Abstract
Aims
Optic nerve hypoplasia (ONH), which is defined as a congenital deficiency of retinal ganglion cells, may also involve more distal layers of the retina. We investigated electrophysiological function of the retina in ONH using electroretinograms (ERGs).
Methods
ERGs were recorded from 48 subjects (3.5–35 months) with unilateral or bilateral ONH. Pattern reversal (4° checks) was presented under chloral hydrate sedation, using an optical system to correct a cycloplegic refraction. A photopic flash stimulus was also used. Fundus photographs were used to measure the disk diameter/disk macula ratio (DD/DM), and to document other clinical signs. Eyes were classified as moderate (0.15–0.3) or severe (<0.15) ONH, and those with DD/DM greater than 0.3 were used as reference eyes.
Results
Pattern ERG recording was completed in 89 eyes and was detectable in 80% of eyes with ONH (61/76 tested) and in all 13 reference eyes. Photopic flash ERGs were of good quality in all eyes. The severity of ONH correlates with the amplitude of the photopic flash b-waves and with the amplitude of the N95 component of the pattern ERG (P<0.01). However, the ERGs to large patterns were well preserved (>3.5 μV) in 10 of 35 eyes with severe ONH. Tortuous retinal vessels in eyes with either moderate or severe ONH were associated with smaller amplitude photopic b-waves and markedly diminished or undetectable pattern ERGs.
Conclusions
This study supports the hypothesis that retinal dysfunction distal to the ganglion cells is common in ONH, but is not predictable on the basis of ONH severity alone. Additionally, tortuous retinal vessels in ONH may be a sign associated with retinal dysfunction.
Similar content being viewed by others
Introduction
Optic nerve hypoplasia (ONH), congenital dysgenesis of the optic nerves, is an important cause of visual dysfunction in childhood. By definition, ONH is a congenital deficiency of retinal ganglion cells and their axons that form the optic nerve. Few histological descriptions of ONH exist, and even fewer with clinicopathological correlation. In general, the only retinal abnormality is deficiency of the retinal ganglion cells and nerve fibre layer.1 ONH may occur as an isolated unilateral or bilateral condition but it is also frequently associated with other congenital anomalies, often as part of the triad of septo-optic dysplasia, De Moisiers Syndrome (ONH with pituitary hormone deficiencies and midline brain lesions).2, 3, 4, 5, 6 Although a number of risk factors have been identified, the pathogenesis of ONH remains unclear and is likely multifactorial.
Visual function in ONH varies with the severity of the condition from nearly normal to no light perception.5, 6, 7 Along the severity continuum, there are some cases with sectoral defects in the optic nerve head, and there is a frequent coincidence of ONH and pallor of these small optic nerve heads.8 Prognosis for vision can be difficult to establish based on clinical observation. Both neuro-radiological studies and anthropomorphic measurements aid in establishing the diagnosis and prognosis for young infants with ONH.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Visual evoked potential (VEP) testing can give a functional measure of vision early in life. In ONH, the amplitude of VEPs to flash stimulation and the presence of VEPs to pattern stimulation are correlated with visual function.7, 14, 17, 18, 19
Typically, ONH is characterized by normal full-field electroretinograms (ERGs) and abnormal VEPs.16, 17, 18, 19 The a-waves of flash ERGs are consistently reported as normal in ONH, but several authors have reported abnormalities in the b-wave.20, 21 Under photopic conditions, the b-wave is generated by the post-receptoral ON and OFF pathways mediated by the depolarizing and hyperpolarizing bipolar cells of the retina, with no significant contribution from ganglion cells.22, 23, 24 Thus, abnormalities in the photopic b-wave are evidence for dysfunction distal to the ganglion cells.
The generators of the pattern ERG are proximal to those of the flash ERG, with a major contribution from spiking cells of the inner retina. Pharmacological blockade of the ganglion cells and spiking amacrine cells with TTX in primates eliminates the negative component of the pattern ERG (N95).23 Generators of the positive component of the pattern ERG (P50) have not been as well localized. They are clearly distinct from those of the N95, as the effect of TTX on P50 is spatially tuned; P50 is largely preserved with large pattern stimuli but it is diminished with a shortened implicit time for small patterns. In clinical disorders causing optic atrophy, and in severe experimentally induced glaucoma in primates, both the P50 and N95 are affected, suggesting that damage is not limited to the spiking neurons of the inner retina.25, 26, 27, 28 Thus, generation of the N95 requires functional spiking cells. P50 for small patterns involves both spiking cells and more distal generators, whereas for large patterns, it may rely entirely on more distal generators, similar to those of focal ERGs that reflect local luminance.23, 27
Pattern electroretinography requires good fixation and a clear retinal image. To date, pattern ERGs in ONH have not been reported. In the ongoing CHLA prospective study of prenatal and clinical risk factors of ONH, an examination with chloral hydrate sedation and electrodiagnostic testing are part of the protocol. Fortuitously, nystagmus is suppressed during sedation. We have devised a strategy for recording pattern ERGs in young children with ONH. In the present series, we report flash ERGs and large pattern ERGs to evaluate and distinguish between the function of the inner retinal spiking cells and the more distal retinal layers in ONH.
Materials and methods
Subjects
A sequential series of 48 subjects (20 girls and 28 boys) recruited to the CHLA prospective study of prenatal and clinical risk factors of ONH are reported here. Inclusion criteria are referral before 3 years of age and a small optic nerve in at least one eye defined by a ratio of less than 0.3 between the disk diameter (DD) and the disk macula (DM) distance (DD/DM).7 Parents gave fully informed consent to participate in the study, which was approved by the hospital ethical committee and conforms to the declaration of Helsinki. A comprehensive protocol of ophthalmology, neuro-radiography, endocrine, and psychometric assessments is carried out annually until 5 years of age. The present report includes electroretinography and ocular biometry collected under sedation at the initial study visit. Normative data collection with a protocol requiring sedation is unethical. Comparisons are made across the range of severity of ONH, including the unaffected eyes of unilateral cases as reference eyes.
The subjects ranged in age from 16 weeks to 37 months of age at the time of ERG testing (median age 14.4 months). Neuro-radiography, including MRI scan data, is available for 31 subjects. Twenty of these subjects (65%) have structural abnormalities in addition to ONH (nine with hypoplasia of the corpus callosum; 11 with absence of the septum pellucidum (including five with both) and five children with other neurological defects). Follow-up data for endocrine function are available for 37 subjects. Nineteen of these children (51%) have hypopituitarism.
Ocular biometry and clinical signs
An experienced paediatric ophthalmologist (MSB) analysed fundus photographs while masked to the identity and clinical status of the subjects. The diameter of the neural tissue of the optic disk (DD) and the distance between the temporal margin of the disk and the centre of the macula (DM) were measured directly from printed photographs (Figure 1). DD/DM ratios of 0.3 or greater are associated with normal vision and were classed as reference eyes; those between 0.3 and 0.15 were classed as moderate ONH; and a DD/DM of 0.15 or less was severe ONH.6 The observer also judged the retinal vessels for tortuosity (ranked within normal limits, tortuous veins only or tortuous veins and arteries) and assessed the presence or absence of double ring sign. Optic nerve colour was classified as normal, temporal pallor, or overall pallor.
ERG methods
Subjects had full mydriasis and cycloplegia with three drops in each eye of a combined solution (1% cyclopentolate and 2.5% phenylepharine). Following this, they were sedated with chloral hydrate (100 mg/kg, orally or by rectal suppository) for electroretinography, fundus examination, and fundus photography. A nurse monitored vital signs until recovery. In all subjects, nystagmus was completely suppressed during sedation.
Conjunctival electrodes (DTL fibre or HK loop electrodes) were inserted and referenced to a silver–silver chloride skin electrode near the ipsilateral outer canthus (Figure 2).29, 30 These two electrode types give very similar pattern ERG data.31 An indifferent electrode was attached to the earlobe or mastoid. Flash ERGs were amplified 5000 times and pattern ERGs were amplified 20 000 times. The amplifier bandwidth was 1–100 Hz.
Flash stimulation
ERGs were recorded monocularly with a light-excluding black patch covering the opposite eye. The eyelid was held open and non-standard brief flash (1.3 cd s/m2) was delivered to the posterior pole at 2.03 Hz from a large screen monitor (25 × 32 degrees). Standard full-field ERG stimuli were not available when this cohort was tested. Data from 100 flashes were stored for off-line processing.
Pattern stimulation
Clear optical images for pattern testing were obtained using a correcting optical system. First, the cycloplegic refraction was measured by retinoscopy. A correction incorporating an additional +1.5 dioptres to compensate for the distance to the stimulus was placed before the eye. The subject, in supine position, was then placed under a front surface mirror and the corneal reflection of the stimulus was centred in the pupil. The eye was opened manually, interrupted with blinks to maintain corneal hydration. Fixation was monitored throughout testing, and recording was paused whenever adjustments to the position of the head and/or mirror were required to maintain centration in the pupil.
The stimulus was a high contrast (95%) checkerboard array of 48 checks (8 × 6) reversing 2.03 times per second on a large high-resolution screen (32 × 24 degrees), positioned 67 cm from the eye. The checks subtended 4° of visual angle, so four check widths would be positioned between the fovea and the optic disk. Raw data for 320 pattern reversals were digitized and stored for off-line processing on a Neuroscan evoked potential system (Neuroscientific, Herondon, PA, USA).
Signal processing
For each subject, the raw data for each electrode consisted of 320 epochs of 200 ms, following each pattern reversal. Noise, blink artifacts, and shifts in the baseline associated with eye movements were retained in the raw data. The signals were then digitally filtered with a band pass of 1–50 Hz. Next, each epoch was adjusted off line to a zero mean baseline (baseline correction routine, Neurosoft Inc.), and then the mean slope by linear regression was also adjusted to zero (linear detrend, Neurosoft Inc.). Following this processing, epochs still containing artefacts greater than ±80μV were rejected. Two separate averaged pattern ERGs were then produced using half of the retained epochs for each average so that reproducibility could be evaluated. The intraclass correlation statistic (Neurosoft Inc.) was used to objectively measure the related variability of the two pattern ERG averages. This statistic ranges from 0 for dissimilar signals to 1 for identical signals. The authors also rated quality subjectively as excellent, good, fair, or poor by observation of the superimposed averages, while masked to the clinical status of the subject. Amplitudes and peak times for the P50 and N95 were recorded from the grand average.
Results
Subjects
Twelve cases were classified as unilateral ONH, as the DD/DM was greater than 0.3 in the better eye. Of the 36 bilateral cases, 32 were symmetrical (89%) with less than a 30% difference between DD/DM of the right and left eyes. Spontaneous nystagmus was present in 90% of the bilateral cases, and 45% of the cases were classified as unilateral.
Eyes were classified as follows: 13 reference eyes (DD/DM>0.3 including one borderline case with DD/DM of 0.31 and 0.34 for the right and left eyes, respectively), 46 eyes with moderate ONH (0.15<DD/DM<0.30), 35 eyes with severe ONH (DD/DM≤0.15), and one severely microphthalmic eye that was excluded from the quantitative analysis. Tortuous retinal veins were apparent in 34 eyes with ONH (41.4%) and in none of the reference eyes. Tortuosity of arteries and veins was observed in 18 eyes (Table 1). Tortuous retinal vessels (ranked none, veins only, or all vessels) are more prevalent in those with more severe ONH (Kendall's rank correlation, τ=0.33, P=0.001). Endocrine dysfunction is also more prevalent in subjects with tortuous retinal vessels (χ2=4.7, P=0.03). Of the 37 subjects with known endocrine outcomes, 13/20 (71%) of those treated or monitored for hypopituatrism have tortuous vessels in at least one eye, whereas only 5/17 (28%) with normal vascular appearance in both eyes have clinically significant endocrine dysfunction.
Pallor of the temporal optic nerve head was evident in 32 eyes, including one fellow eye of a unilateral case. An additional 20 eyes with ONH showed pallor of the entire disk, demonstrating the frequent concurrence of optic atrophy and ONH8 (Table 1). The prevalence and extent of optic nerve pallor increased with the severity of ONH (τ=0.24, P<0.001). Double ring sign was evident in 78 eyes (82%) including five of the 13 eyes classified as reference eyes (Table 1). The association between double ring sign and the severity of ONH is also significant (τ=0.33, P<0.001).
There were no differences in the severity of ONH or the presence of the above clinical signs based on the age of the subjects. All eyes had a clinically normal maculae except one eye with severe ONH and mild foveal hypoplasia. The same eye had irregular disk margins, and an additional eye with moderate ONH had mylention in the nerve fibre layer. The DD/DM ratio was estimated in these two cases.
Flash ERGs
Non-standard photopic flash ERGs with clearly reproduced a- and b-waves were recorded in all eyes, except the microphthalmic eye that was not tested (Figure 3). The a-wave showed no association with the severity of ONH. However, b-wave amplitude was significantly smaller in eyes with more severe ONH based on the DD/DM ratio (r2=0.08, P<0.01). Figure 4 demonstrates this association with the group mean amplitudes, although the association is not apparent in individual examples, as the b-wave is variable with considerable overlap between ONH groups. The ratio of the b-wave/a-wave amplitudes also varies with the severity of ONH: mean a:b ratios were 2.0, 1.8, and 1.6 for eyes classified as fellow, moderate, and severe ONH, respectively (τ=0.14, P=0.01). Neither the photopic b-wave amplitude nor the a:b ratio was significantly associated with the presence of vessel tortuosity, endocrine dysfunction, or neuro-radiographical abnormalities, or with the age of the subject. There were no eyes with severely diminished b-waves (negative ERGs) or with very large b-waves using this single stimulus.
Mean values are shown for the a-wave and b-wave amplitudes for eyes classified as severe and moderate ONH and for fellow eyes (DD/DM≤0.15, ≤0.3, and ≥0.3, respectively). B-wave amplitude increases with DD/DM category (P<0.01) but there is no significant association with a-wave amplitude (P>0.1). Bars indicate standard errors.
Large pattern ERGs
Pattern ERGs were completed and technically acceptable in 89/96 eyes. The eyes excluded were one microphthalmic eye, four eyes not tested when a child awoke from sedation (two eyes in one case of moderate bilateral ONH, one eye from a severe bilateral case, and one eye from a moderate bilateral case), and two further eyes with high noise levels (one eye from two separate cases of moderate bilateral ONH). Mean values for the intraclass statistic (indicating the reproducibility) were 0.71, 0.51, and 0.44 for pattern ERGs rated as good, fair, and undetectable, respectively. This intraclass measure of reproducibility shows a strong association with the subjective quality rating (ANOVA, P<0.001). Although this statistic could be useful to quantify reproduction of the pattern ERGs, we found the subjective rating more reliable, as the intraclass value can be artificially high or low when artefact or noise is present in one or both of the pattern ERG averages.
Overall, 65 of the 89 eyes tested had clearly detectable and reproducable pattern ERGs (rated good or fair quality). Thirty-one eyes had P50 to N95 amplitudes greater than 3.5 μV, 34 eyes had clearly detectable pattern ERGs of smaller amplitude, and 24 eyes had undetectable pattern ERGs (Figure 5). All 13 reference eyes had detectable pattern ERGs to large checks (Table 1). In eyes with ONH, pattern ERGs were detectable in 76 and 60% of eyes with moderate and severe ONH, respectively. Thus, undetectable pattern ERGs were more prevalent in eyes with more severe ONH (τ=−0.24, P<0.001 for PERG detected vs DD/DM). The amplitude from P50 to N95 was significantly correlated with the DD/DM ratio (Spearman's ρ=0.26, P<0.02) (Figure 6). The trends for P50 amplitude and for the implicit times of both components did not reach significance. Within each ONH severity category, all aspects of these pattern ERGs were invariant with age.
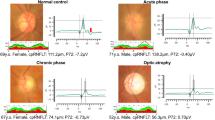
Typical pattern ERGs with chloral hydrate sedation to a 4° alternating checkerboard are illustrated for eyes with severe and moderate ONH and for a fellow eye. Pattern ERG amplitude is associated with the severity of ONH; however, there were large inter-individual variations. Arrow indicates pattern reversal.
Mean values are shown for the pattern ERG P50 and N95 amplitudes for severe ONH, moderate ONH, and for fellow eyes based on the DD/DM classification (≤0.15, ≤0.3, and ≥0.3, respectively). Both P50 and N95 amplitude are significantly associated with the DD/DM category (P<0.05 and P<0.02, respectively). Bars indicate standard errors.
In this population of children with ONH, the prevalence of small or absent pattern ERGs in either eye did not differ between those with and without endocrine dysfunction, or between those with and without other neurological dysfunction (t-tests P>0.4). Vascular tortuosity was, however, strongly associated with small or absent pattern ERGs (τ=0.25, P<0.001 for tortuosity ranked all vessels, veins only, or none vs P50–N95 amplitude). Only one of 15 eyes with tortuosity of both veins and arteries had a large-amplitude pattern ERG (>3.5 μV), whereas large amplitudes were recordable in 44% of eyes with clinically normal vessels (Table 2).
Discussion
The present series of 48 infants show the expected spectrum of ONH severity and of associated conditions, including endocrine dysfunction and neural malformations.4, 5, 32, 33, 34 Although reports of ERGs in ONH are few, they agree that flash ERGs are normal in the majority of cases.17, 18, 19, 20, 21, 34, 35 However, normal ERGs are not universal and a variety of ERG abnormalities have been reported in ONH. Subnormal b-wave amplitude is reported in up to one-third of eyes and, in a few cases, ONH has been associated with absent scotopic b-waves, suggesting retinal dysfunction similar to that in congenital stationary night blindness.20, 21 Severe ERG abnormalities have been reported occasionally in eyes with microphthalmos, colobomata, or other defects.20, 21, 32 In contrast, a few cases of ONH with supranormal b-wave amplitudes have been reported for the scotopic ERG19 or the photopic ERG.35 These could be explained by selective dysfunction involving the depolarizing bipolar cells, or by disinhibition of retinal function in the absence of ganglion cell-mediated feedback.17, 19, 36
The photopic flash ERG recorded in the present series was a non-standard, which limits interpretation to comparisons within the subject group.37 The posterior pole was illuminated evenly by the flashing screen, but the remaining retina was illuminated unevenly with scattered light. The stimulus was below the luminance level for the standard photopic ERG, which is already well below the saturation level for the a-wave. For the photopic b-wave, the luminance response function, or photopic hill, is complex owing to interactions between the hyperpolarizing and depolarizing bipolar cells of the retina.24 In healthy eyes, the flash used in the present study would elicit b-wave amplitudes in the rising phase of this function, where both depolarizing and hyperpolarizing bipolar cells add to the b-wave amplitude. Thus, the association of smaller b-waves with more severe ONH supports the contention that one or both populations of bipolar cells may be involved, but does not isolate specific deficits. Our ongoing studies of the luminance-response function of the full-field photopic ERG will investigate the photopic b-wave more thoroughly.
In alert healthy infants, pattern ERGs can be recorded using a skin electrode on the lower eyelid.38, 39 However, cooperation and good fixation during many stimulus presentations is required so the clinical utility of this strategy is limited.17 Pattern ERG recording without sedation would be unreliable or impossible in subjects with visual impairment and nystagmus. The present study demonstrates a strategy for recording pattern ERGs under sedation in infants and young children with an appropriate optical system. As the procedure requires sedation, we have relied on reference eyes (those with DD/DM ratios within the range associated with normal vision) for comparisons. Clearly, these eyes are not a normal control group; some eyes show definite signs associated with mild ONH. However, the fact that ERGs to large checks were recorded in all 13 of the reference eyes in this series supports the validity of the technique. Technical difficulties, for example, inaccurate refraction or a poorly centred stimulus, could contribute to diminished amplitudes, but poor technique would not completely extinguish ERGs to these large checks.40 We contend that those infants with ONH and undetectable pattern ERGs have severe dysfunction of both the ganglion cells and of the more distal retinal cells.
Overall, the present study demonstrates that the congenital deficiency of ganglion cells in ONH produces the expected effects on the ERG to large checks. Specifically, the N95 is more affected than the P50 and the abnormalities are associated with the severity of the ONH. Pharmacological blockade of ganglion cells and spiking amacrine cells of the inner retina abolished most of the N95 and produced an earlier, diminished P50, indicating a more distal contribution to the P50.24 Similarly, when ganglion cell death is associated with optic atrophy, the N95 is severely diminished and the P50 is moderately diminished and earlier.26, 27, 28, 41, 42, 43
The optimal check size for recording pattern ERGs in adults is between 0.75 and 1.0°, and the available data suggest that larger checks would be optimal for infants.38 For large field sizes, there is very little attenuation of either P50 or N95 amplitude for large check stimuli,28, 44, 45 but there is an increasing proportion of the response, which can be accounted for by local luminance modulation akin to the focal ERG.46 For the present 4° checks, a pattern-specific component is expected but this will derive primarily from stimulation of the outer part of our stimulus field, between 12 and 25° from the fovea.46
Although that diminished or extinguished pattern ERGs are common in ONH, within this cohort there is substantial heterogeneity. In clinically similar cases of ONH, the ERG to large checks ranges from a completely undetectable response to a large-amplitude signal with well-preserved P50 and N95 components. Such heterogeneity suggests that there may be substantial variations in the cellular deficits associated with ONH and, quite likely, different aetiological processes.
There are many possible processes that could cause ganglion cell deficiency. ONH could arise from some initial failure of sufficient cellular differentiation. However, the pathfinding processes for ganglion cells in the retina, optic nerve head and optic chiasm and formation of the retinotopic connections in the brainstem is complex.47, 48, 49 Deficiency in any one of these processes could lead to failure of accurate functional connections and excessive apoptosis in the ganglion cell layer. The relationships between the quantity and function of ganglion cells and the development of the more distal retinal cells are poorly understood. Differences in the timing of the ganglion cell loss could, however, be associated with the functional differences we observe among clinically similar cases of ONH. It is interesting that the present study shows no association between the b-wave and the P50 amplitudes in the same eyes.
The presence of tortuous vessels has led to the theory that the primary lesion in ONH may be vascular.50, 51 In the present study, we report a clear association between vascular tortuosity and retinal dyfunction, as indicated by absent pattern ERGs. This supports a link between vascular morphology and retinal dysfunction in a subset of our cases. Two hypotheses regarding the mechanism for retinal dysfunction in ONH remain viable: retinal dysgenesis may be a primary lesion leading to failure of ganglion cell development, or a primary deficit of ganglion cell development may be associated with retrograde retinal degeneration.
The present series has demonstrated associations between ONH and retinal electrophysiology. Specifically, normal photopic a-waves are evidence that the photoreceptor layer is functional in all of our cases. The mixed results for tests of inner nuclear layer and ganglion cell layer functions suggest that retinal dysfunction is a variable feature of ONH. The strong association between pattern ERG abnormality and tortuous retinal vessels suggests that this vascular sign may be an indicator of retinal dysfunction distal to the ganglion cells.
References
Hotchkiss ML, Green WR . Optic nerve aplasia and hypoplasia. J Pediatr Ophthalmol Strabismus 1979; 16: 225–240.
DeMosier G . Median cranioencephalic dysraphias and olfacto-genital dysplasia. World Neurol 1962; 3: 485–503.
Hoyt CS . Optic nerve hypoplasia, changing perspective. Aust NZ J Ophthalmol 1986; 14: 325–331.
Zeki SM, Dutton GN . Mini review: optic nerve hypoplasia in children. Br J Ophthalmol 1990; 74: 300–304.
Skarf B, Hoyt CS . Optic nerve hypoplasia in children. Association with anomalies of the endocrine and CNS. Arch Ophthalmol 1984; 102: 62–67.
Lambert SR, Hoyt CS, Narahara MH . Optic nerve hypoplasia. Surv Ophthalmol 1987; 32: 1–9.
Borchert M, McCulloch DL, Rother C, Stout AU . Clinical assessment, optic disk measurements and visual-evoked potential in optic-nerve hypoplasia. Am J Ophthalmol 1995; 120: 605–612.
Hoyt CS, Good WV . Do we really know the difference between optic nerve hypoplasia and atrophy? Eye 1992; 6: 201–204.
Zeki SM, Dudgeon J, Dutton GN . Reappraisal of the ratio of disc to macula/disc diameter in optic nerve hypoplasia. Br J Ophthalmol 1991; 75: 538–541.
Borchert MS, McCulloch DL . Clinical value of VEP and disc size in optic nerve hypoplasia. Invest Ophthalmol Vis Sci 1992; 33(Suppl 4): 225.
Waugh MC, Chong WK, Sonksen P . Neuroimaging in children with congenital disorders of the peripheral visual system. Dev Med Child Neurol 1998; 40: 812–819.
Hellstrom A, Wiklund LM, Svensson E . Diagnostic value of magnetic resonance imaging and planimetric measurement of optic disc size in confirming optic nerve hypoplasia. J AAPOS 1999; 3: 104–108.
Barr DB, Weir CR, Purdie AT . An appraisal of the disc–macula distance to disc diameter ratio in the assessment of optic disc size. Ophthal Physiol Opt 1999; 19: 365–375.
Weiss AH, Kelly JP . Acuity, ophthalmoscopy and visually evoked potentials in the prediction of visual outcome in infants with bilateral optic nerve hypoplasia. J AAPOS 2003; 7: 108–115.
Alvarez E, Wakakura M, Khan Z, Dutton GN . The disc–macula to disc diameter ratio: a new test for confirming optic nerve hypoplasia in children. J Pediatr Ophthalmol Strab 1988; 25: 151–154.
Ouvrier RA, Billson F . Optic-nerve hypoplasia — a review. J Child Neurol 1986; 1: 181–188.
Kriss A, Russell-Eggitt I . Electrophysiological assessment of visual pathway function in infants. Eye 1992; 6: 145–153.
Borchert MS, Van Boemel GB, McCulloch DL . Correlation of VEP and ERG with visual outcome in infants with optic nerve hypoplasia. Invest Ophthalmol Vis Sci 2000; 41: 1274.
Sprague JB, Wilson WB . Electrophysiologic findings in bilateral optic nerve hypoplasia. Arch Ophthalmol 1981; 99: 1028–1029.
Cibis GW, Fitzgerald KM . Optic nerve hypoplasia in association with brain anomalies and an abnormal electroretinogram. Doc Ophthalmol 1994; 86: 11–22.
Janáky M, Deak A, Pelle Z, Benedek G . Electrophysiological alterations in patients with optic nerve hypoplasia. Doc Ophthalmol 1994; 86: 247–257.
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA . Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 2004; 45: 3827–3837.
Viswanathan S, Frishman L, Robson JG . The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci 2000; 41: 2797–2810.
Uneo S, Kondo M, Niwa Y, Terasaki H, Miyaki Y . Luminance dependence of neural components that underlies the primate photopic electroretinogram. Invest Ophthalmol Vis Sci 2004; 45: 1033–1040.
Holder GE, Votruba M, Cazrter AC, Bhattacharya SS, Fitzke FW, Moore AT . Electrophysiological findings in dominant optic atrophy (DOA) linking the OPA1 locus on chromosome 3q 28–qter. Doc Ophthalmol 1999; 95: 217–288.
Harrison JM, O’Connor PS, Young RSL, Kincaid M, Bentley R . The pattern ERG in man following resection of the optic nerve. Invest Ophthalmol Vis Sci 1987; 28: 492–499.
Holder GE . Pattern electroretinography (PERG) in an integrated approach to visual pathway diagnosis. Prog Retinal Eye Res 2001; 20: 531–561.
Bach M . Electrophysiological approaches for early detection of glaucoma. Eur J Ophthalmol 2001; 11(Suppl 2): S41–S49.
Dawson WW, Trick GL, Litzkow CA . Improved electrode for electroretinography. Invest Ophthalmol Vis Sci 1989; 18: 988–991.
Hawlina M, Konec B . New non-corneal ‘HK-loop’ electrode for clinical ERG. Doc Ophthalmol 1992; 81: 253–259.
McCulloch DL, van Boemel GB, Borchert MS . Comparison of corneal, conjunctival and skin electrodes for pattern electroretinograms. Doc Ophthalmol 1998; 94: 327–340.
Margolith D, Jan JE, McCormick AQ, Tze WJ, Lapointe J . Clinical spectrum of optic nerve hypoplasia. Review of 51 patients. Dev Med Child Neurol 1984; 71: 427–433.
Siatkowski RM, Sanchez JC, Andrade R, Alvarez A . The clinical, neuroradiographic, and endocrinologic profile of patients with bilateral optic nerve hypoplasia. Ophthalmology 1997; 104: 493–496.
Francoise J, DeRouck A . Electroretinographical study of the hypoplasia of the optic nerve. Ophthalmologica 1976; 172: 308–330.
Kriss A, Lloyd C, Taylor D . Flash ERG and VEP findings in young patients with optic nerve hypoplasia. Int Soc Clin Electrophys Vision XXX Symposium, Vienna, May 1992.
Zucker CL, Dowling JE . Centrifugal fibers synapse on dopaminergic interplexiform cells in the teleost retina. Nature 1987; 330: 166–168.
Marmor MF, Holder GE, Seeliger MW, Yamamoto S . Standard for clinical electroretinography (2004 update). Doc Ophthalmol 2004; 108: 107–114.
Fiorentini A, Pirchio M, Sandini G . Development of retinal acuity in infants evaluated with pattern electroretinography. Hum Neurobiol 1984; 3: 93–95.
Fiorentini A, Trimarchi C . Development of temporal properties of pattern electroretinogram and visual evoked potentials in infants. Vis Res 1992; 32: 1609–1621.
Bach M, Mathieu M . Different effect of dioptric defocus vs light scatter on the pattern electroretinogram (PERG). Doc Ophthalmol 2004; 108: 99–106.
Mashima Y, Oguchi Y . Clinical study of the pattern electroretinogram in patients with optic nerve damage. Doc Ophthalmol 1985; 61: 91–96.
Nesher R, Trick GL . The pattern electroretinogram in retinal and optic nerve disease. Doc Ophthalmol 1991; 77: 225–235.
Bach M, Gerling J, Geiger K . Optic atrophy reduces the pattern electroretinogram for both fine and coarse stimulus patterns. Clin Vis Sci 1992; 7: 327–333.
Török B, Meyer M, Wildberger H . The influence of pattern size on amplitude, latency and wave form of retinal and cortical potentials elicited by checkerboard pattern reversal and stimulus onset-offset. Electroenceph Clin Neurophysiol 1992; 84: 13–19.
Bach M, Holder GE . Check size tuning of the pattern electroretinogram: a reappraisal. Doc Ophthalmol 1996; 92: 193–202.
Thompson DA, Drasdo N . The origins of luminance and pattern responses of the pattern electroretinogram. Int J Psychophysiol 1994; 16: 219–227.
Oster SF, Deiner M, Birgbauer E, Sretavan DW . Ganglion cell axon pathfinding in the retina and optic nerve. Sem Cell Dev Biol 2004; 15: 125–136.
Mann F, Harris WA, Hold CE . New views on retinal axon development: a navigational guide. Int J Dev Biol 2004; 48: 957–964.
Stuermer C, Bastmeyer M . The retinal axon's pathfinding to the optic disk. Prog Neurobiol 2000; 62: 197–214.
Lubinsky MS . Hypothesis: septo-optic dysplasia is a vascular disruption sequence. Am J Med Genet 1997; 69: 235–236.
Hellstrom A, Wiklund LM, Svensson E, Albertsson-Wikland K, Stromland K . Optic nerve hypoplasia with isolated tortuosity of the retinal veins — A marker of endocrinopathy. Arch Ophthalmol 1999; 117: 880–884.
Acknowledgements
This research was supported in part by The One Small Voice Foundation, the NIH NCRR GCRC Grant M01 RR-43, and was performed at the GCRC at Childrens Hospital Los Angeles. Travel costs were supported by the Carnegie Trust for the Universities of Scotland. We thank Wendy McNamara, Angela Groundland, and Caroline Chaplin for assistance with data and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Some of the material in this paper was presented to the Association for Research in Vision and Ophthalmology (ARVO), April 2002
Rights and permissions
About this article
Cite this article
McCulloch, D., Garcia-Fillion, P., van Boemel, G. et al. Retinal function in infants with optic nerve hypoplasia: electroretinograms to large patterns and photopic flash. Eye 21, 712–720 (2007). https://doi.org/10.1038/sj.eye.6702309
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702309
Keywords
This article is cited by
-
Repeated measurements of ERGs and VEPs using chloral hydrate sedation and propofol anesthesia in young children
Documenta Ophthalmologica (2021)
-
Predictive value of N95 waveforms of pattern electroretinograms (PERGs) in children with optic nerve hypoplasia (ONH)
Documenta Ophthalmologica (2017)
-
Comparison of human expert and computer-automated systems using magnitude-squared coherence (MSC) and bootstrap distribution statistics for the interpretation of pattern electroretinograms (PERGs) in infants with optic nerve hypoplasia (ONH)
Documenta Ophthalmologica (2015)
-
L1CAM mutation in association with X-linked hydrocephalus and Hirschsprung’s disease
Pediatric Surgery International (2009)
-
Light-adapted electroretinograms in optic nerve hypoplasia
Documenta Ophthalmologica (2009)