Abstract
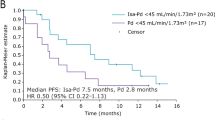
Renal impairment is associated with poor prognosis in multiple myeloma (MM). This subgroup analysis of the phase 3 Assessment of Proteasome Inhibition for Extending Remissions (APEX) study of bortezomib vs high-dose dexamethasone assessed efficacy and safety in patients with relapsed MM with varying degrees of renal impairment (creatinine clearance (CrCl) <30, 30–50, 51–80 and >80 ml min−1). Time to progression (TTP), overall survival (OS) and safety were compared between subgroups with CrCl ⩽50 ml min−1 (severe-to-moderate) and >50 ml min−1 (no/mild impairment). Response rates with bortezomib were similar (36–47%) and time to response rapid (0.7–1.6 months) across subgroups. Although the trend was toward shorter TTP/OS in bortezomib patients with severe-to-moderate vs no/mild impairment, differences were not significant. OS was significantly shorter in dexamethasone patients with CrCl ⩽50 vs >50 ml min−1 (P=0.003), indicating that bortezomib is more effective than dexamethasone in overcoming the detrimental effect of renal impairment. Safety profile of bortezomib was comparable between subgroups. With dexamethasone, grade 3/4 adverse events (AEs), serious AEs and discontinuations for AEs were significantly elevated in patients with CrCl ⩽50 vs >50 ml min−1. These results indicate that bortezomib is active and well tolerated in patients with relapsed MM with varying degrees of renal insufficiency. Efficacy/safety were not substantially affected by severe-to-moderate vs no/mild impairment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Penfield JG . Multiple myeloma in end-stage renal disease. Semin Dial 2006; 19: 329–334.
Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J . Renal function in newly diagnosed multiple myeloma—a demographic study of 1353 patients. The nordic myeloma study group. Eur J Haematol 1994; 53: 207–212.
Knudsen LM, Hjorth M, Hippe E . Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic myeloma study group. Eur J Haematol 2000; 65: 175–181.
Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom medical research council trials between 1980 and 2002—medical research council adult leukaemia working party. J Clin Oncol 2005; 23: 9219–9226.
Alexanian R, Barlogie B, Dixon D . Renal failure in multiple myeloma. Pathogenesis and prognostic implications. Arch Intern Med 1990; 150: 1693–1695.
Clark AD, Shetty A, Soutar R . Renal failure and multiple myeloma: pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev 1999; 13: 79–90.
Bladé J, Fernandez-Llama P, Bosch F, Montoliu J, Lens XM, Montoto S et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998; 158: 1889–1893.
Carlson K . Melphalan 200 mg/m2 with blood stem cell support as first-line myeloma therapy: impact of glomerular filtration rate on engraftment, transplantation-related toxicity and survival. Bone Marrow Transplant 2005; 35: 985–990.
Knudsen LM, Nielsen B, Gimsing P, Geisler C . Autologous stem cell transplantation in multiple myeloma: outcome in patients with renal failure. Eur J Haematol 2005; 75: 27–33.
Torra R, Blade J, Cases A, Lopez-Pedret J, Montserrat E, Rozman C et al. Patients with multiple myeloma requiring long-term dialysis: presenting features, response to therapy, and outcome in a series of 20 cases. Br J Haematol 1995; 91: 854–859.
Bladé J, Rosinol L . Renal, hematologic and infectious complications in multiple myeloma. Best Pract Res Clin Haematol 2005; 18: 635–652.
Jagannath S, Barlogie B, Berenson JR, Singhal S, Alexanian R, Srkalovic G et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impaired renal function. Cancer 2005; 103: 1195–1200.
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78: 21–33.
Sakhuja V, Jha V, Varma S, Joshi K, Gupta KL, Sud K et al. Renal involvement in multiple myeloma: a 10-year study. Ren Fail 2000; 22: 465–477.
Cavo M, Galieni P, Zuffa E, Baccarani M, Gobbi M, Tura S . Prognostic variables and clinical staging in multiple myeloma. Blood 1989; 74: 1774–1780.
Zangari M, Barlogie B, Thertulien R, Jacobson J, Eddleman P, Fink L et al. Thalidomide and deep vein thrombosis in multiple myeloma: risk factors and effect on survival. Clin Lymphoma 2003; 4: 32–35.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420.
Mileshkin L, Biagi JJ, Mitchell P, Underhill C, Grigg A, Bell R et al. Multicenter phase 2 trial of thalidomide in relapsed/refractory multiple myeloma: adverse prognostic impact of advanced age. Blood 2003; 102: 69–77.
San Miguel JF, Lahuerta JJ, Garcia-Sanz R, Alegre A, Blade J, Martinez R et al. Are myeloma patients with renal failure candidates for autologous stem cell transplantation? Hematol J 2000; 1: 28–36.
Kyle RA, Rajkumar SV . Multiple myeloma. N Engl J Med 2004; 351: 1860–1873.
Kane RC, Farrell AT, Sridhara R, Pazdur R . United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 2006; 12: 2955–2960.
Millennium Pharmaceuticals Inc. VELCADE® (bortezomib) for Injection. Prescribing Information. Cambridge, MA, USA, 2007; Issued October 2007, Rev 7.
Mulkerin D, Remick S, Ramanathan R, Hamilton A, Takimoto C, Davies P et al. A dose-escalating and pharmacologic study of bortezomib in adult cancer patients with impaired renal function. J Clin Oncol 2006; 24: 87s.
Mohrbacher A, Levine AM . Reversal of advanced renal dysfunction on bortezomib treatment in multiple myeloma patients. J Clin Oncol 2005; 23: 612s.
Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood 2007; 109: 2604–2606.
Malani AK, Gupta V, Rangineni R . Bortezomib and dexamethasone in previously untreated multiple myeloma associated with renal failure and reversal of renal failure. Acta Haematol 2006; 116: 255–258.
Terpos E, Katodritou E, Tsiftsakis E, Verrou E, Christoulas D, Anagnostopoulos A et al. Cystatin-C: an early marker of renal impairment and an independent predictive factor for survival in multiple myeloma. Reduction post bortezomib therapy. Haematologica 2007; 92: PO–227.
Ludwig H, Drach J, Graf H, Lang A, Meran JG . Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica 2007; 92: 1411–1414.
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352: 2487–2498.
Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 2007; 110: 3557–3560.
Bladé J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma subcommittee of the EBMT. European group for blood and marrow transplant. Br J Haematol 1998; 102: 1115–1123.
Cockcroft DW, Gault MH . Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41.
Acknowledgements
This research was supported by Millennium Pharmaceuticals, Inc. and Johnson & Johnson Pharmaceutical Research & Development LLC. We thank Steve Hill and Rosemary Washbrook for their assistance in drafting the manuscript. Steve Hill is a medical writer and Rosemary Washbrook is a medical editor with Gardiner-Caldwell London. We also thank the APEX Management Team; Dalton W, Anderson K, Harousseau J and San-Miguel J and the APEX Investigators; Abubakr Y, Agura E, Alexanian R, Alsina M, Andre M, Attal M, Avigan D, Baccarani M, Bahlis N, Barbui T, Barton, K, Belch A, Bensinger W, Ben-Yehuda D, Berdeja J, Bjorkstrand B, Bladé J, Boccadoro M, Boue F, Bourhis J, Bron D, Catlett J, Cavenagh J, Cavet J, Chanan-Khan A, Coiffier B, Comenzo R, Craddock C, Dearden C, Delforge M, Densmore J, Doyen C, Durk H, Ehninger G, Einsele H, Engelhardt M, Facon T, Fay J, Fehrenbacher L, Feremans W, Fermand JP, Fernandez H, Giguere J, Glasmacher A, Glass J, Goldschmidt H, Gordon P, Gramatzki M, Gruber A, Gyan E, Hamm J, Hegewisch-Becker S, Huber C, Hulin C, Hussein M, Ifthikharuddin J, Irwin D, Jackson G, Jagannath S, Jagasia M, Jakubowiak A, Klein A, Kobbe G, Kovacs M, Krishnan A, Kropff M, Kuter D, Lacy M, Lenhoff S, Limentani S, Lokhorst H, Lonial S, Ludwig H, Mandelli F, Marie JP, Marsden GJ, Martin T, Mason J, Mavromatis B, Morris C, Morrison V, Nowrousian M, Orlowski R, Pecora A, Phelan J, Posada, J, Rahemtulla A, Rai K, Reece D, Richardson P, Rowe JM, Schilder R, Schmidt W, Schuster M, Sezer O, Shadduck R, Shustik C, Siegel D, Singhal S, Sonneveld P, Sotto JJ, Stadtmauer E, Tarantolo S, Van Droogenbroeck J, Van Oers MH, Vellenga E, Vesole D, Vij R, Zachee P and Zangari M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Statement of originality
The authors confirm that this manuscript contains original material.
Rights and permissions
About this article
Cite this article
San-Miguel, J., Richardson, P., Sonneveld, P. et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia 22, 842–849 (2008). https://doi.org/10.1038/sj.leu.2405087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2405087
Keywords
This article is cited by
-
Acute myeloma kidney and SARS-COV2 infection with dialysis need: never say never - a case report
BMC Nephrology (2023)
-
Prophylactic or pre-emptive therapies to prevent relapse after allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2023)
-
Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA-MM subgroup analysis
Leukemia (2021)
-
Real-world renal function among patients with multiple myeloma in the United States
Blood Cancer Journal (2021)
-
Peripheral neuropathy following bortezomib therapy in multiple myeloma patients: association with cumulative dose, heparanase, and TNF-α
Annals of Hematology (2019)