Abstract
OBJECTIVE: To investigate the implications of technology choice between automated auditory brainstem response (AABR) and transiently evoked otoacoustic emissions (TEOAE) on service provision for a universal newborn hearing screening (UNHS) program.
METHOD: Over a 4-day period, we offered to perform AABR hearing screening on a cohort of 48 well babies in the maternity unit and outpatient department of our busy district hospital. Those parents that consented were asked to sign a consent form and their babies were then screened using the Natus ALGO Model 2e color newborn hearing screener supplied on loan from Neonatal Perspectives Ltd, Manchester, UK. We recorded the patient age at testing, test duration, results obtained (as a pass/refer) and any problems that we experienced with the screening progress, together with parent or user perceived differences between this technology and the current TEOAE screen. A single user carried out all screening. Having collected the AABR data, we then analyzed the implications of the results in relation to service provision in our hospital, utilizing historical data on TEOAE screening.
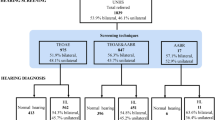
RESULTS: Forty-four mothers, from 48, consented to having their baby screened by AABR and we were able to achieve a result in all 44 babies that we screened. At the standard test criteria of 35 dBnHL, a total 42 babies passed the initial screen in both ears and 2 referred in a single ear only. The test duration was less than 5 minutes for 36 of 44 babies. Applying these results to a model of UNHS generated a per screen cost of £15.98 for a two-stage OAE/AABR program and £14.25 for an AABR-only program. Parents found the AABR test acceptable and we found that being able to discuss the screen and hearing with the parents while the screen was taking place both time-efficient and reassuring to parents. In our experience and using our screening model, the OAE/AABR two-stage approach would have generated 509 infants for second-stage screening (AABR stage) before full audiological follow up and the AABR-only approach would have generated 72 infants for second stage.
CONCLUSIONS: Testing with the Natus ALGO Model 2e color newborn hearing screener proved to be practical, time-, and cost-efficient. The low initial referral rate would not only save money within our hospital, but serves to keep parental anxiety at a minimum. The high tolerance of ambient noise allowed flexibility in our screening location and timing, improving our ability to screen before discharge. In our setting, the adoption of AABR as our primary screen is more practical and less expensive than TEOAE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iley, K., Addis, R. Impact of Technology Choice on Service Provision for Universal Newborn Hearing Screening Within a Busy District Hospital. J Perinatol 20 (Suppl 1), S122–S127 (2000). https://doi.org/10.1038/sj.jp.7200443
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7200443
This article is cited by
-
Specific guidelines for assessing and improving the methodological quality of economic evaluations of newborn screening
BMC Health Services Research (2012)