Abstract
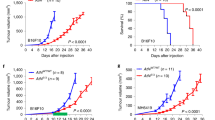
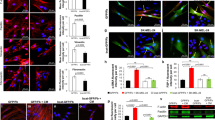
Fibroblast activation protein-α (FAP) is a cell surface serine protease expressed at sites of tissue remodeling in embryonic development. FAP is not expressed by mature somatic tissues except activated melanocytes and fibroblasts in wound healing or tumor stroma. FAP expression is specifically silenced in proliferating melanocytic cells during malignant transformation. To study the role of FAP as a tumor suppressor, the gene for mouse fap was cloned and mutated at the catalytic domain (FAP serine mutant, FSM). We found that expression of FAP or FSM at physiologic levels in mouse melanoma cells abrogated tumorigenicity. Remarkably, the mutant form FSM lacking specific serine protease activity was a more potent tumor suppressor. Tumor rejection was not due to adaptive immune responses because RAG1−/− mice challenged with melanoma cells expressing either FAP or FSM were not tumorigenic. In in vitro assays, FAP or FSM expression restored contact inhibition, led to cell cycle arrest at G0/G1 phase, and increased susceptibility to stress-induced apoptosis. Cell death in FAP+ or FSM+ melanoma cells was readily triggered by depletion of survival factors from the media, leading to subsequent activation of caspases via the intrinsic pathway. These results show that expression of FAP is a tumor suppressor that abrogates tumorigenicity through regulation of cell growth and survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Bennett DC, Cooper PJ and Hart IR . (1987). Int. J. Cancer, 39, 414–418.
Berkelhammer J, Oxenhandler RW, Hook Jr RR and Hennessy JM . (1982). Cancer Res., 42, 3157–3163.
Brown DD, Wang Z, Furlow JD, Kanamori A, Schwartzman RA, Remo BF and Pinder A . (1996). Proc. Natl. Acad. Sci. USA, 93, 1924–1929.
Cheng JD, Dunbrack Jr RL, Valianou M, Rogatko A, Alpaugh RK and Weiner LM . (2002). Cancer Res., 62, 4767–4772.
Desmouliere A . (1995). Cell Biol. Int., 19, 471–476.
Garin-Chesa P, Old LJ and Rettig WJ . (1990). Proc. Natl. Acad. Sci. USA, 87, 7235–7239.
Hearing VJ, Cannon GB, Vieira WD, Jimenez-Atienzar M, Kameyama K and Law LW . (1988). Int. J. Cancer, 41, 275–282.
Hearing VJ, Vieira WD and Law LW . (1985). Int. J. Cancer, 35, 403–409.
Hofmann UB, Westphal JR, Van Muijen GN and Ruiter DJ . (2000). J. Invest. Dermatol., 115, 337–344.
Houghton AN, Albino AP, Cordon-Cardo C, Davis LJ and Eisinger M . (1988). J. Exp. Med., 167, 197–212.
Houghton AN, Real FX, Davis LJ, Cordon-Cardo C and Old LJ . (1987). J. Exp. Med., 165, 812–829.
Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU and Garin-Chesa P . (2003). J. Invest. Dermatol., 120, 182–188.
Morrison ME, Vijayasaradhi S, Engelstein D, Albino AP and Houghton AN . (1993). J. Exp. Med., 177, 1135–1143.
Niedermeyer J, Garin-Chesa P, Kriz M, Hilberg F, Mueller E, Bamberger U, Rettig WJ and Schnapp A . (2001). Int. J. Dev. Biol., 45, 445–447.
Niedermeyer J, Scanlan MJ, Garin-Chesa P, Daiber C, Fiebig HH, Old LJ, Rettig WJ and Schnapp A . (1997). Int. J. Cancer, 71, 383–389.
Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ and Rettig WJ . (1999). J. Biol. Chem., 274, 36505–36512.
Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Ozer HL, Schwab M, Albino AP and Old LJ . (1993). Cancer Res., 53, 3327–3335.
Rettig WJ, Su SL, Fortunato SR, Scanlan MJ, Raj BK, Garin-Chesa P, Healey JH and Old LJ . (1994). Int. J. Cancer, 58, 385–392.
Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, Old LJ and Rettig WJ . (1994). Proc. Natl. Acad. Sci. USA, 91, 5657–5661.
Sviderskaya EV, Wakeling WF and Bennett DC . (1995). Development, 121, 1547–1557.
Tsujimoto H, Nishizuka S, Redpath JL and Stanbridge EJ . (1999). Mol. Carcinogen., 26, 298–304.
Tuxhorn JA, Ayala GE and Rowley DR . (2001). J. Urol., 166, 2472–2483.
Wesley UV, Albino AP, Tiwari S and Houghton AN . (1999). J. Exp. Med., 190, 311–322.
Acknowledgements
TR-M was a recipient of a CRI grant and EVS was supported by Wellcome Trust Grant 064583/KS/JH. This work was supported by the Quentin J Kennedy Foundation, Swim Across America and the Peter J Sharp/Breast Cancer Research Foundation. We would like to acknowledge Rodica Stan, PhD and Kathy Panageas, PhD for their help in the elaboration of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramirez-Montagut, T., Blachere, N., Sviderskaya, E. et al. FAPα, a surface peptidase expressed during wound healing, is a tumor suppressor. Oncogene 23, 5435–5446 (2004). https://doi.org/10.1038/sj.onc.1207730
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207730
Keywords
This article is cited by
-
The role of fibroblast activation protein in health and malignancy
Cancer and Metastasis Reviews (2020)
-
Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment
Oncogene (2019)
-
Integral membrane protease fibroblast activation protein sensitizes fibrosarcoma to chemotherapy and alters cell death mechanisms
Apoptosis (2015)
-
FAP-α (Fibroblast activation protein-α) is involved in the control of human breast cancer cell line growth and motility via the FAK pathway
BMC Cell Biology (2014)
-
Fibroblast-activation protein: valuable marker of cutaneous epithelial malignancy
Archives of Dermatological Research (2014)