Abstract
Objective:
The objective of this study is to establish a prevalence rate and characterize risk factors for NICU graduates who demonstrate the AN electrophysiologic profile.
Study Design:
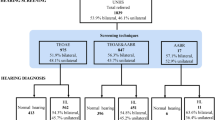
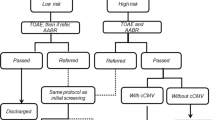
This retrospective study examined infants admitted to the NICU at Kapi'olani Medical Center for Women and Children in Honolulu, HI from 1999 through 2003. Infants were screened with automated ABR. Diagnostic testing and OAE were performed before discharge if the ABR was abnormal. Hospital courses of 24 AN, 71 SNHL and 95 gestational age (GA)-matched control infants with normal hearing were reviewed.
Result:
With a SNHL prevalence of 16.7/1000, the rate for AN was 5.6/1000 NICU infants. Compared to infants with SNHL, infants with AN were significantly younger (GA 28.3±4.8 AN vs 32.9±5.2 weeks SNHL, P<0.0001) and smaller (BW 1318±894 AN vs 1968±1006 g SNHL). Nearly two-thirds of the AN infants were ELBW and had significantly longer hospital stays compared to SNHL infants of the same birth weight group. Exposure to furosemide, aminoglycosides, vancomycin or dexamethasone was associated with increased AN but not SNHL. Peak bilirubin level correlated with SNHL but not AN.
Conclusion:
Low birth weight NICU infants are at significant risk for AN. ELBW infants are at significantly higher risk for both AN and SNHL. Infants admitted to the NICU should be routinely screened by automated ABR and if abnormal, further evaluation should be started before hospital discharge. Early identification of AN will result in better understanding of this disorder and lead to the development of appropriate intervention strategies.
Auditory neuropathy (AN) is a condition in which transmission of sound to the brain is abnormal. This is reflected as an electrophysiologic profile of normal otoacoustic emissions (OAE), with abnormal auditory brainstem evoked responses (ABR). Functionally speech perception is impaired and management strategies remain controversial. AN can be missed if high-risk newborns are screened for hearing loss with only OAE testing. The rate of sensorineural hearing loss (SNHL) in high-risk nursery infants is 10 times greater compared with normal term newborns. Therefore, we hypothesize that infants from the neonatal intensive care unit (NICU) are at significantly higher risk for AN than normal term infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Deltenre P, Mansbach SL, Bozet C, Christinaens F, Barthelmy P, Paulissen D . Auditory neuropathy with preserved cochlear microphonics and secondary loss of otoacoustic emissions. Audiology 1999; 38: 187–195.
Wang Q, Gu R, Han D, Yang W . Familial auditory neuropathy. Laryngoscope 2003; 113: 1623–1629.
Rance G, Beer DE, Cone-Wesson B, Shepherd RK, Dowell RC, King AM et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear 1999; 20: 238–252.
Rubin RA, Balow B, Fisch RO . Neonatal serum bilirubin levels related to cognitive development at ages 4 through 7 years. J Pediatr 1979; 94: 601–604.
Odell GB, Storey GN, Rosenberg LA . Studies in kernicterus III. The saturation of serum proteins with bilirubin during neonatal life and its relationship to brain damage at five years. J Pediatr 1970; 76: 12–21.
Scheidt PC, Mellitis ED, Hardy JB, Drage JS, Boggs TR . Toxicity to bilirubin in neonates: infant development during the first year in relation to maximal neonatal serum bilirubin concentration. J Pediatr 1977; 92: 292–297.
Salamy A, Eldredge L, Tooley WH . Neonatal status and hearing loss in high-risk infants. J Pediatr 1989; 114: 847–852.
Madden C, Rutter M, Hilbert L, Greinwald Jr JH, Choo DI . Clinical and audiological features in auditory neuropathy. Arch Otolaryngol Head Neck Surg 2002; 128: 1026–1030.
Cone-Wesson B, Vohr BR, Sininger YS . Identification of neonatal hearing impairment: infants with hearing loss. Ear Hear 2000; 21: 488–507.
Kraus N, Ozdamar O, Stein L . Absent auditory brain stem response; peripheral hearing loss or brain stem dysfunction? Laryngoscope 1984; 94: 400–406.
Starr A, Picton TW, Sininger Y, Hood L, Berlin C . Auditory neuropathy. Brain 1996; 199: 741–753.
Berg AL, Spitzer JB, Towers HM, Bartosiewicz C, Diamond BE . Newborn hearing screening in the NICU: profile of failed auditory brainstem response/passed otoacoustic emission. Pediatrics 2005; 116: 933–938.
Davis H, Hirsh SK . A slow brainstem response for low-frequency audiometry. Audiology 1979; 18: 445–461.
Stein L, Trembly K, Pasternak J, Banerjee S, Lindemann K, Kraus N . Brainstem abnormalities in neonates with normal otoacoustic emissions. Semin Hear 1996; 17: 197–213.
Deltenre P, Mansbach AL, Bozet C, Clercx A, Hecox KE . Auditory neuropathy: a report on three cases with early onsets and major neonatal illnesses. Electroencephalogr Clin Neurophysiol 1997; 104: 17–22.
Shaia WT, Shapiro SM, Spencer RF . The jaundiced Gunn rat model of auditory neuropathy/dys-synchrony. Laryngoscope 2005; 115: 2167–2173.
Grojean S, Koziel V, Ver P, Daval JL . Bilirubin induces apoptosis via activation of NMDA receptors in developing rat brain neurons. Exp Neurol 2000; 166: 334–341.
Rodrigues CMP, Sola S, Brites D . Bilirubin induces apoptosis via the mitochondrial pathway in developing rat brain neurons. Hepatology 2002; 35: 1186–1195.
Plateel M, Teissier E, Cecchelli R . Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem 1997; 68: 874–877.
Joint Committee on Infant Hearing; American Academy of Audiology; American Academy of Pediatrics; American Speech-Language-Hearing Association; Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2000; 106: 798–817.
Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics 1998; 101: E7.
O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver III RG et al. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator-dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics 1999; 104: 15–21.
Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed 2000; 83: F177–F181.
Murphy BP, Inder TE, Huppi TS, Warfield S, Zientara GP, Kikinis R et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics 2001; 107: 217–221.
Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 2001; 344: 95–101.
Rybak LP . Furosemide ototoxicity: clinical and experimental aspects. Laryngoscope 1985; 95: 1–14.
Iwamoto LM, Fujiwara N, Nakamura KT, Wada RK . Na-K-2Cl cotransporter inhibition impairs human lung cellular proliferation. Am J Physiol (Lung Cell Mol Physiol) 2004; 287: L510–L514.
de Hoog M, van Zanten BA, Hop WC, Overbosch E, Weisglas-Kuperus N, van den Anker JN . Newborn hearing screening: tobramycin and vancomycin are not risk factors for hearing loss. J Pediatr 2003; 142: 41–46.
Rais-Bahrami K, Majd M, Veszelovsky E, Short BL . Use of furosemide and hearing loss in neonatal intensive care survivors. Am J Perinatol 2004; 21: 329–332.
Cone-Wesson B . Auditory neuropathy: evaluation and habilitation of a hearing disability. Infants Young Child 2004; 17: 69–81.
Berlin CI, Hood L, Morlet T, Rose K, Brashears S . Auditory neuropathy/dys-synchrony: diagnosis and management. MRDD Res Rev 2003; 9: 225–231.
Acknowledgements
We thank Ms Patty Iwamoto from the University of Hawaii Clinical Research Center for help with data collection and Drs Randal Wada, Kenneth Nakamura and Charles Neal for their advice and review of the manuscript. This investigation was supported by Hawaii Pacific Health Center for Health Outcomes and by a Research Centers in Minority Institutions award, P20 RR011091, from the National Center for Research Resources, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xoinis, K., Weirather, Y., Mavoori, H. et al. Extremely low birth weight infants are at high risk for auditory neuropathy. J Perinatol 27, 718–723 (2007). https://doi.org/10.1038/sj.jp.7211803
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211803
Keywords
This article is cited by
-
Choline supplementation mitigates effects of bilirubin in cerebellar granule neurons in vitro
Pediatric Research (2024)
-
Prevalence and Auditory Characteristics of Auditory Neuropathy Spectrum Disorder in Adult Population with Sensory Neural Hearing Loss: A Hospital Based Study in South India
Indian Journal of Otolaryngology and Head & Neck Surgery (2023)
-
Comparison of Otoacoustic Emission (OAE) and Brainstem Evoked Response Audiometry (BERA) in High Risk Infants and Children under 5 Years of Age for Hearing Assessment in Western India: A Modification in Screening Protocol
Indian Journal of Otolaryngology and Head & Neck Surgery (2022)
-
Supra-Threshold Hearing Sensitivity Disorders and Mild Permanent Hearing Loss: Neglected Cause of Hidden Hearing Loss and Speech Defects
Indian Journal of Otolaryngology and Head & Neck Surgery (2022)
-
Bilirubin inhibits lipid raft dependent functions of L1 cell adhesion molecule in rat pup cerebellar granule neurons
Pediatric Research (2021)