Abstract
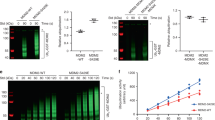
The HDM2 protein is a key regulator of the tumour suppressor, p53. Control of HDM2 function is critical for normal cell proliferation and stress responses, and it is becoming evident that multiple modifications of HDM2 can regulate its function within cells. In this study we show that HDM2 associated with the serine-threonine kinase, Akt, in response to growth factor stimulation of human primary cells. This association was concurrent with phosphorylation of Akt (at Ser 473), and resulted in elevated expression of HDM2 and enhanced nuclear localization. However, analysis of HDM2 proteins mutated at the consensus Akt recognition sites at serines 166 and 186 indicated that modification at these residues was not sufficient for the increased expression of the protein, which was blocked by the PI3 kinase inhibitor LY294002. Tryptic peptide and mutational analyses revealed evidence for an Akt phosphorylation site in HDM2 additional to the two consensus sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ashcroft M, Stephens RM, Hallberg B, Downward J, Kaplan DR . 1999 Oncogene 18: 4586–4597
Ashcroft M, Taya Y, Vousden KH . 2000 Mol. Cell. Biol. 20: 3224–3233
Buschmann T, Fuchs SY, Lee C-G, Pan Z-Q, Ronai Z . 2000 Cell 101: 753–762
Cahilly-Snyder L, Yang-Feng T, Francke U, George DL . 1987 Somat. Cell Mol. Genet. 13: 235–244
Chen JD, Marechal V, Levine AJ . 1993 Mol. Cell. Biol. 13: 4107–4114
Chen J, Lin J, Levine AJ . 1995 Mol. Med. 1: 142–152
Fakharzadeh SS, Trusko SP, George DL . 1991 EMBO J. 10: 1565–1569
Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM . 2000 J. Biol. Chem 275: 8945–8951
Finlay CA . 1993 Mol. Cell. Biol. 13: 301–306
Franke TF, Kaplan DR, Cantley LC, Toker A . 1997 Science 275: 628–630
Freedman DA, Wu L, Levine AJ . 1999 Cell Mol. Life Sci. 55: 96–107
Gotz C, Kartarius S, Scholtes P, Nastainczyk W, Montenarh M . 1999 Eur. J. Biochem. 266: 493–501
Guerra B, Gotz C, Wagner P, Montenarh M, Issinger OG . 1997 Oncogene 14: 2683–2688
Hay TJ, Meek DW . 2000 FEBS Lett. 478: 183–186
Honda R, Yasuda H . 2000 Oncogene 19: 1473–1476
Kane LP, Shapiro VS, Stokoe D, Weiss A . 1999 Curr. Biol. 9: 601–604
Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D . 1999 Proc. Natl. Acad. Sci. USA 96: 14973–14977
Kubbutat MHG, Vousden KH . 1998 Mol. Med. Today 4: 250–256
Leri A, Liu Y, Claudio PP, Kajstura J, Wang X, Wang S, Kang P, Malhotra A, Anversa P . 1999 Am. J. Pathol. 154: 567–580
Leveillard T, Wasylyk B . 1997 J. Biol. Chem. 272: 30651–30661
Lohrum MAE, Ashcroft M, Kubbutat MHG, Vousden KH . 2000 Nature Cell Biol. 2: 179–181
Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O, Moas M, Buschmann T, Ronai Z, Shiloh Y, Kastan MB, Katzir E, Oren M . 2001 Genes Dev. 15: 1067–1077
Mayo LD, Turchi JJ, Berberich SJ . 1997 Cancer Res. 57: 5013–5016
Mayo LD, Donner DB . 2001 Proc. Natl. Acad. Sci. USA 98: 11598–11603
Morrison DK, Heidecker G, Rapp UR, Copeland TD . 1993 J. Biol. Chem. 268: 17309–17316
Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B . 1992 Nature 358: 80–83
Olson DC, Marechal V, Momand J, Chen J, Romocki C, Levine AJ . 1993 Oncogene 8: 2353–2360
Picksley SM, Vojtesek B, Sparks A, Lane DP . 1994 Oncogene 9: 2523–2529
Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T, McMahon M, Oren M, McCormick F . 2000 Cell 103: 321–330
Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ . 1998 EMBO J. 17: 554–564
Shaulian E, Resnitzky D, Shifman O, Blandino G, Amsterdam A, Yayon A, Oren M . 1997 Oncogene 15: 2717–2725
Stott F, Bates SA, James M, McConnell BB, Starborg M, Brookes S, Palmero I, Hara E, Ryan KM, Vousden KH, Peters G . 1998 EMBO J. 17: 5001–5014
Thut CJ, Goodrich JA, Tjian R . 1997 Genes Dev. 11: 1974–1986
Weber JD, Kuo M-L, Bothner B, DiGiammarino EL, Kriwacki RW, Roussel MF, Sherr CJ . 2000 Mol. Cell. Biol. 20: 2517–2528
Woods DB, Vousden KH . 2001 Exp. Cell Res. 264: 56–66
Zhang T, Prives C . 2001 J. Biol. Chem. 276: 29702–29710
Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung M-C . 2001 Nature Cell Biol. 3: 973–982
Acknowledgements
We would like to thank David Stokoe (UCSF) for providing an expression construct containing full-length Akt. We are also grateful to Debbie Morrison and Jurgen Muller (NCI), for helpful discussions and assistance with 2D phosphoamino-acid analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashcroft, M., Ludwig, R., Woods, D. et al. Phosphorylation of HDM2 by Akt. Oncogene 21, 1955–1962 (2002). https://doi.org/10.1038/sj.onc.1205276
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205276
Keywords
This article is cited by
-
Akt: a key transducer in cancer
Journal of Biomedical Science (2022)
-
Regulation of p53 by the 14-3-3 protein interaction network: new opportunities for drug discovery in cancer
Cell Death Discovery (2020)
-
Pim kinases in hematological malignancies: where are we now and where are we going?
Journal of Hematology & Oncology (2014)
-
Novel dual-color immunohistochemical methods for detecting ERG–PTEN and ERG–SPINK1 status in prostate carcinoma
Modern Pathology (2013)
-
Mapping genetic alterations causing chemoresistance in cancer: identifying the roads by tracking the drivers
Oncogene (2013)