Summary:
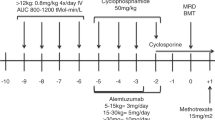
Infectious complications due to adenovirus are of increasing concern after allogeneic stem cell transplantation. Over the past 4 years, we have modified our conditioning regimens to use alemtuzumab in preference to anti-thymocyte globulin (ATG) for pediatric patients receiving stem cell transplants from alternate donors. Recent reports in adult studies implicate alemtuzumab as a risk factor for adenovirus infection. We therefore evaluated the incidence of adenovirus infection in pediatric patients receiving either ATG or alemtuzumab in their conditioning regimens. Of the 111 patients evaluated, a total of 54 patients received ATG and 57 patients received alemtuzumab. In total, 35/111 (32%) patients were infected by adenovirus, and 9/111 (8%) had adenovirus disease (AD). Adenovirus infection was greater in the alemtuzumab group than the ATG group (23/57 vs 12/54) (P=0.039) and disseminated AD was more frequent in the alemtuzumab group vs the ATG group (8/57 and 1/54 respectively) (P=0.032). The presence of Grade 3–4 graft-versus-host disease was a risk factor for adenovirus infection. Our findings highlight the fact that adenovirus infection is a frequent complication after stem cell transplantation from alternate donors in the pediatric population and that alemtuzumab increases the risk of infection compared to ATG. This work will help in identifying at-risk populations for our upcoming immunotherapy trial using adoptively transferred donor-derived adenovirus-specific cytotoxic T lymphocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Horwitz MS . Adenoviruses. Fields Virology. Lippincott, Williams & Wilkins: Philadelphia, PA, Baltimore, MD, 2002.
Hale GA, Heslop HE, Krance RA et al. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant 1999; 23: 277–282.
Flomenberg P, Babbitt J, Drobyski WR et al. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis 1994; 169: 775–781.
Shenk TE . Adenoviridae: The Viruses and Their Replication. Fields Virology. Lippincott, Williams & Wilkins: Philadelphia, PA, Baltimore, MD, 2002.
Lion T, Baumgartinger R, Watzinger F et al. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood 2003; 102: 1114–1120.
Baldwin A, Kingman H, Darville M et al. Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplant 2000; 26: 1333–1338.
Schilham MW, Claas EC, van Zaane W et al. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin Infect Dis 2002; 35: 526–532.
Bruno B, Gooley T, Hackman RC et al. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant 2003; 9: 341–352.
Carter BA, Karpen SJ, Quiros-Tejeira RE et al. Intravenous Cidofovir therapy for disseminated adenovirus in a pediatric liver transplant recipient. Transplantation 2002; 74: 1050–1052.
Hoffman JA, Shah AJ, Ross LA, Kapoor N . Adenovirus infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001; 7: 388–394.
Ljungman P, Ribaud P, Eyrich M et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2003; 31: 481–486.
Hromas R, Cornetta K, Srour E et al. Donor leukocyte infusion as therapy of life-threatening adenovirus infections after T-cell-depleted bone marrow transplantation. Blood 1994; 84: 1689–1690.
Chakrabarti S, Mautner V, Osman H et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood 2002; 100: 1619–1627.
Avivi I, Chakrabarti S, Milligan DW et al. Incidence and outcome of adenovirus disease in transplant recipients after reduced-intensity conditioning with alemtuzumab. Biol Blood Marrow Transplant 2004; 10: 186–194.
Wagner HJ, Cheng YC, Huls MH et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood 2004; 103: 3979–3981.
Hongeng S, Krance RA, Bowman LC et al. Outcomes of transplantation with matched-sibling and unrelated-donor bone marrow in children with leukemia. Lancet 1997; 350: 767–770.
Deeg HJ, Amylon ID, Harris RE et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant 2001; 7: 208–215.
Krance RA, Kuehnle I, Rill DR et al. Hematopoietic and immunomodulatory effects of lytic CD45 monoclonal antibodies in patients with hematologic malignancy. Biol Blood Marrow Transplant 2003; 9: 273–281.
Kalbfleisch JD, Prentice RL . The Statistical Analysis of Failure Time Data. 1st edn. John-Wiley: New York, 1980.
Gray RJ . A class of K-sample tests for comparing the culmulative incidence of a competing risk. Ann Statist 1988; 16: 1141–1154.
Heim A, Ebnet C, Harste G, Pring-Akerblom P . Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol 2003; 70: 228–239.
Leen AM, Sili U, Savoldo B et al. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood 2004; 103: 1011–1019.
Leen AM, Sili U, Vanin EF et al. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood 2004; 104: 2432–2440.
Acknowledgements
This work was supported in part by a Doris Duke Distinguished Clinical Scientist Award to HEH and an Amy Strelzer Manasevit Scholar award from the NMDP to CMB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myers, G., Krance, R., Weiss, H. et al. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transplant 36, 1001–1008 (2005). https://doi.org/10.1038/sj.bmt.1705164
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705164
Keywords
This article is cited by
-
Antiviral cellular therapy for enhancing T-cell reconstitution before or after hematopoietic stem cell transplantation (ACES): a two-arm, open label phase II interventional trial of pediatric patients with risk factor assessment
Nature Communications (2024)
-
Matched related hematopoietic cell transplant for sickle cell disease with alemtuzumab: the Texas Children’s Hospital experience
Bone Marrow Transplantation (2021)
-
A survey on incidence and management of adenovirus infection after allogeneic HSCT
Bone Marrow Transplantation (2019)
-
Bacteremia during neutropenic episodes in children undergoing hematopoietic stem cell transplantation with ciprofloxacin and penicillin prophylaxis
International Journal of Hematology (2017)