Abstract
OBJECTIVE: To evaluate the efficacy and side effects of an herbal formulation to promote weight loss, as compared to placebo.
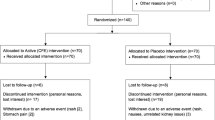
DESIGN: 12-week multicenter double-blind, placebo-controlled, randomized parallel groups design. Study conducted at three clinical sites in New York State. Subjects were randomized to receive either the ‘active’ product or a ‘placebo’ supplement for 12 weeks. Minimal steps were taken to influence lifestyle changes with regard to diet or exercise.
SUBJECTS: 102 overweight/obese (30<BMI≤39.9 kg/m2) volunteers between the ages of 18 and 65 y.
MAIN OUTCOME MEASURES: Weight, percent body fat, fat mass, waist circumference, BMI, blood pressure, and pulse measured at 2 days, 1 week, 2 weeks, 4 weeks, 8 weeks, and 12 weeks postrandomization.
RESULTS: Subjects receiving the ‘active’ treatment experienced, on average, an additional 1.5 kg of weight loss compared with subjects receiving the placebo. In addition, subjects receiving the ‘active’ treatment experienced greater reductions in BMI and waist circumference over the 12-week period. No differences were observed with respect to percent body fat, fat mass, diastolic or systolic blood pressure, pulse, the occurrence of any adverse event, or the occurrence of any presumed treatment-related adverse event. Testing of the study product by two independent laboratories indicated that it had only approximately half of the intended amount of ephedrine alkaloids and caffeine.
CONCLUSIONS: Over the 12-week trial, subjects on the active treatment experienced significantly greater weight loss than subjects on placebo, without an increase in blood pressure, pulse, or the rate of adverse events. These benefits were achieved in the absence of any lifestyle treatment to change dietary or exercise behavior and with lower doses of ephedrine alkaloids and caffeine than those commonly utilized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Flegal KM, Carroll MD, Ogden CL, Johnson CL . Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002; 288: 1728–1732.
Allison DB, Pi-Sunyer FX . Obesity treatment: examining the premises. Endocr Pract 1995; 1: 353–364.
Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB . How many deaths are attributable to obesity? JAMA 1999; 282: 1530–1538.
Fontaine KR, Redden D, Wang C, Westfall A, Allison DB . Years of life lost due to obesity. JAMA 2003; 289: 187–193.
Goldstein DJ . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 1992; 16: 397–415.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403.
Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF . Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med 2003; 138: 383–389.
Yang D, Fontaine KR, Wang C, Allison DB . Con: Weight loss causes increased mortality. Obes Rev 2003; 4: 9–16.
Ayyad C, Andersen T . Long-term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999. Obes Rev 2000; 1: 113–119.
Dhurandhar N, Allison DB . The pharmacologic treatment of obesity. The Econ Neurosci 2000; 2: 42–52.
Allison DB, Saunders S . Three prescription weight loss medications—a review. Weight Control Digest 1999; 9: 827–832.
Haddock CK, Poston WSC, Dill PL, Foreyt JP, Ericsson M . Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord 2002; 26: 262–273.
Bosello O, Carruba MO, Ferrannini E, Rotella CM . Sibutramine lost and found. Eat Weight Disord 2002; 7: 161–167.
Allison DB, Fontaine KR, Heshka S, Mentore JL, Heymsfield SB . Alternative treatments for weight loss: a critical review. Crit Rev Food Sci Nutr 2001; 41: 1–28.
Greenway F . The safety and efficacy of pharmaceutical and herbal caffeine and ephedrine use as a weight loss agent. Obes Rev 2001; 2: 199–211.
Ephedra Working Group. Report of Ephedra Working Group to the National Advisory Council for Complementary and Alternative Medicine, 2003, http://nccam.nih.gov/health/alerts/ephedra/working-group.pdf.
Astrup A, Toubro S, Cannon S, Hein P, Madsen J . Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study. Metabolism 1991; 40: 323–329.
Astrup A, Breum L, Toubro S, Hein P, Quaade F . The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine, and placebo in obese subjects on an energy restricted diet. A double blind trial. Int J Obes Relat Metab Disord 1992; 16: 269–277.
Dulloo AG . Ephedrine, xanthines, and prostaglandin-inhibitors: actions and interactions in the stimulation of thermogenesis. Int J Obes Relat Metab Disord 1993; (Suppl 1): S35–S40.
Greenway FL, Heber D . Herbal and alternative approaches to obesity. In: Bray GA, Bouchard C (eds). Handbook of Obesity. Marcel Dekker, Inc.: New York; 2004. pp 329–358.
Committee on Military Nutrition Research and Food and Nutrition Board. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations. Institute of Medicine National Academy Press: Washington DC; 2001.
Gadbury GL, Coffey CS, Allison DB . Modern statistical methods for obesity trial data: beyond LOCF. Obes Rev 2003; 4: 175–184.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD . SAS System for Mixed Models. SAS Institute: Cary, NC; 1996.
Shekelle P, Hardy M, Morton SC, Maglione M, Suttorp M, Roth E, Jungvig L, Mojica WA, Gagné J, Rhodes S, McKinnon E . Ephedra and ephedrine for weight loss and athletic performance enhancement: clinical efficacy and side effects. Evidence Report/Technology Assessment No. 76 (Prepared Fpage: by Southern California Evidence-based Practice Center, RAND, under Contract No 290-97-0001, Task Order No. 9). AHRQ Publication No. 03-E022. Agency for Healthcare Research and Quality: Rockville, MD, February 2003.
Porta M, Jick H, Habakangas JA . Follow-up study of pseudoephedrine users. Ann Allergy 1986; 57: 340–342.
National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Ephedrine Sulfate (CAS No. 134-72-5) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl Toxicol Program Tech Rep Ser 1986; 307: 1–186.
Gurley BJ, Gardner SF, Hubbard MA . Content versus label claims in ephedra-containing dietary supplements. Am J Health Syst Pharm 2000; 57: 963–969.
Malchow-Moller A, Larsen S, Hey H, Stokholm KH, Juhl E, Quaade F . Ephedrine as an anorectic: the story of the ‘Elsinore pill’. Int J Obes Metab Disord 1981; 5: 183–187.
Ku YR, Chag LY, Ho LK, Lin JH . Analysis of synthetic anti-diabetic drugs in adulterated traditional Chinese medicines by high-performance capillary electrophoresis. J Pharm Biomed Anal 2003; 33: 329–334.
FDA. Consumer alert: FDA plans regulation prohibiting sale of ephedra-containing dietary supplements and advises consumers to stop using these products, 2004, http://www.fda.gov/oc/initiatives/ephedra/december2003/advisory.htmlAccessed: 1/4/2004.
Acknowledgements
Supported by Pinnacle, Inc., the manufacturer of the product tested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coffey, C., Steiner, D., Baker, B. et al. A randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. Int J Obes 28, 1411–1419 (2004). https://doi.org/10.1038/sj.ijo.0802784
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802784
Keywords
This article is cited by
-
Therapeutic potential of herbal medicine for the management of hyperlipidemia: latest updates
Environmental Science and Pollution Research (2022)
-
Effect of Daesiho-tang on obesity with non-alcoholic fatty liver disease: a study protocol for a randomised, double-blind, placebo-controlled pilot trial
Trials (2020)
-
Anti-obesogenic and antidiabetic effects of plants and mushrooms
Nature Reviews Endocrinology (2017)
-
A pilot study to evaluate the effect of Taeumjowi-tang on obesity in Korean adults: study protocol for a randomised, double-blind, placebo-controlled, multicentre trial
Trials (2012)
-
Food Supplements for Body Weight Reduction: A Systematic Review of Systematic Reviews
Obesity (2011)