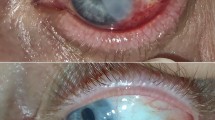
Abstract
Aim:
To compare the efficacy and complications of conjunctival limbal autograft (CLAU) and amniotic membrane transplantation (AMT) vsintraoperative mitomycin C (MMC) and AMT for treatment of recurrent pterygium.
Methods:
Forty eyes of 40 patients with recurrent pterygium underwent CLAU and AMT (20 eyes) or intraoperative MMC (0.02%, 3 min) and AMT (20 eyes). Three eyes (15%) had symblepharon before surgery in each group. Recurrence was compared in each group by using χ2 test.
Results:
No major postoperative complications occurred during 6–19 months of follow-up. In CLAU/AMT group, no pterygium recurrence was observed. Recurrence occurred in four eyes (20%) in MMC/AMT group after 3 and 4 months (P-value=0.035, χ2 test). No recurrence of pterygium or symblepharon was seen in six eyes with recurrent pterygium and symblepharon (three eyes in each group).
Conclusion:
CLAU with AMT seems to be more effective than intraoperative MMC with AMT for treatment of recurrent pterygium.
Similar content being viewed by others
Introduction
There are various surgical procedures for pterygium; however, recurrence remains a major concern. Recurrence after primary excision varies from 24 to 89%.1 Recurrent pterygium is more difficult to treat than primary pterygium because it is often accompanied by increased conjunctival inflammation and accelerated corneal involvement.2
Surgical procedures for treatment of pterygium (including recurrent pterygium) include excision with mitomycin C (MMC), conjunctival autograft, limbal autograft, amniotic membrane transplantation, and lamellar keratoplasty.3
Limbal stem cells may play an important role in the pathogenesis of pterygium.2 There is a general agreement that the limbal stem cells are damaged in the region of pterygium,4 and therefore limbal autograft can be recommended for pterygium treatment. Shimazaki et al5 treated 27 eyes with recurrent pterygium or advanced pterygium with conjunctival limbal autograft (CLAU) and showed a recurrence of 7.4%. This result indicates that CLAU may be effective for treatment of recurrent and advanced pterygium.5
Amniotic membrane has antiadhesive and anti-inflammatory properties and is felt to decrease inflammation, neovascularization, and fibrosis, and promote epithelialization.6
Recurrent pterygium is a condition in which partial stem cell deficiency and inflammation coexist, and limbal autograft and amniotic membrane transplantation are expected to be effective.
Xi XH et al7 compared AMT and AMT/CLAU in treatment of recurrent pterygium or pseudopterygium, and showed lower recurrences in the latter group. Shimazaki et al8 also demonstrated no recurrence in their patients when treated with AMT and CLAU.
MMC use, intraoperatively or postoperatively, is one of adjunctive treatments that can significantly reduce the rate of pterygium recurrence. Recurrence rates are reported to be 5.4–21% when MMC is used alone in treating primary pterygium.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Recurrence rate of recurrent pterygium is still as high as 12.5–19.2% in using MMC alone,12, 13 and 12.5–33.3% when using AMT alone.13, 14 It is therefore expected that there is no single and simple method achieving satisfactory results for complicated cases, especially in eyes with severe symblepharon and motility restriction. Hence, a combined approach may be more successful in treating recurrent advanced pterygium.14, 15
We conducted a study to compare the efficacy and complications of two combined approaches. One approach that has no serious complication is to replace the damaged stem cells, but it is technically difficult and may not be possible in all eyes. Another approach is to inhibit the proliferation of fibrovascular tissue, which is technically easy but has rare serious complications (intraoperative MMC). To the best of our knowledge, this study was the first randomized clinical trial study that compared efficacy and complications of these two treatment approaches in the management of recurrent pterygium.
Materials and methods
This study was conducted in accordance with the declaration of Helsinki Principles. Use of human amniotic membrane for surgery and operative procedure was approved by the ethics committee of Tehran University of Medical Sciences.
This study was a prospective randomized controlled trial. From April 2004 to July 2005, 40 eyes of 40 patients with recurrent pterygium were randomly assigned to receive excision of pterygium followed by either CLAU combined with AMT or AMT combined with intraoperative MMC in Farabi Eye Hospital, Tehran, Iran.
All patients with recurrent pterygium (number of previous surgery ≥1), size of pterygium head on the cornea equal or greater than 2 mm and presence of indications for pterygium surgery such as ocular discomfort refractory to medical treatment or cosmetic purposes were included. Patients with glaucoma, vitreoretinal disorders, pseudopterygium, connective tissue disorders, systemic vasculitis, and diabetes mellitus were excluded from the study. Informed consent was obtained from all subjects before surgery. One surgeon performed all operations.
Surgical procedure
Preserved human amniotic membrane was obtained from a community-based hospital affiliated with Tehran University of Medical Sciences. All necessary procedures including inform consent from pregnant donors, serological tests to rule out hepatitis B, C virus, HIV, human T-cell lymphotropic virus, processing and storage, were performed by the tissue culture unit of the same hospital. Retrobulbar anaesthesia with 2% lidocaine and bupivacaine 0.25 % (2 : 1) was administered to all patients before surgery.
In CLAU group, pterygium was completely resected from the cornea and the body of the pterygium was dissected and excised with Westcott scissors. Abnormal scarring tissue on the corneal surface was polished. Minimal cautery was used to control bleeding. Donor tissue consisted of corneal–limbal–conjunctival tissue that was harvested from supra-temporal part of the same eye or fellow eye. Autograft contained 0.5 mm clear cornea, limbus, and 1 mm of the adjacent bulbar conjunctiva. The length of autograft was equal to the excised limbus at the pterygium site. A front cutting edged calibrated diamond knife was set to 150 μm. The knife was used to make a circumferential corneal incision, parallel to limbus, and two radial incisions, extending from either end of circumferential incision to the limbus. An angled beveled blade (crescent knife) was used to dissect the 150 μm of corneal tissue and emerged beyond the limbus, under conjunctiva. Then one millimeter of conjunctiva that was attached to the corneal–limbal tissue, along the limbal border, was excised. Then, the calibrated diamond knife was set to 100 μm (an allowance of 50 μm was made for the lack of epithelium) and a bed was then fashioned at the recipient site. The donor tissue was sutured into the recipient site with two interrupted 10-0 nylon sutures at either ends of tissue.16 Bare sclera region was covered with amniotic membrane (basement membrane was upside), and then sutured to adjacent normal bulbar conjunctiva with interrupted 10-0 nylon.6 Sutures of amniotic membrane and corneal limbal tissue were removed 2 and 3 weeks after surgery, respectively.
In the second group (AMT with intraoperative MMC 0.02%), procedure was performed as follows: after resection of pterygium head and excision of pterygium body, a weak-cell sponge soaked with 0.02% MMC was placed on the bare sclera for 3 min and then rinsed with normal saline serum for 5 min. The bare sclera was then covered with amniotic membrane (basement membrane was upside) and sutured to adjacent normal bulbar conjunctiva with interrupted 10-0 nylon.6 Sutures of amniotic membrane were removed 2 weeks after surgery.
Postoperative treatment consisted of topical chloramphenicol, topical betamethasone, and artificial tear, 4 times daily for first four weeks. Then betamethasone was changed to fluorometholone, four times daily, for the remaining 2 weeks. All patients were followed up daily until corneal epithelial defect healed, 1 week, 2 weeks, 1, 2, 3, 6 months, then every 3 months. Minimum duration of the follow-up period was 6 months. Recurrence was defined as any fibrovascular tissue that extended over the clear cornea (corneal recurrence). In each follow-up visit, patients were examined for recurrence and any potential postoperative complications such as iritis, persistent corneal epithelial defect, corneal perforation, scleral necrosis, microbial infection, and cataract formation. Student's t-test was used to analyse continuous variables such as age and size of pterygium and χ2 test was used to analyse categorical variables such as gender and recurrence rate. P-value of less than 0.05 was considered significant.
Results
Between April 2004 and July 2005, 40 eyes of 40 patients with recurrent pterygium were randomly assigned to receive excision of pterygium followed by CLAU with AMT (20 eyes) or intraoperative MMC with AMT (20 eyes). The characteristics of patients are shown in Table 1.
Range of patients’ age was 23–74 years. Thirty-eight patients (19 patients in each group) had undergone simple pterygium excision once (bare sclera). Two (one patient in each group, P=1.00, χ2 test) patients had the surgery twice in which the techniques of surgery were simple excision (bare sclera) in the first operation and rotational conjunctival flap in the second operation. There were no intraoperative complications. In the first postoperative week, some patients had mild ocular pain, foreign body sensation, lacrimation, and photophobia. Epithelial defect of cornea healed within 5 days in all eyes. Four patients in the CLAU/AMT group and three patients in the MMC/AMT group showed significant oedema of amniotic membrane in the first few postoperative days that spontaneously resolved few days later. The donor area in the CLAU/AMT group healed without complications in all patients (ie, pseudopterygium or pannus formation). Follow-up ranged from 6 to 19 months. During the follow-up period, pterygium did not recur in any eyes in the CLAU/AMT group, but recurred in four eyes (20%) in the MMC/AMT group (P-value=0.035, χ2 test). Recurrences were seen 3 months postoperatively in two eyes and 4 months postoperatively in two other eyes. Six eyes that had symblpharon before surgery achieved normal eye movement after surgery.
No iritis or rise in intraocular pressure was observed in the MMC/AMT group. Scleral and corneal melting was not noticed in the MMC/AMT group.
Discussion
Limbal autograft acts as a barrier against conjunctival invasion of cornea and it is used to correct limbal dysfunction.2 Although pathogenesis of pterygium is still unclear, it is believed that damage of limbal stem cell may have a role in pterygium formation.2, 17 Consequently, limbal autograft can be recommended for treatment of recurrent pterygium.2, 14, 15 Excision of all subconjunctival fibrovascular tissue might leave a relatively large bare sclera, which requires considerable conjunctiva to cover it. This may not be possible in some patients, but can be easily performed with amniotic membrane.2 Owing to probable antiadhesive, anti-inflammatory and, antifibrotic properties of amniotic membrane, use of amniotic membrane reduces pterygium recurrence.15, 18, 19 Therefore, combined use of limbal autograft and amniotic membrane not only eliminates technical limitation of limbal conjunctival autograft, but may also decrease the recurrence rate of pterygium further. This assumption was evaluated in our study. Recurrence rate of recurrent pterygium is 0–18.2% when using limbal conjunctival autograft in various studies.20, 21, 22, 23, 24, 25, 26, 27 Whereas recurrence rate of limbal conjunctival autograft in some studies is comparable with CLAU/AMT in our study,23, 24, 25 in many others the rate is higher than CLAU/AMT in our cases.20, 21, 22, 26, 27
Another treatment approach for treatment of recurrent pterygium is use of intraoperative MMC on bare sclera. Recurrence rate of this approach is 12.5–19.2%.12, 13 Advantages of this approach is the relatively low recurrence rate and lack of technical difficulty. Disadvantages are rare, but devasting complications including scleral ulceration and necrosis, secondary glaucoma, corneal perforation, cataract formation, iritis, and irreversible damage to stem cells may occur.1, 12 In this study, in addition to intraoperative MMC, AMT was used to evaluate whether the use of amniotic membrane in addition to intraoperative MMC may further reduce recurrence rate of pterygium. Addition of AMT to MMC may also make ocular surface reconstruction possible even after extensive fibrovascular tissue removal.8, 14, 19
Our study included 40 eyes of 40 patients with recurrent pterygium. Twenty eyes were treated with MMC (0.02%, 3 min) and AMT. Another 20 eyes were treated with limbal autograft and AMT. Particular challenging cases were three patients with pterygium and symblepharon in each group. During follow-up period, pterygium recurred in four eyes of the MMC/AMT group (20%), in two patients after 3 months and in two others after 4 months. But no recurrence occurred in the CLAU/AMT group. Recurrence of pterygium was significantly lower in the CLAU/AMT group than the MMC/AMT group (P-value=0.035, χ2 test). No major complications occurred intraoperatively or during follow-up period in either group. Recurrence of symblepharon was not observed in any patients.
To the best of our knowledge, this study was the first clinical trial study that compared efficacy and complications of this two treatment approaches in the management of recurrent pterygium, but each treatment modality has been evaluated alone, or in comparison with other treatment modalities in several studies.
Shimazaki et al5 treated 11 eyes with recurrent pterygium and 16 eyes with advanced pterygium with CLAU. Slight recurrence was noted in only two eyes (7.4%). This result indicated that CLAU might be effective for treatment of recurrent and advanced pterygium. In another study Shimazaki et al8 treated four patients with recurrent pterygium and severe symblepharon with AMT and CLAU. Recurrence of symblpharon was not observed in any patients. Xi et al17 treated 48 eyes of recurrent pterygium or pseudopterygium with AMT and AMT combined CLAU. All cases were followed up for 12 months. No recurrence was found in amniotic membrane with CLAU group. But three recurrences occurred in the AMT group. They concluded that AMT with LSCAT is more effective in the treatment of recurrence or pseudopterygium than AMT. Sangwan et al15 reported the result of a combined surgical procedure of pterygium excision with simultaneous amniotic membrane transplant, conjunctival limbal autograft, and MMC application in the management of two cases of chronically recurring pterygium. Niether of them had recurrence during the follow-up period of 2 years. Yao et al14 also showed that combined intraoperative MMC, amniotic membrane graft and limbal conjunctival autograft were successful approaches for treating multirecurrent pterygia with severe symblepharon to restore the ocular surface integrity and prevent recurrence.
In summary, after reviewing the other studies concerning CLAU/AMT,12, 13, 28 it seems that results of all these studies (including our study) were comparable, and all proposed CLAU/AMT as an effective and safe method in the treatment of recurrent or advanced pterygium.
By considering the recurrence rate of AMT/MMC (20%) in our study and reported recurrence rate in using AMT (12.5–33.3%)13, 14 or intraoperative MMC (12.5–19.2%)12, 13, 28 alone in other studies, it seems that simultaneous use of AMT and MMC may not be more effective than either modality alone. But this impression needs a randomized controlled trial. Thus, even considering the limited number of cases in this study, we concluded that CLAU/AMT is more effective in treatment of recurrent pterygium than MMC/AMT.
References
Young AL, Leung GY, Wong AK, Cheng LL, Lam DS . A randomized trial comparing 0.02% mitomycin C and limbal conjunctival autograft after excision of primary pterygium. Br J ophthalmol 2004; 88: 995–997.
Dekaris I, Gabric N, Karaman Z, Mravicic I, Kastelan S . Limbal–conjunctival autograft transplantation for recurrent pterygium. Eur J Ophthalmol 2002; 12: 177–182.
Buratto L, Phillips RL, Carito G . Pterygium Surgery. Slack Incorporated: Milan, 2000.
Basti S, Rao SK . Current status of limbal conjunctival autograft. Curr Opin Ophthalmol 2000; 11: 224–232.
Shimazaki J, Yang HY, Tsubota K . Limbal autograft transplantation for recurrent and advanced pterygia. Ophthalmic Surg Lasers 1996; 27: 917–923.
Ti SE, Tesng SC . Management of primary and recurrent pterygium using amniotic membrane transplantation. Curr Opin Ophthalmol 2002; 13: 204–212.
Xi XH, Jiang DY, Tang LS . Transplantation of amniotic membrane and amniotic membrane combined with limbal autograft for patients with complicated pterygium (abstract). Hunan Yi Ke Da Xue Xue Bao 2003; 28: 149–151.
Shimazaki J, Shinozaki N, Tsubota K . Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol 1998; 82: 235–240.
Ma DH, See LC, Liau SB, Tsai RJ . Amniotic membrane graft for primary pterygium: comparision with conjunctival autograft and topical mitomycin C treatment. Br J ophthalmol 2000; 84: 973–978.
Yanyali AC, Talu H, Alp BN, Karabas L, Ay GM, Caglar Y . Intraoperative mitomycin C in the treatment of pterygium. Cornea 2000; 19: 471–473.
Avisar R, Gaton DD, Loya N, Appel I, Weinberger D . Intraoperative mitomycin C 0.02% for pterygium: effect of duration of application on recurrence rate. Cornea 2003; 22: 102–104.
Mastropasqua L, Carpineto P, Ciancaglini M, Gallenga EP . Long term results of intraoperative mitomycin C in the treatment of recurrent pterygium. Br J Ophthalmol 1996; 80: 288–291.
Ma DH, See LC, Hwang YS, Wang SF . Comparison of amniotic membrane graft alone or combined with intraoperative mitomycicin C to prevent recurrence after excision of recurrent pterygia. Cornea 2005; 24: 141–150.
Yao YF, Qiu WY, Zhang YM, Tseng SC . Mitomycin C, amniotic membrane transplantation and limbal conjunctival autograft for treating multirecurrent pterygia with symblepharon and motility restriction. Graefes Arch Clin Exp Ophthalmol 2006; 244: 232–236.
Sangwan VS, Murthy SI, Bansal AK, Rao GN . Surgical treatment of chronically recurring pterygium. Cornea 2003; 22: 63–65.
Dua HS, Azuara-Blanco A . Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol 2000; 84: 273–278.
Kwok LS, Coroneo MT . A model for pterygium formation. Cornea 1994; 13: 219–224.
Tekin NF, Kaynak S, Saatci AO, Cingil G . Preserved human amniotic membrane transplantation in the treatment of primary pterygium. Ophthalmic Surg Lasers 2001; 32: 464–469.
Solomon A, Pries RT, Tseng SC . Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology 2001; 108: 449–460.
Dekaris I, Gabric N, Karaman Z, Mravicic I, Kastelan S . Limbal–conjunctival autograft transplantation for recurrent pterygium. Eur J Ophthalmol 2002; 12: 177–182.
Dekaris I, Gabric N, Karaman Z, Mravicic I, Kastelan S, Spoljaric N . Pterygium treatment with limbal–conjunctival autograft transplantation. Coll Antropol 2001; 25: 7–12.
Wong AK, Rao SK, Leung AT, Poon AS, Lam DS . Inferior limbal–conjunctival autograft transplantation for recurrent pterygium. Indian J Ophthalmol 2000; 48: 21–24.
Gris O, Guell JL, del Campo Z . Limbal–conjunctival autograft transplantation for the treatment of recurrent pterygium. Ophthalmology 2000; 107: 270–273.
Rao SK, Lekha T, Mukesh BN, Sitalakshmi G, Padmanabhan P . Conjunctival-limbal autografts for primary and recurrent pterygia: technique and results. Indian J Ophthalmol 1998; 46: 203–209.
Al Fayez M . Limbal vs conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 2002; 109: 1752–1755.
Mutlu FM, Sobaci G, Tatar T, Yildirim E . A comparative study of recurrent pterygium surgery: limbal conjunctival autograft transplantation vs mitomycin C with conjunctival flap. Ophthalmology 1999; 106: 817–821.
Guler M, Sobaci G, Ilker S, Ozturk F, Mutlu FM, Yildirim E . Limbal–conjunctival autograft transplantation in cases with recurrent pterygium. Acta Ophthalmol (Copenhagen) 1994; 72: 721–726.
Kawasaki S, Uno T, Shimamura I, Ohashi Y . Outcome of surgery for recurrent pterygium using intraoperative application of mitomycin C and amniotic membrane transplantation (abstract). Nippon Gank Gakkai Zasshi 2003; 107: 316–321.
Acknowledgements
This study was supported by a grant from Tehran University of Medical Sciences. We thank Dr Ehsanollaah Shafigh Ardestaani for his invaluable help. Ethic approval:The review board and ethical committee of Eye Research Center of Tehran University of Medical Sciences approved the trial. Competing interests:There is no competing interest. This study was supported by a grant from the Tehran University of Medical Science (TUMS). Informed consent:Written Informed consent was obtained from all the patients after complete explanation
Author information
Authors and Affiliations
Corresponding author
Additional information
We state that our only interest is academic and we have no financial interest in this publication
Rights and permissions
About this article
Cite this article
Fallah, M., Golabdar, M., Amozadeh, J. et al. Transplantation of conjunctival limbal autograft and amniotic membrane vs mitomycin C and amniotic membrane in treatment of recurrent pterygium. Eye 22, 420–424 (2008). https://doi.org/10.1038/sj.eye.6702657
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702657
Keywords
This article is cited by
-
The effect of different pterygium surgery techniques on the ocular surface parameters in different durations: a systematic review and meta-analysis
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Safety and efficacy of pterygium extended removal followed by extended conjunctival transplant for recurrent pterygia
International Ophthalmology (2022)
-
Long-term follow-up of transplantation of preserved limbal allograft and amniotic membrane for recurrent pterygium
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)
-
Tissue Engineering and Regenerative Medicine in Iran: Current State of Research and Future Outlook
Molecular Biotechnology (2015)
-
Comparison of conjunctival autograft transplantation and amniotic membrane transplantation for pterygium: a meta-analysis
Graefe's Archive for Clinical and Experimental Ophthalmology (2012)