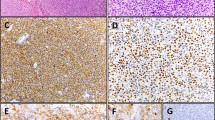
Abstract
Mucosa-associated lymphoid tissue (MALT) lymphoma is a heterogeneous form of a B-cell non-Hodgkin's lymphoma with extranodal location. The gastrointestinal tract is the most common site of disease, but involvement of multiple other organ systems has been documented. Four translocations, t(11;18)(q21;q21), t(1;14)(p22;q32), t(14;18)(q32;q21) and t(3;14)(p13;q32), are specifically associated with MALT lymphoma. Remarkably, the genes targeted by at least three of these translocations are involved in one and the same pathway, leading to the activation of nuclear factor-κB (NF-κB). This review presents MALT lymphoma as a model of how sustained inflammation increases the risk of genotoxic insults and how these genetic events initiate oncogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jaffe E, Harris N, Stein H, Vardiman J . World Health Organisation Classification of Tumours: Pathology and Genetics: Tumours of Haemopoietic and Lymphoid Tissues. IAR Press: Lyon, France, 2004, 157–161.
Suarez F, Lortholary O, Hermine O, Lecuit M . Infection-associated lymphomas derived from marginal-zone B cells: a model of antigen-driven lymphoproliferations. Blood 2006; 107: 3034–3044.
Baens M, Steyls A, Dierlamm J, De Wolf-Peeters C, Marynen P . Structure of the MLT gene and molecular characterization of the genomic breakpoint junctions in the t(11;18)(q21;q21) of marginal zone B-cell lymphomas of MALT type. Genes Chromosomes Cancer 2000; 29: 281–291.
Streubel B, Lamprecht A, Dierlamm J, Cerroni L, Stolte M, Ott G et al. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood 2003; 101: 2335–2339.
Willis TG, Jadayel DM, Du MQ, Peng HZ, Perry AR, Abdul-Rauf M et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 1999; 96: 35–45.
Wlodarska I, Veyt E, De Paepe P, Vandenberghe P, Nooijen P, Theate I et al. FOXP1, a gene highly expressed in a subset of diffuse large B-cell lymphoma, is recurrently targeted by genomic aberrations. Leukemia 2005; 19: 1299–1305.
Thome M . CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol 2004; 4: 348–359.
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N et al. Helicobacter pylori infection and the risk of gastric-carcinoma. N Engl J Med 1991; 325: 1127–1131.
Wotherspoon AC, Ortizhidalgo C, Falzon MR, Isaacson PG . Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338: 1175–1176.
Doglioni C, Wotherspoon AC, Moschini A, Deboni M, Isaacson PG . High-incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339: 834–835.
Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330: 1267–1271.
Hussell T, Isaacson PG, Crabtree JE, Spencer J . The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid-tissue to Helicobacter-pylori. Lancet 1993; 342: 571–574.
Enno A, O'Rourke J, Braye S, Howlett R, Lee A . Antigen-dependent progression of mucosa-associated lymphoid tissue (MALT)-type lymphoma in the stomach – effects of antimicrobial therapy on gastric MALT lymphoma in mice. Am J Pathol 1998; 152: 1625–1632.
Wotherspoon AC, Doglioni C, Diss TC, Pan LX, Moschini A, Deboni M et al. Regression of primary low-grade B-cell gastric lymphoma of Mucosa-associated lymphoid-tissue type after eradication of Helicobacter pylori. Lancet 1993; 342: 575–577.
Royer B, CazalsHatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T et al. Lymphomas in patients with Sjogren's syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997; 90: 766–775.
Derringer GA, Thompson LDR, Frommelt RA, Bijwaard KE, Heffess CS, Abbondanzo SL . Malignant lymphoma of the thyroid gland – a clinicopathologic study of 108 cases. Am J Surg Pathol 2000; 24: 623–639.
Tierens A, Delabie J, Pittaluga S, Driessen A, De Wolf-Peeters C . Mutation analysis of the rearranged immunoglobulin heavy chain genes of marginal zone cell lymphomas indicates an origin from different marginal zone B lymphocyte subsets. Blood 1998; 91: 2381–2386.
Du M, Diss TC, Xu C, Peng H, Isaacson PG, Pan L . Ongoing mutation in MALT lymphoma immunoglobulin gene suggests that antigen stimulation plays a role in the clonal expansion. Leukemia 1996; 10: 1190–1197.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420: 860–867.
Goossens T, Klein U, Kuppers R . Frequent occurrence of deletions and duplications during somatic hypermutation: Implications for oncogene translocations and heavy chain disease. Proc Natl Acad Sci USA 1998; 95: 2463–2468.
Sagaert X, Laurent M, Baens M, Wlodarska I, De Wolf-Peeters C . MALT1 and BCL10 aberrations in MALT lymphomas and their effect on the expression of BCL10 in the tumour cells. Mod Pathol 2006; 19: 225–232.
Streubel B, Simonitsch-Klupp I, Mullauer L, Lamprecht A, Huber D, Siebert R et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia 2004; 18: 1722–1726.
Starostik P, Patzner J, Greiner A, Schwarz S, Kalla J, Ott G et al. Gastric marginal zone B-cell lymphomas of MALT type develop along 2 distinct pathogenetic pathways. Blood 2002; 99: 3–9.
Liu HX, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye HT, Molina T, Bouhnik Y et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357: 39–40.
Ye HT, Liu HX, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood 2003; 102: 1012–1018.
Roy N, Deveraux QL, Takahashi R, Zhou O, Ambrosini G, Altieri D et al. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. Blood 1997; 90: 2645.
Uren AG, O'Rourke K, Aravind L, Pisabarro MT, Seshagiri S, Koonin EV et al. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 2000; 6: 961–967.
Liu HX, Hamoudi RA, Ye HT, Ruskone-Fourmestraux A, Dogan A, Isaacson PG et al. t(11;18)(q21;q21) of mucosa-associated lymphoid tissue lymphoma results from illegitimate non-homologous end joining following double strand breaks. Br J Haematol 2004; 125: 318–329.
Ye HT, Dogan A, Karran L, Willis TG, Chen LL, Wlodarska I et al. BCL10 expression in normal and neoplastic lymphoid tissue – nuclear localization in MALT lymphoma. Am J Pathol 2000; 157: 1147–1154.
Ruland J, Duncan GS, Elia A, Barrantes ID, Nguyen L, Plyte S et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κkappa B and neural tube closure. Cell 2001; 104: 33–42.
Xue LQ, Morris SW, Orihuela C, Tuomanen E, Cui XL, Wen RR et al. Defective development and function of Bcl10-deficient follicular, marginal zone and B1B cells. Nat Immunol 2003; 4: 857–865.
Lucas P, Yonezumi M, Inohara N, McAllister-Lucas L, Abazeed M, Chen F et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-κkappa B signaling pathway. J Biol Chem 2001; 276: 19012–19019.
Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A . T(3;4)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia 2005; 19: 652–658.
Banham AH, Beasley N, Campo E, Fernandez PL, Fidler C, Gatter K et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res 2001; 61: 8820–8829.
Katoh M, Katoh M . Human FOX gene family (Review). Int J Oncol 2004; 25: 1495–1500.
Sagaert X, De Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol 2006; 24: 2490–2497.
Hayden MS, Ghosh S . Signaling to NF-kappa B. Genes Dev 2004; 18: 2195–2224.
Ruefli-Brasse AA, French DM, Dixit VM . Regulation of NF-κ B-dependent lymphocyte activation and development by paracaspase. Science 2003; 302: 1581–1584.
Ruland J, Duncan GS, Wakeham A, Mak TW . Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 2003; 19: 749–758.
Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κ B activation. Nat Immunol 2002; 3: 836–843.
Che TJ, You Y, Wang DH, Tanner MJ, Dixit VM, Lin X . MALT1/paracaspase is a signaling component downstream of CARMA1 and mediates T cell receptor-induced NF-κ B activation. J Biol Chem 2004; 279: 15870–15876.
Sun LJ, Deng L, Ea CK, Xia ZP, Chen ZJJ . The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell 2004; 14: 289–301.
Stilo R, Liguoro D, Di JB, Formisano S, Consiglio E, Leonardi A et al. Physical and functional interaction of CARMA1 and CARMA3 with Iκ kinase gamma-NFκB essential modulator. J Biol Chem 2004; 279: 34323–34331.
Zhou HL, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M et al. Bcl10 activates the NF-κ B pathway through ubiquitination of NEMO. Nature 2004; 427: 167–171.
Hara H, Bakal C, Wada T, Bouchard D, Rottapel R, Saito T et al. The molecular adapter Carma1 controls entry of IκB kinase into the central immune synapse. J Exp Med 2004; 200: 1167–1177.
Lee KY, D'Acquisto F, Hayden MS, Shim JH, Ghosh S . PDK1 nucleates T cell receptor-induced signaling complex for NF-κB activation. Science 2005; 308: 114–118.
Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL et al. Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity 2005; 23: 561–574.
Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B et al. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-κB activation. Immunity 2005; 23: 575–585.
Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ . Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-κB activation. Curr Biol 2006; 16: 1666–1671.
Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J et al. Requirement for caspase-8 in NF-κB activation by antigen receptor. Science 2005; 307: 1465–1468.
Zhou HL, Du MQ, Dixit VM . Constitutive NF-κ B activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell 2005; 7: 425–431.
Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV . The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995; 83: 1243–1252.
Park YC, Burkitt V, Villa AR, Tong L, Wu H . Structural basis for self-association and receptor recognition of human TRAF2. Nature 1999; 398: 533–538.
Varfolomeev E, Wayson SM, Dixit VM, Fairbrother WJ, Vucic D . The inhibitor of apoptosis protein fusion c-IAP2/MALT1 stimulates NF-κ B activation independently of TRAF1 and TRAF2. J Biol Chem 2006; 281: 29022–29029.
Baens M, Fevery S, Sagaert X, Noels H, Hagens S, Broeckx V et al. Selective expansion of marginal zone B cells in E mu-API2-MALT1 mice is linked to enhanced I κ B kinase gamma polyubiquitination. Cancer Res 2006; 66: 5270–5277.
Ho L, Davis RE, Conne B, Chappuis R, Berczy M, Mhawech P et al. MALT1 and the API2-MALT1 fusion act between CD40 and IKK and confer NF-κ B-dependent proliferative advantage and resistance against FAS-induced cell death in B cells. Blood 2005; 105: 2891–2899.
Hosokawa Y, Suzuki H, Suzuki Y, Takahashi R, Seto M . Antiapoptotic function of apoptosis inhibitor 2-MALT1 fusion protein involved in t(11;18)(q21;q21) mucosa-associated lymphoid tissue lymphoma. Cancer Res 2004; 64: 3452–3457.
Hu SM, Du MQ, Park SM, Alcivar A, Qu L, Gupta S et al. cIAP2 is a ubiquitin protein ligase for BCL10 and is dysregulated in mucosa-associated lymphoid tissue lymphomas. J Clin Invest 2006; 116: 174–181.
Nakagawa M, Hosokawa Y, Yonezumi M, Izumiyama K, Suzuki R, Tsuzuki S et al. MALT1 contains nuclear export signals and regulates cytoplasmic localization of BCL10. Blood 2005; 106: 4210–4216.
Kuo SH, Chen LT, Yeh KH, Wu MS, Hsu HC, Yeh PY et al. Nuclear expression of BCL10 or nuclear factor kappa B predicts Helicobacter pylori-independent status of early-stage, high-grade gastric mucosa-associated lymphoid tissue lymphomas. J Clin Oncol 2004; 22: 3491–3497.
Franco R, Camacho FI, Caleo A, Staibano S, Bifano D, De RA et al. Nuclear bcl10 expression characterizes a group of ocular adnexa MALT lymphomas with shorter failure-free survival. Mod Pathol 2006; 19: 1055–1067.
Vejabhuti C, Harris GJ, Erickson BA, Nishino H, Chevez-Barrios P, Chang CC . BCL10 expression in ocular adnexal lymphomas. Am J Ophthalmol 2005; 140: 836–843.
Gallardo F, Bellosillo B, Espinet B, Pujol RM, Estrach T, Servitje O et al. Aberrant nuclear BCL10 expression and lack of t(11;18)(q21;q21) in primary cutaneous marginal zone B-cell lymphoma. Hum Pathol 2006; 37: 867–873.
Li C, Inagaki H, Kuo TT, Hu S, Okabe M, Eimoto T . Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol 2003; 27: 1061–1069.
Liu Y, Dong W, Chen L, Zhang P, Qi Y . Characterization of Bcl10 as a potential transcriptional activator that interacts with general transcription factor TFIIB. Biochem Biophys Res Commun 2004; 320: 1–6.
Yeh PY, Kuo SH, Yeh KH, Chuang SE, Hsu CH, Chang WC et al. A pathway for tumor necrosis factor-alpha-induced Bcl10 nuclear translocation. Bcl10 is up-regulated by NF-κB and phosphorylated by Akt1 and then complexes with Bcl3 to enter the nucleus. J Biol Chem 2006; 281: 167–175.
Stoffel A, Chaurushiya M, Singh B, Levine AJ . Activation of NF-κ B and inhibition of p53-mediated apoptosis by API2/mucosa-associated lymphoid tissue 1 fusions promote oncogenesis. Proc Natl Acad Sci USA 2004; 101: 9079–9084.
Yamasaki R, Yokota K, Okada H, Hayashi S, Mizuno M, Yoshino T et al. Immune response in Helicobacter pylori-induced low-grade gastric-mucosa-associated lymphoid tissue (MALT) lymphoma. J Med Microbiol 2004; 53: 21–29.
Izumiyama K, Nakagawa M, Yonezumi M, Kasugai Y, Suzuki R, Suzuki H et al. Stability and subcellular localization of API2-MALT1 chimeric protein involved in t(11;18)(q21;q21) MALT lymphoma. Oncogene 2003; 22: 8085–8092.
Hosokawa Y, Suzuki H, Nakagawa M, Lee TH, Seto M . AP12-MALT1 fusion protein induces transcriptional activation of the API2 gene through NF-κ B binding elements: evidence for a positive feed-back loop pathway resulting in unremitting NF-κ B activation. Biochem Biophys Res Commun 2005; 334: 51–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sagaert, X., De Wolf-Peeters, C., Noels, H. et al. The pathogenesis of MALT lymphomas: where do we stand?. Leukemia 21, 389–396 (2007). https://doi.org/10.1038/sj.leu.2404517
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404517
Keywords
This article is cited by
-
Manifold role of ubiquitin in Helicobacter pylori infection and gastric cancer
Cellular and Molecular Life Sciences (2021)
-
Hematologic malignancies of the gastrointestinal luminal tract
Abdominal Radiology (2020)
-
Epidemiological characterization, genetic alterations of Helicobacter pylori infection in chronic gastric disorder and prognostic values of heterozygosity loss in chromosome 3p
Molecular Biology Reports (2019)
-
Lymphome de MALT colorectal et manifestation orale chez un enfant drépanocytaire noir Africain
Journal Africain d'Hépato-Gastroentérologie (2014)