Abstract
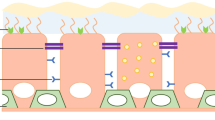
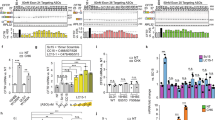
Nonviral vectors have been shown to be a safe and valid alternative to recombinant viruses for gene therapy of cystic fibrosis (CF). Nevertheless, gene transfer efficiency needs to be increased before clinical efficacy is likely in man. One barrier to increased efficacy is normal airway mucus. Using an ex vivo model of sheep tracheal epithelium, we show that this barrier can, in part, be overcome by treatment with the mucolytic agents, Nacystelyn or N-acetylcysteine using either a cationic lipid or a cationic polymer as the gene transfer agent. Further, in vivo application of either Nacystelyn or the anticholinergic glycopyrrolate, both clinically used agents, resulted in increased reporter gene expression in the mouse lung, but no significant correction of the bioelectric defect in CF null mice. These results, whilst unlikely to be sufficient in themselves to achieve clinically relevant gene therapy, may be a further useful step in the attainment of this goal. Gene Therapy (2001) 8, 1380–1386.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zabner J et al. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelium of patients with cystic fibrosis Cell 1993 75: 207–216
Hay JG, McElvaney NG, Herena J, Crystal RG . Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA gene transfer vector Hum Gene Ther 1995 6: 1487–1496
Caplen NJ et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis Nat Med 1995 1: 39–46
Porteous DJ et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis Gene Therapy 1997 4: 210–218
Alton EW et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial Lancet 1999 353: 947–954
Kitson C et al. The extra- and intracellular barriers to lipid and adenovirus-mediated pulmonary gene transfer in native sheep airway epithelium Gene Therapy 1999 6: 534–546
Zabner J et al. Cellular and molecular barriers to gene transfer by a cationic lipid J Biol Chem 1995 270: 18997–19007
Matsui H, Johnson LG, Randell SH, Boucher RC . Loss of binding and entry of liposome–DNA complexes decreases transfection efficiency in differentiated airway epithelial cells J Biol Chem 1997 272: 1117–1126
Fasbender A, Zabner J, Zeiher BG, Welsh MJ . A low rate of cell proliferation and reduced DNA uptake limit cationic lipid-mediated gene transfer to primary cultures of ciliated human airway epithelia Gene Therapy 1997 4: 1173–1180
Pickles RJ et al. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer J Virol 1998 72: 6014–6023
Sandberg JW et al. Improving access to intestinal stem cells as a step toward intestinal gene transfer Hum Gene Ther 1994 5: 323–329
Tomkiewicz RP et al. Mucolytic treatment with N-acetylcysteine L-lysinate metered dose inhaler in dogs: airway epithelial function changes Eur Respir J 1994 7: 81–87
Tomkiewicz RP et al. A comparison of a new mucolytic N-acetylcysteine L-lysinate with N-acetylcysteine: airway epithelial function and mucus changes in dog Pulm Pharmacol 1995 8: 259–265
Dasgupta B, King M . Reduction in viscoelasticity in cystic fibrosis sputum in vitro using combined treatment with Nacystelyn and rhDNase Pediatr Pulmonol 1996 22: 161–166
Thorburn JR et al. Comparison of the effects of atropine and glycopyrrolate on pulmonary mechanics in patients undergoing fiberoptic bronchoscopy Anesth Analg 1986 65: 1285–1289
Stern M et al. The effect of mucolytic agents on gene transfer across a CF sputum barrier in vitro Gene Therapy 1998 5: 91–98
Virella-Lowell I et al. Inhibition of recombinant adeno-associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients Gene Therapy 2000 7: 1783–1789
Pickles RJ et al. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer J Virol 2000 74: 6050–6057
Clamp JR . The properties of the mucus gel layer in the gastrointestinal tract. In: Brostoff J, Challacombe SJ (eds) Food Allergy and Intolerance WB Saunders: London 1987 190–205
Gillissen A et al. Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitro Respir Med 1997 91: 159–168
Pack RJ, Al-Ugaily LH, Morris G . The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscopy study J Anat 1981 132: 71–84
Sehgal A, Presente A, Engelhardt JF . Developmental expression patterns of CFTR in ferret tracheal surface airway and submucosal gland epithelia Am J Respir Cell Mol Biol 1996 15: 122–131
Coyne CB, Kelly MM, Boucher RC, Johnson LG . Enhanced epithelial gene transfer by modulation of tight junctions with sodium caprate Am J Respir Cell Mol Biol 2000 23: 602–609
Yonemitsu Y et al. Efficient gene transfer to airway epithelium using recombinant sendai virus Nat Biotechnol 2000 18: 970–973
Ferrari S et al. Exgen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo Gene Therapy 1997 4: 1100–1106
Gorman CM et al. Efficient in vivo delivery of DNA to pulmonary cells using the novel lipid EDMPC Gene Therapy 1997 4: 983–992
Lee ER et al. Detailed analysis of structures and formulations of cationic lipids for efficient gene transfer to the lung Hum Gene Ther 1996 7: 1701–1717
Smith SN et al. The in vivo effects of Milrinone on the airways of cystic fibrosis mice and human subjects Am J Respir Cell Mol Biol 1999 20: 129–134
Acknowledgements
We would like to thank Tony Phillips for his support and discussions, Karen Palin and Mike Holton for logistics and supply of the EDMPC:Chol complexes. L-PEI was kindly provided by Prof JP Behr (Université L Pasteur, Illkirch, France). SF was supported by a CF Trust Fellowship, EWFWA by Wellcome Trust Senior Clinical Fellowship and the studies by the Cystic Fibrosis Research Trust.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferrari, S., Kitson, C., Farley, R. et al. Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium. Gene Ther 8, 1380–1386 (2001). https://doi.org/10.1038/sj.gt.3301525
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301525
Keywords
This article is cited by
-
The Influence of Functional Carrier Particles (FCPs) on the Molecular Transport Rate Through the Reconstructed Bronchial Mucus: In Vitro Studies
Transport in Porous Media (2015)
-
Pseudomonas aeruginosa infection in cystic fibrosis lung disease and new perspectives of treatment: a review
European Journal of Clinical Microbiology & Infectious Diseases (2013)
-
Advances in Cell and Gene-based Therapies for Cystic Fibrosis Lung Disease
Molecular Therapy (2012)
-
N-acetylcysteine Enhances Cystic Fibrosis Sputum Penetration and Airway Gene Transfer by Highly Compacted DNA Nanoparticles
Molecular Therapy (2011)
-
An ovine tracheal explant culture model for allergic airway inflammation
Journal of Inflammation (2010)