009).
MRS, particularly 1H MRS, can also be used to detect the metabolic changes that accompany a positive response to treatment (Aboagye and Bhujwalla, 1999; Glunde et al, 2006). The relative lack of sensitivity of MRS, however, limits the spatial and temporal resolution of 1H spectroscopic imaging experiments and in many cases only single voxel studies are performed (Hu et al, 2009). Although 1H MRS experiments are performed widely in the clinic, this lack of sensitivity has limited their routine use. This situation may change with the recent introduction of a dynamic nuclear polarisation (DNP) technique that can increase sensitivity in the solution state 13C MRS experiment by >10 000-fold (Ardenkjaer-Larsen et al, 2003). The enormous gain in sensitivity means that, following injection of a hyperpolarised 13C-labelled cell substrate, there is sufficient signal to image the molecule in vivo, and, more importantly, its metabolic conversion into other cell metabolites. Whereas 1H MRS measurements provide a largely static picture of the levels of tissue metabolites, this labelling technique enables dynamic imaging of cellular metabolism. The principal drawback of the method, however, is the short half-life of the polarisation; for [1-13C]pyruvate this is ∼30 s in vivo, which means that the material must be injected and imaged within ∼5 min. Thus, in order to image metabolism using this technique, the hyperpolarised 13C-labelled substrate must be taken up rapidly by the cell and its subsequent metabolism must be very fast. Even then, it is often only possible to monitor a single enzyme-catalysed reaction (Gallagher et al, 2009a). Nevertheless, the technique has already shown promise for detecting treatment response in tumours (Day et al, 2007; Witney et al, 2009).
The exchange of hyperpolarised 13C label between [1-13C]pyruvate and lactate, in the reaction catalysed by lactate dehydrogenase (LDH), was shown to decrease in a drug-treated murine lymphoma in vivo (Day et al, 2007), where decreased label flux was due to a number of factors, including: DNA damage-mediated activation of polyADP-ribose polymerase (PARP) and consequent depletion of the NAD(H) coenzyme pool, a loss of LDH activity, and a reduction in tumour cellularity (Day et al, 2007; Witney et al, 2009). A similar study in the same tumour model using [1,4-13C2]fumarate, showed that the rate of the fumarase catalysed conversion of fumarate to malate was a measure of subsequent drug-induced cellular necrosis (Gallagher et al, 2009b). With a clinical trial using hyperpolarised [1-13C]pyruvate about to start in prostate cancer (Brindle, 2008b), there is a reasonable expectation that the technique could translate to the clinic, where it offers a new functional imaging approach to detect early tumour responses to treatment.
We show here, in a model of human breast adenocarcinoma, that a combination of hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate can be used to detect response to doxorubicin treatment before there is any detectable change in tumour size. There was a decrease in labelled lactate production, reflecting a DNA damage response and initiation of the apoptotic program, and an increase in labelled malate production, reflecting the onset of cellular necrosis.
Materials and methods
Cell culture
MDA-MB-231 cells (European Collection of Cell Cultures, Salisbury, Wiltshire, UK) were grown in RPMI 1640 media, supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Invitrogen, Paisley, Refrewshire, UK). Cell number and viability were monitored using trypan blue staining. Cell death was induced by addition of doxorubicin (1 μg ml−1 final concentration; Sigma-Aldrich Co. Ltd, Poole, Dorset, UK).
Flow cytometry
Apoptosis and necrosis were measured by flow cytometry. Cells were trypsinised (0.25% trypsin; 1 mM EDTA) and harvested by centrifugation (1300 g, 3 min). Detached cells present in the media before trypsinisation were retained and pooled with the trypsinised cells. Cell pellets (1 × 106 cells) were washed once in ice-cold HEPES-buffered saline (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) and resuspended in 100 μl of the same buffer. Annexin V-Pacific Blue (5 μl per 100 μl of cell suspension) and SYTOX Red (Invitrogen) (5 nM) were added to the cells and incubated for 15 min at 20°C. The resulting mixture was washed once in ice-cold HEPES-buffered saline, kept briefly on ice and then analysed in an LSRII cytometer (BD Biosciences, Rockville, MD, USA), with 20 000 cells counted per event. Viable cells were identified as cells that stain with neither annexin V nor SYTOX Red; apoptotic cells as cells that stain with annexin V, but not SYTOX Red; and necrotic cells as cells that stain with both annexin V and SYTOX Red. Cellular NADH content was assessed from measurements of autofluorescence at 455 nm following excitation at 350 nm (Day et al, 2007; Witney et al, 2009). Cytosolic NADH has been shown previously to make a significant contribution to this autofluorescence (Aubin, 1979) and it is the cytosolic, rather than the mitochondrial NAD(H) pool, that is depleted the following activation of PARP (Ying et al, 2005).
Western blots
PolyADP-ribose polymerase cleavage was assessed by western blotting as described previously (Witney et al, 2009). Membranes were probed using polyclonal rabbit anti-PARP1 antibody (Abcam, Cambridge, Cambridgeshire, UK, 1 : 1000). A rabbit anti-actin antibody (Sigma-Aldrich Co. Ltd, 1 : 1000) was used as a loading control and a peroxidase-conjugated donkey anti-rabbit IgG antibody (Jackson ImmunoResearch Europe Ltd., Newmarket, UK, 1 : 10 000) as the secondary antibody. Proteins were visualised using the ECLplus kit (GE Healthcare, Chalfont St Giles, Bucks, UK). Blots were scanned (PowerLook III, Umax Systems GmbH, Willich, Germany) and signal quantification was performed by densitometry using scanning analysis software (TotalLab TL120, Nonlinear Dynamics, Newcastle upon Tyne, UK).
[1-13C]Pyruvate and [1,4-13C2]fumarate hyperpolarisation
A 44 mg sample of 91% [1-13C]pyruvic acid, containing 15 mM of the trityl radical OXO63 (GE Healthcare, Little Chalfont, UK) and 1.5 mM ProHance (Bracco Diagnostics Inc, Princeton, NJ, USA), was prepared with a 40 mg sample of [1,4-13C2]fumaric acid dissolved in DMSO, containing 15 mM of the trityl radical AH-111501 and 0.5 mM of a gadolinium chelate, 3-Gd, (GE Healthcare, Little Chalfont, UK). Aliquots (10 μl) of [1,4-13C2]fumaric acid were dropped into liquid nitrogen to form pellets, which were placed in the DNP polariser with previously frozen [1-13C]pyruvic acid. Polarisation was performed using a microwave source at 93.982 GHz and 100 mW for 1 h. The frozen sample was dissolved at 180°C using 6 ml of a 40 mM phosphate buffer, containing 120 mM NaOH and 100 mg l−1 EDTA. The dissolved solution contained 20 mM [1,4-13C2]fumarate and 75 mM [1-13C]pyruvate. The sample was dissolved in <5 s and polarisation levels were between 18–25%, determined by comparing the signal intensity from the hyperpolarised sample to that observed at thermal equilibrium (Gallagher et al, 2008). For in vivo studies, [1-13C]pyruvic acid and [1,4-13C2]fumaric acid pellets were hyperpolarised separately, with 40 mM HEPES, 94 mM NaOH, 30 mM NaCl, 100 mg l−1 EDTA and 40 mM phosphate buffer, 40 mM NaOH, 50 mM NaCl, 100 mg l−1 EDTA used as the dissolution buffers, respectively.
13C MRS measurements on cells
Cells (2–6 × 107) were examined in a 10-mm NMR tube, using a broadband probe (Varian NMR Instruments, Palo Alto, CA, USA) in a 9.4-T vertical wide-bore magnet (Oxford Instruments, Oxfordshire, UK) interfaced to a Varian INOVA console (Varian NMR Instruments, Paulo Alto, CA, USA). The sample temperature was maintained at 37°C. Hyperpolarised [1-13C]pyruvate (75 mM), hyperpolarised [1,4-13C2]fumarate (20 mM) and nonhyperpolarised, unlabelled lactate (75 mM) were injected into the cell suspension and single transient 13C spectra were acquired every second for 240 s, using a 6° flip angle pulse and a spectral width of 32 kHz. The area under each peak was used to calculate concentration of the labelled metabolites. The two inequivalent malate peaks, 13C-labelled at the C-1 and C-4 position, were combined to produce a single measurement of intensity (Gallagher et al, 2009b). The peak intensities of hyperpolarised metabolites were fitted to two-site exchange models describing the fluxes of hyperpolarised 13C label between pyruvate and lactate (kP), and fumarate and malate (kF; Day et al, 2007).
Tumour implantation
Female severe combined immunodeficiency mice were purchased at 6–8 weeks of age from Harlan UK Ltd (Bicester, UK). Tumour cells (1 × 107) were injected subcutaneously into the shaved flanks of mice. Tumour size was measured using callipers and is reported as the product of the two largest perpendicular diameters (mm2). Mice were imaged 24 h before treatment with an i.v. injection of 10 mg doxorubicin per kg body weight and at 24 and 48 h post-treatment. All procedures were carried out in accordance with the Animals (Scientific Procedures) Act of 1986 (UK) and were designed with reference to the UK Co-ordinating Committee on Cancer Research Guidelines for the Welfare of Animals in Experimental Neoplasia.
13C MRS and 1H MRI measurements on tumours
Mice were anesthetised with an intraperitoneal injection of 10 ml per kg body weight of a 5 : 4 : 31 mixture of Hypnorm (VetaPharma Ltd, Leeds, West Yorkshire, UK), Hypnovel (Roche, Basel, Switzerland) and saline and a catheter inserted into a tail vein. A 25 mm diameter surface coil tuned to 13C (100 MHz) was positioned over the tumour. The entire assembly was placed in a quadrature 1H-tuned volume coil (Varian), in a 9.4-T vertical wide-bore magnet. Transverse 1H images were acquired from the tumour using a spin-echo pulse sequence (repetition time, 1.5 s; echo time, 30 ms; field of view, 32 mm × 32 mm; data matrix, 256 × 256; slice thickness, 2 mm; 11 slices). Following injection of hyperpolarised [1-13C]pyruvate, which was accomplished within 3 s, 128 single transient spectra were collected from a 6 mm tumour slice over a period of 128 s (every sixteenth spectrum was collected from the entire sensitive volume of the surface coil). Spectra were acquired using a slice-selective 600 μs sinc pulse, with a nominal flip angle of 5°. C-1 lactate and pyruvate peak intensities were fitted to the modified Bloch equations for two-site exchange to obtain an apparent rate constant for label exchange between pyruvate and lactate (kP), as described previously (Day et al, 2007). Following [1-13C]pyruvate injection, [1,4-13C2]fumarate was polarised and injected into the animal, which was still anesthetised. This was achieved within 1 h of the previous injection. Owing to the low-malate signals observed, dynamic measurements of fumarate conversion to malate could not be performed in vivo. Instead, a single 13C spectrum, collected from a 6 mm tumour slice, was acquired 20 s after injection of 0.2 ml fumarate using a nominal flip angle of 20°; a time at which the maximum malate peak intensity had been observed in a previous dynamic study in a drug-treated murine lymphoma tumour (Gallagher et al, 2009b). Data were expressed as the malate/fumarate ratio, calculated from the intensities of the [1,4-13C2]fumarate resonance and the combined resonances from [1-13C]malate and [4-13C]malate.
Statistics
Results are expressed as the mean±s.d. Significant differences between values were determined using a Mann–Whitney nonparametric test. Statistical analysis was performed using GraphPad Prism version 5.0 for windows (GraphPad Software, San Diego, CA, USA). Differences between treatment groups were considered significant if P<0.05.
Results
Measurement of cell death following drug treatment in vitro
Drug-induced apoptosis and necrosis of human breast cancer cells (MDA-MB-231) were assessed by flow cytometry, following staining of the cells with Annexin V-Pacific Blue and SYTOX Red, respectively (Figure 1). There were significant increases in apoptosis and necrosis by 48 h after treatment with doxorubicin, which is a commonly used chemotherapeutic drug (Gewirtz, 1999; Figure 1A). Annexin V binding to apoptotic cells correlated with the induction of caspase-3 activity at 48 h, as indicated by PARP cleavage (Kaufmann et al, 1993). There was a small decrease in the level of intact PARP at 48 h, with a 26 and 20% decrease compared with cells at 0 h in two independent experiments, with the level of uncleaved PARP becoming almost undetectable at 72 h (Figure 1A, inset). The apoptotic fraction peaked at 72 h, before declining at 96 h, whereas the necrotic fraction was increased at 72 h and further increased at 96 h.
Cell death and NADH depletion in MDA-MB-231 cells following doxorubicin treatment. (A) Cell fraction undergoing apoptosis (▪) and necrosis (○), as determined by flow cytometry. Apoptotic cells were detected using annexin V Pacific Blue (λ Ex/Em=410/455) and necrotic cells using SYTOX Red nuclear staining (λ Ex/Em=640/658). Inset: Representative western blot of PARP expression in MDA-MB-231 cells following doxorubicin treatment. Actin was used as a loading control. (B) Changes in the cellular NADH pool following treatment. The NADH pool was measured by recording changes in UV autofluorescence, as detected by flow cytometry (λ Ex/Em=350/455).
Monitoring treatment response in cells with hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate
Addition of hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate to MDA-MB-231 cells, resulted in an increase in the lactate carboxyl signal intensity as the hyperpolarised 13C label was transferred from pyruvate, followed by a decrease in signal intensity due to decay of the polarisation (Figures 2 and 3). At 72 h after doxorubicin treatment, there was a 48% decrease (n=4; P<0.01) in 13C label exchange between pyruvate and lactate (Figures 2, 3A and B), which was accompanied by a 77% decrease in NADH autofluorescence (P<0.01; Figure 1B). At 96 h after drug treatment there was a 72% (n=4; P<0.01) decrease in label exchange, by which time the NADH autofluorescence had decreased by 89±1% (P<0.01). There was no detectable flux of label from hyperpolarised [1,4-13C2]fumarate to hyperpolarised [1,4-13C2]-labelled malate until 72 h after doxorubicin treatment (Figure 2), when the apparent rate constant (kF) for label flux was 2.8 × 10−4±1.4 × 10−4 s−1 (n=4). By 96 h, the rate constant had increased by 55% to 6.1 × 10−4±0.8 × 10−4 s−1 (n=4; P<0.01; Figure 3C and D). There was a good correlation between the extent of cellular necrosis, and the rate constant describing label flux between fumarate and malate (R2=0.96; Figure 4).
Representative 13C spectra of cell suspensions 15 s after the addition of 75 mM hyperpolarised [1-13C]pyruvate, 20 mM hyperpolarised [1,4-13C2]fumarate and 75 mM unlabelled lactate. Cells were either untreated (A), or had been treated with doxorubicin for 72 h (B) or 96 h (C). The labelled peaks are: 1, [1-13C]lactate; 2, [1,4-13C2]malate; 3, [1-13C]pyruvate hydrate; 4, [1,4-13C2]fumarate; 5, [1-13C]pyruvate.
Effect of doxorubicin-induced cell death on flux of hyperpolarised 13C label between pyruvate and lactate, and between fumarate and malate in an MDA-MB-231 cell suspension. (A) [1-13C]lactate peak intensities after addition of 75 mM hyperpolarised [1-13C]pyruvate, 20 mM hyperpolarised [1,4-13C2]fumarate and 75 mM unlabelled lactate to MDA-MB-231 cell suspensions that had been treated with doxorubicin or were untreated. Spectra were corrected for cell number and scaled to the initial pyruvate signal intensity to correct for variation in polarisation levels. Shaded regions represent 1 s.e.m. (n=4 for all treatment groups). (B) Changes in [1-13C]pyruvate–[1-13C]lactate exchange rate constant following doxorubicin treatment. The exchange rate constant (kP) was derived by fitting changes in signal peak intensity to the modified Bloch equations for two-site exchange. Mean values (n=4) and s.d. are shown (*P<0.05, **P<0.01). (C) Total [1,4-13C2]malate peak intensities after addition of 75 mM hyperpolarised [1-13C]pyruvate, 20 mM hyperpolarised [1,4-13C2]fumarate and 75 mM unlabelled lactate to MDA-MB-231 cell suspensions that had been treated with doxorubicin or were untreated. Spectra were corrected for cell number and scaled to the initial fumarate signal intensity to correct for variation in polarisation levels. Shaded regions represent 1 s.e.m. (n=4 for all treatment groups). (D) Changes in the rate constant describing hyperpolarised 13C label flux between [1,4-13C2]fumarate and [1,4-13C2]malate following doxorubicin treatment. The rate constant (kF) was derived by fitting changes in signal peak intensities to the modified Bloch equations for two-site exchange. Mean values (n=4) and s.d. are shown (*P<0.05, **P<0.01).
Correlation between cellular necrosis and the rate constant describing hyperpolarised 13C label flux between [1,4-13C2]fumarate and malate. Necrosis was monitored by flow cytometric analysis of SYTOX Red dead cell staining (λ Ex/Em=640/658). In separate experiments, the rate constant (kF) was derived by fitting changes in signal peak intensities to the modified Bloch equations for two-site exchange. Mean values (n=3–4) and s.d. are shown.
Monitoring treatment response in tumours with hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate
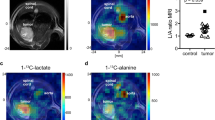
Intravenous injection (0.2 ml) of co-hyperpolarised [1-13C]pyruvate (75 mM) and [1,4-13C2]fumarate (20 mM) into MDA-MB-231 tumour-bearing mice resulted in the appearance of signals from [1-13C]pyruvate, [1-13C]lactate and [1,4-13C2]fumarate in the tumours. There were no detectable signals from [1,4-13C2]malate, because of their masking by resonances from labelled lactate and pyruvate hydrate (data not shown). For this reason, [1-13C]pyruvate and [1,4-13C2]fumarate experiments were performed separately. There was a 49% decrease in pyruvate–lactate exchange at 24 h after doxorubicin treatment, where the rate constant (kP) decreased from 0.075±0.002 s−1 in untreated tumours to 0.038±0.025 s−1 (P=0.15; n=3 animals). By 48 h, the rate constant had decreased by 73% to 0.020±0.002 s−1 (P=0.0017, n=3). There was no significant change in tumour size during this period, with the mean tumour volume of 68.0±12.9 mm2 (n=10 animals) in untreated animals remaining at 65.9±17.3 mm2 (n=6) and 62.5±16.3 mm2 (n=6) at 24 and 48 h post-treatment, respectively. Representative spectra from an untreated tumour and a tumour at 48 h after doxorubicin treatment are shown in Figure 5A. The maximum amplitude of the [1,4-13C2]malate signals relative to the signal from [1,4-13C2]fumarate 20 s post-injection increased from 0.017±0.013 in untreated tumours to 0.090±0.050 at 24 h after doxorubicin treatment (n=4; P<0.05), a 5.4-fold increase (Figure 5B).
Detection of treatment response in implanted MDA-MB-231 tumours. Representative 13C spectra from untreated (upper spectra) or doxorubicin-treated (lower spectra) tumours 20 s post-injection of either hyperpolarised [1-13C]pyruvate (A), or hyperpolarised [1,4-13C2]fumarate (B). The peaks are: 1, [1-13C]lactate; 2, [1-13C]pyruvate hydrate; 3, [1-13C]alanine; 4, [1-13C]pyruvate; 5, [1,4-13C2]fumarate; 6, [1,4-13C2]malate. Spectra were taken 48 and 24 h post-doxorubicin treatment for the pyruvate and fumarate experiments, respectively.
Discussion
Imaging currently has an important role in the management of breast cancer patients by enabling assessment of treatment response following primary or neoadjuvant therapy. Therapeutic response is assessed primarily by measurements of tumour shrinkage, using a combination of computed tomography (CT) and/or MRI (reviewed in Ollivier et al (2005)). However, there are problems with these imaging modalities. CT has the advantages of simplicity, speed and availability, however, changes in tumour size are frequently overestimated in diffuse or multinodular tumours (Ollivier et al, 2005). Changes in tumour size can also be overestimated by MRI, particularly with smaller tumours and if there is surrounding tissue oedema (Merchant et al, 1993). Dynamic contrast-enhanced MRI can provide further diagnostic information, with the intensity of contrast enhancement shown to predict the presence of residual tumour after preoperative chemotherapy (Gilles et al, 1994). There is a need, however, to develop imaging methods that can detect treatment response more sensitively and before there is any change in tumour size.
FDG-PET is being used increasingly in the clinic as a functional imaging technique for detection, staging and early response monitoring in breast cancer (Eubank and Mankoff, 2005). Although currently most useful as a staging tool, initial studies have shown FDG-PET to be capable of predicting pathological response in patients with locally advanced breast cancer following a single course of neoadjuvant chemotherapy (Biersack et al, 2004; Krak et al, 2004). However, the presence of infected or inflamed tissue, and high uptake by infiltrating immune cells can mask changes in tumour FDG uptake (Strauss, 1996; Zhuang et al, 2005). Patients also receive a high radiation dose, with scans being costly, time-consuming and are only available in a few selected centres. Complementary methods for the detecting treatment response in breast cancer are therefore still required that provide alternative biological readouts that can be used in conjunction with existing methods in a multimodality approach.
Treatment of murine EL-4 lymphoma cells with a topoisomerase II inhibitor and DNA damaging agent, etoposide, was shown previously to inhibit the rate of hyperpolarised 13C label exchange between [1-13C]pyruvate and lactate, catalysed by the enzyme LDH. The decreased exchange was explained by induction of a DNA damage response and consequent induction of PARP, for which the coenzyme NAD+ is a substrate (Day et al, 2007; Witney et al, 2009). We have shown here that doxorubicin treatment of the oestrogen receptor negative-human breast cancer cell line, MDA-MB-231, in vitro resulted in a marked decrease in the rate of hyperpolarised 13C label exchange between [1-13C]pyruvate and lactate and that this was correlated with a decrease in the concentration of the cellular NAD(H) coenzyme pool. Doxorubicin, like etoposide, is a topoisomerase II inhibitor and a DNA damaging agent that induces PARP activation (Munoz-Gamez et al, 2005). Therefore, this hyperpolarised [1-13C]pyruvate method for detecting response to genotoxic agents would appear to be generally applicable to other tumour types.
The hyperpolarised [1-13C]pyruvate experiment shows that the tumour cell has responded to treatment, but not that it has died. Moreover, it provides ‘negative contrast’, in that there is a decrease in lactate signal intensity in those tumours that have responded to treatment (Day et al, 2007; Witney et al, 2009). Such a decrease in lactate signal could also be due to other factors, such as reduced delivery of pyruvate to the tumour. In contrast, hyperpolarised 13C-labelled [1,4-13C2]fumarate provides positive contrast and appears to be a marker of cell death (Gallagher et al, 2009b). Fumarate shows relatively slow plasma membrane transport, and thus, in viable cells there is no detectable conversion of fumarate to malate within the relatively short lifetime of the 13C polarisation. However, in necrotic cells, where the plasma membrane permeability barrier has been compromised, fumarate is rapidly converted to malate and the hyperpolarised [1,4-13C2]malate signal provides a positive marker of tumour cell necrosis. Treatment of MDA-MB-231 cells with doxorubicin resulted in the production of significant levels of labelled malate by 72 h after drug treatment, a time at which there was also a significant decrease in the rate of labelled lactate production. There was no detectable labelled malate produced in untreated cells. The rate of labelled malate production showed a good correlation with the level of tumour cell necrosis, as has been observed previously (Gallagher et al, 2009b). As with the hyperpolarised [1-13C]pyruvate method for detecting response to genotoxic agents, these experiments have demonstrated that the hyperpolarised [1,4-13C2]fumarate experiment could be a generic method for detecting tumour cell death post-treatment.
Experiments with hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate were also performed in implanted MDA-MB-231 tumours, where we have shown a response to doxorubicin treatment in the absence of any detectable change in tumour size. Co-hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate preparations could not be used in vivo as malate production was masked by overlapping signals from lactate and pyruvate hydrate formed from the labelled pyruvate in these lower resolution 13C spectra. When the pyruvate was injected alone, a significant decrease in pyruvate–lactate exchange was observed at 48 h post-therapy at the maximum tolerated dose of 10 mg kg−1 doxorubicin. This relatively slow response to therapy is consistent with that observed in cultured cells and reflects the relative resistance of MDA-MB-231 tumours to genotoxic agents (Fang et al, 2007). At 24 h, after treatment with doxorubicin and 20 s after the injection of hyperpolarised [1,4-13C2]fumarate the hyperpolarised 13C malate/fumarate ratio increased 5.4-fold (Figure 5B), indicating the onset of tumour cell necrosis. At this time point, a decrease in pyruvate–lactate exchange was also observed, but did not reach significance. Therapy response was observed earlier in tumours than in the cell experiments, with the larger number of tumour cells in vivo giving an increase in sensitivity.
The level of tumour cell death after drug treatment has been shown, in preclinical and clinical studies, to be a good prognostic indicator for treatment outcome, including in breast cancer (Chang et al, 2000). However, early evidence of treatment response does not necessarily indicate a favourable long-term outcome. For example, an early decrease in FDG uptake in oesophageal adenocarcinoma in the clinic following neoaduvant treatment did not correlate with a histopathological response in the longer term (Smithers et al, 2008) and in head and neck cancers treated with radiotherapy uptake of l-[methyl-11C]methionine was reduced, but there was no correlation with treatment outcome (Nuutinen et al, 1999). However, in the case of the MDA-MB-231 human breast cancer model used in this study, a single treatment with doxorubicin, at a slightly lower dose than used here, has been shown to produce a long-term treatment response, as indicated by a tumour growth delay of ∼20 days (Mason et al, 2008). Thus, the imaging methods that we have described here can detect early evidence of a treatment response that is correlated with outcome in the longer term.
In summary, we have shown that hyperpolarised 13C experiments for detecting early evidence of treatment response, which had previously only been demonstrated in an implanted murine lymphoma tumour model that responds very rapidly to treatment with a genotoxic agent (Day et al, 2007; Witney et al, 2009), also work in a human breast tumour xenograft that is much more resistant to treatment with these agents. The hyperpolarised [1-13C]pyruvate experiment provided early evidence of a DNA damage response, whereas the [1,4-13C2]fumarate experiment indicated the presence of tumour cell necrosis post-treatment. Both experiments indicated the presence of a treatment response in the absence of any change in tumour size. Translation of this technique to the clinic could offer a new method for monitoring treatment response in breast cancer.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aboagye EO, Bhujwalla ZM (1999) Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res 59 (1): 80–84
Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K (2003) Increase in signal-to-noise ratio of >10 000 times in liquid-state NMR. Proc Natl Acad Sci USA 100 (18): 10158–10163
Aubin JE (1979) Autofluorescence of viable cultured mammalian cells. J Histochem Cytochem 27 (1): 36–43
Biersack HJ, Bender H, Palmedo H (2004) FDG-PET in monitoring therapy of breast cancer. Eur J Nucl Med Mol Imaging 31 (Suppl 1): S112–S117
Brindle K (2008a) New approaches for imaging tumour responses to treatment. Nat Rev Cancer 8 (2): 94–107
Brindle KM (2008b) Abstractions. Nature 453 (7197): xi
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434 (7035): 913–917
Chang J, Ormerod M, Powles TJ, Allred DC, Ashley SE, Dowsett M (2000) Apoptosis and proliferation as predictors of chemotherapy response in patients with breast carcinoma. Cancer 89 (11): 2145–2152
Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM (2007) Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med 13 (11): 1382–1387
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45 (2): 228–247
Eubank WB, Mankoff DA (2005) Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med 35 (2): 84–99
Fang Y, Sullivan R, Graham CH (2007) Confluence-dependent resistance to doxorubicin in human MDA-MB-231 breast carcinoma cells requires hypoxia-inducible factor-1 activity. Exp Cell Res 313 (5): 867–877
Gallagher F, Kettunen M, Brindle K (2009a) Biomedical applications of hyperpolarized 13C magnetic resonance imaging. Prog NMR Spectrosc 55: 285–295
Gallagher FA, Kettunen MI, Day SE, Lerche M, Brindle KM (2008) 13C MR spectroscopy measurements of glutaminase activity in human hepatocellular carcinoma cells using hyperpolarized 13C-labeled glutamine. Magn Reson Med 60 (2): 253–257
Gallagher FA, Kettunen MI, Hu DE, Jensen PR, Zandt RI, Karlsson M, Gisselsson A, Nelson SK, Witney TH, Bohndiek SE, Hansson G, Peitersen T, Lerche MH, Brindle KM (2009b) Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci USA 106 (47): 19801–19806
Gewirtz DA (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57 (7): 727–741
Gilles R, Guinebretiere JM, Toussaint C, Spielman M, Rietjens M, Petit JY, Contesso G, Masselot J, Vanel D (1994) Locally advanced breast cancer: contrast-enhanced subtraction MR imaging of response to preoperative chemotherapy. Radiology 191 (3): 633–638
Glunde K, Jacobs MA, Bhujwalla ZM (2006) Choline metabolism in cancer: implications for diagnosis and therapy. Expert Rev Mol Diag 6: 821–829
Hu JN, Feng WZ, Hua J, Jiang Q, Xuan Y, Li T, Haacke EM (2009) A high spatial resolution in vivo 1H magnetic resonance spectroscopic imaging technique for the human breast at 3 T. Medical Physics 36 (11): 4870–4877
Hu L, Zaloudek C, Mills GB, Gray J, Jaffe RB (2000) In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin Cancer Res 6 (3): 880–886
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG (1993) Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res 53 (17): 3976–3985
Krak NC, Hoekstra OS, Lammertsma AA (2004) Measuring response to chemotherapy in locally advanced breast cancer: methodological considerations. Eur J Nucl Med Mol Imaging 31 (Suppl 1): S103–S111
Mason KA, Valdecanas D, Hunter NR, Milas L (2008) INO-1001, a novel inhibitor of poly(ADP-ribose) polymerase, enhances tumor response to doxorubicin. Invest New Drugs 26 (1): 1–5
Merchant TE, Obertop H, de Graaf PW (1993) Advantages of magnetic resonance imaging in breast surgery treatment planning. Breast Cancer Res Treat 25 (3): 257–264
Munoz-Gamez JA, Martin-Oliva D, Aguilar-Quesada R, Canuelo A, Nunez MI, Valenzuela MT, Ruiz de Almodovar JM, De Murcia G, Oliver FJ (2005) PARP inhibition sensitizes p53-deficient breast cancer cells to doxorubicin-induced apoptosis. Biochem J 386 (Part 1): 119–125
Neves AA, Brindle KM (2006) Assessing responses to cancer therapy using molecular imaging. Biochim Biophys Acta 1766: 242–261
Nuutinen J, Jyrkkio S, Lehikoinen P, Lindholm P, Minn H (1999) Evaluation of early response to radiotherapy in head and neck cancer measured with [11C]methionine-positron emission tomography. Radiother Oncol 52 (3): 225–232
Ollivier L, Balu-Maestro C, Leclere J (2005) Imaging in evaluation of response to neoadjuvant breast cancer treatment. Cancer Imaging 5 (1): 27–31
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3 (10): 721–732
Smithers BM, Couper GC, Thomas JM, Wong D, Gotley DC, Martin I, Harvey JA, Thomson DB, Walpole ET, Watts N, Burmeister BH (2008) Positron emission tomography and pathological evidence of response to neoadjuvant therapy in adenocarcinoma of the esophagus. Dis Esophagus 21 (2): 151–158
Strauss LG (1996) Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med 23 (10): 1409–1415
Weissleder R, Pittet MJ (2008) Imaging in the era of molecular oncology. Nature 452 (7187): 580–589
Witney TH, Kettunen MI, Day SE, Hu DE, Neves AA, Gallagher FA, Fulton SM, Brindle KM (2009) A comparison between radiolabeled fluorodeoxyglucose uptake and hyperpolarized (13)C-labeled pyruvate utilization as methods for detecting tumor response to treatment. Neoplasia 11 (6): 574–582
Ying W, Alano CC, Garnier P, Swanson RA (2005) NAD+ as a metabolic link between DNA damage and cell death. J Neurosci Res 79 (1–2): 216–223
Zhuang H, Yu JQ, Alavi A (2005) Applications of fluorodeoxyglucose-PET imaging in the detection of infection and inflammation and other benign disorders. Radiol Clin North Am 43 (1): 121–134
Acknowledgements
The work was supported by a Cancer Research UK Programme Grant (to KMB; C197/A3514). THW was in receipt of a GE Healthcare-BBSRC CASE studentship and FAG a Cancer Research UK and Royal College of Radiologists (UK) clinical research training fellowship and funding from the National Institute for Health Research Cambridge Biomedical Research Centre. RN was supported by a ‘Short-Term Scientific Mission’ funded by the COST D38 ‘Metal-based systems for Molecular Imaging’. COST is supported by the EU RTD Framework Programme. The polariser and related materials were provided by GE Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Witney, T., Kettunen, M., Hu, De. et al. Detecting treatment response in a model of human breast adenocarcinoma using hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate. Br J Cancer 103, 1400–1406 (2010). https://doi.org/10.1038/sj.bjc.6605945
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605945
Keywords
This article is cited by
-
Developing a metabolic clearance rate framework as a translational analysis approach for hyperpolarized 13C magnetic resonance imaging
Scientific Reports (2023)
-
Hyperpolarisierte 13C‑Magnetresonanztomographie – ein Fenster in den Stoffwechsel
Die Radiologie (2022)
-
The use of hyperpolarised 13C-MRI in clinical body imaging to probe cancer metabolism
British Journal of Cancer (2021)
-
A new horizon of DNP technology: application to in-vivo 13C magnetic resonance spectroscopy and imaging
Biophysical Reviews (2013)