Abstract
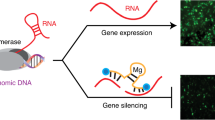
Background: RNA interference (RNAi) is a cellular pathway of gene silencing in a sequence-specific manner at the messenger RNA level. The basic mechanism behind RNAi is the breaking of a double-stranded RNA (dsRNA) matching a specific gene sequence into short pieces called short interfering RNA, which trigger the degradation of mRNA that matches its sequence. In this study, we explored the effects of RNAi in reducing the target gene expression in human myeloid leukemia cell lines. Methods: Four myeloid leukemia cell lines (HL-60, U937, THP-1, and K562) were transfected with dsRNA duplexes corresponding to the endogenous c-raf and bcl-2 genes and the gene expression inhibition was assessed. The effect of RNAi on cell differentiation was studied; the apoptosis induction and the sensitization of the leukemia cell lines to etoposide and daunorubicin were quantified by flowcytometric methods. Results: Transfection of the myeloid leukemia cell lines with dsRNA corresponding to c-raf and bcl-2 genes decreased the expression of Raf-1 and Bcl-2 proteins. RNAi for c-raf gene blocked the appearance of the monocytic differentiation induced by treatment with TPA. Combined RNAi for c-raf and bcl-2 induced apoptosis in HL-60, U937, and THP-1 cells and increased chemosensitivity to etoposide and daunorubicin. Conclusions: RNAi is a functional pathway in human myeloid leukemia cell lines and combined RNAi of c-raf and bcl-2 genes may represent a novel approach to leukemia, providing a means to overcome the resistance to chemotherapeutic agents and ultimately to augment the efficacy of chemotherapy in myeloid leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE . Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806 –811.
Clemens JC, Worby CA, Siminson-Leff N, et al. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci USA. 2000;97:6499–6503.
Misquitta L, Peterson BM . Targeted disruption of gene function in Drosophila by RNA interference: a role for nautilus in embryonic somatic muscle formation. Proc Natl Acad Sci USA. 1999;96:1451–1456.
Hammond SM, Bernstein E, Beach D, Hannon GJ . An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296.
Kennerdell JR, Carthew RW . Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled2 act in the winglass pathway. Cell. 1998;95:1017–1026.
Ngo H, Tschudi C, Gull K, Ullu E . Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc Natl Acad Sci USA. 1998;95:14687–14692.
Sanchez-Alvarado A, Newmark PA . Double-stranded RNA specifically disrupt gene expression during planarian regeneration. Proc Natl Acad Sci USA. 1999;96:5049–5054.
Lohmann JU, Endl I, Bosch TC . Silencing of developmental genes in Hydra. Dev Biol. 1999;214:211–214.
Sharp PA . RNA interference 2001. Genes Dev. 2001;15:485–490.
Marx S . Interfering with gene expression. Science. 2000;288:1370–1372.
Nykanen A, Haley B, Zamore PD . ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–321.
Elbashir SM, Lendeckel W, Tuschl T . RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200.
Hunter T, Hunt T, Jackson RJ, Robertson HD . The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975;250:409–417.
Bass BL . The short answer. Nature. 2001;11:428–429.
Minks MA, West DK, Benvin S, Baglioni C . Structural requirements of double-stranded RNA for the activation of 2′,5′-oligo (A) polymerase and protein kinase of interferon-treated HeLa cells. J Biol Chem. 1979;254:10180–10183.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21-nucleotide RNAs mediate interference in cultured mammalian cells. Nature. 2001;411:494–498.
Funato T, Kozawa K, Fujimaki S, Kaku M . Increased sensitivity to cytosine arabinoside in human leukemia by c-raf-1 antisense oligonucleotides. Anticancer Drugs. 2001;12:325–329.
Miyashita T, Reed JC . BCL-2 gene transfer increases relative resistance of S49.1 and WEH17.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992;52:5407–5411.
Miyashita T, Reed JC . Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157.
Wang HG, Miyashita T, Takayama S, et al. Apoptosis regulation by interaction of Bcl-2 protein and Raf-1 kinase. Cancer Res. 1994;9:2751–2756.
Okuda K, Matulonis U, Salgia R, Kanakura Y, Druker B, Griffin JD . Factor independence of human myeloid leukemia cell lines is associated with increased phosphorylation of the proto-oncogene Raf-1. Exp Hematol. 1994;22:1111–1117.
Collins SJ, Gallo RC, Gallagher RE . Continuous growth and differentiation of human myeloid leukemic cells in suspension culture. Nature. 1977;270:347–349.
Sundstrom C, Nilsson K . Establishment and characterization of a human histocytic cell line (U-937). Int J Cancer. 1976;17:565–577.
Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K . Establishment and characterization of a human acute monocytic leukemic cell line (THP-1). Int J Cancer. 1980;26:171–176.
Lozzio CB, Lozzio BB . Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334.
Monia BP, Johnston JF, Geiger T, Muller M, Fabbro D . Antitumor activity of a phosphorothioate antisense oligodeoxynucleotide targeted against C-raf kinase. Nat Med. 1996;2:668–675.
Konopleva M, Tari AM, Estrov Z, et al. Liposomal Bcl-2 antisense oligonucleotides enhance proliferation, sensitive acute myeloid leukemia to cytosine-arabinoside, and induce apoptosis independent of other antiapoptotic proteins. Blood. 2000;95:3929–3938.
Duriez PJ, Shah GM . Cleavage of poly (ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem Cell Biol. 1997;75:337–349.
Zamzami N, Marchetti P, Castedo M, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed cell death. J Exp Med. 1995;181:1661–1672.
Kharbanda S, Saleem A, Emoto Y, Stone R, Rapp U, Kufe D . Activation of Raf-1 and mitogen-activated protein kinases during monocytic differentiation of human myeloid leukemia cells. J Biol Chem. 1994;269:872–878.
Amarante-Mendes GP, Naekyung KC, Liu L, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome c and activation of caspase-3. Blood. 1998;91:1700–1705.
Benito A, Lerga A, Silva M, Leon J, Fernandez-Luna JL . Apoptosis of human myeloid leukemia cells induced by an inhibitor of protein phosphatases (okadaic acid) is prevented by Bcl-2 and Bcl-X(L). Leukemia. 1997;11:940–944.
Stiewe T, Parssanedjad K, Esche H, Opalka B, Putzer BM . E1A overcomes the apoptosis block in BCR-ABL+ leukemia cells and renders cells susceptible to induction of apoptosis by chemotherapeutic agents. Cancer Res. 2000;60:3957–3964.
Smith BD, Bambach BJ, Vala MS, et al. Inhibited apoptosis and drug resistance in acute myeloid leukemia. Br J Haematol. 1998;102:1042–1049.
Wang HG, Rapp UR, Reed JC . Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638.
Olivier R, Otter I, Monney L, Wartmann M, Borner C . Bcl-2 does not require Raf kinase activity for its death-protective function. Biochem J. 1997;324:75–83.
Zhong J, Troppmair J, Rapp UR . Independent control of cell survival by Raf-1 and Bcl-2 at the mitochondria. Oncogene. 2001;20:4807–4816.
Yang T, Kozopas KM, Craig RW . The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical, to those of Bcl-2. J Cell Biol. 1995;128:1173–1184.
Kitada S, Andersen J, Akar S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood. 1998;91:3379–3389.
Pepper C, Bentley P, Hoy T . Regulation of clinical chemoresistance by bcl-2 and bax oncoproteins in B-cell chronic lymphocytic leukemia. Br J Haematol. 1996;95:513–517.
Krontiris TG . Oncogenes. N Engl J Med. 1995;333:303–306.
Levitzki A, Gazit A . Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788.
Nakayama K, Negishi I, Kuika K, Sawa H, Loh D . Targeted disruption of Bcl-2αβ in mice: occurrence of gray hair, polycystic disease and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704.
Schimmer AD, Hedley DW, Penn LZ, Minden MD . Receptor- and mitochondrial apoptosis in acute leukemia: a translational view. Blood. 2001;98:3541–3553.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cioca, D., Aoki, Y. & Kiyosawa, K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther 10, 125–133 (2003). https://doi.org/10.1038/sj.cgt.7700544
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700544
Keywords
This article is cited by
-
Use of polymeric CXCR4 inhibitors as siRNA delivery vehicles for the treatment of acute myeloid leukemia
Cancer Gene Therapy (2020)
-
Nano-based delivery of RNAi in cancer therapy
Molecular Cancer (2017)
-
siRNA-loaded PEGylated porous silicon nanoparticles for lung cancer therapy
Journal of Nanoparticle Research (2014)
-
Proliferation inhibition and apoptosis induction of imatinib-resistant chronic myeloid leukemia cells via PPP2R5C down-regulation
Journal of Hematology & Oncology (2013)
-
RNA Interference—A Silent but an Efficient Therapeutic Tool
Applied Biochemistry and Biotechnology (2013)