Abstract
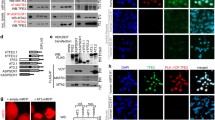
Previously, we reported that the paralogous zinc-finger proteins – CTCF and brother of the regulator of imprinted sites (BORIS), directly contribute to transcriptional regulation of NY-ESO-1 in lung cancer cells. To further examine mechanisms that mediate expression of this cancer-testis gene, we performed software-guided analysis of the NY-ESO-1 promoter region, which revealed several potential Sp1-binding motifs. Sequential 5-aza-2′deoxycytidine/depsipeptide FK228 treatment markedly induced BORIS expression and enhanced nuclear translocation of Sp1 in lung cancer cells. Transient transfection assays using promoter–reporter constructs, as well as gel-shift and chromatin immunoprecipitation experiments revealed that NY-ESO-1 promoter activity coincided with occupancy of the proximal Sp1-binding site in lung cancer cells. Mutations within the Sp1 recognition sequence specifically eliminated binding of Sp1 to this motif in vitro, and markedly diminished NY-ESO-1 promoter activity in vivo. siRNA-mediated inhibition of Sp1 expression decreased NY-ESO-1 promoter activity, whereas knock down of CTCF expression augmented NY-ESO-1 transcription in lung cancer cells. Co-immunoprecipitation experiments indicated that Sp1 physically interacts with BORIS but not with CTCF in vivo. Collectively, these findings suggest that BORIS recruits Sp1 to mediate de-repression of NY-ESO-1 during pulmonary carcinogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Albertini V, Jain A, Vignati S, Napoli S, Rinaldi A, Kwee I et al. (2006). Novel GC-rich DNA-binding compound produced by a genetically engineered mutant of the mithramycin producer Streptomyces argillaceus exhibits improved transcriptional repressor activity: implications for cancer therapy. Nucleic Acids Res 34: 1721–1734.
De Plaen E, Naerhuyzen B, De Smet C, Szikora JP, Boon T . (1997). Alternative promoters of gene MAGE4a. Genomics 40: 305–313.
Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E et al. (2006). NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 95: 1–30.
Hong JA, Kang Y, Abdullaev Z, Flanagan PT, Pack SD, Fischette MR et al. (2005). Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res 65: 7763–7774.
Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A et al. (2004). Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol 25: 461–468.
Huang W, Zhao S, Ammanamanchi S, Brattain M, Venkatasubbarao K, Freeman JW . (2005). Trichostatin A induces transforming growth factor beta type II receptor promoter activity and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1. NF-Y complex. J Biol Chem 280: 10047–10054.
Hung JJ, Wang YT, Chang WC . (2006). Sp1 deacetylation induced by phorbol ester recruits p300 to activate 12(S)-lipoxygenase gene transcription. Mol Cell Biol 26: 1770–1785.
Husmann M, Dragneva Y, Romahn E, Jehnichen P . (2000). Nuclear receptors modulate the interaction of Sp1 and GC-rich DNA via ternary complex formation. Biochem J 352 (Pt 3): 763–772.
James SR, Link PA, Karpf AR . (2006). Epigenetic regulation of X-linked cancer/germline antigen genes by DNMT1 and DNMT3b. Oncogene 25: 6975–6985.
Klenova EM, Morse III HC, Ohlsson R, Lobanenkov VV . (2002). The novel BORIS+CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol 12: 399–414.
Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I et al. (2002). BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci USA 99: 6806–6811.
Nguyen DM, Schrump WD, Chen GA, Tsai W, Nguyen P, Trepel JB et al. (2004). Abrogation of p21 expression by flavopiridol enhances depsipeptide-mediated apoptosis in malignant pleural mesothelioma cells. Clin Cancer Res 10: 1813–1825.
Ohlsson R, Renkawitz R, Lobanenkov V . (2001). CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet 17: 520–527.
Persengiev SP, Raval PJ, Rabinovitch S, Millette CF, Kilpatrick DL . (1996). Transcription factor Sp1 is expressed by three different developmentally regulated messenger ribonucleic acids in mouse spermatogenic cells. Endocrinology 137: 638–646.
Pore N, Liu S, Shu HK, Li B, Haas-Kogan D, Stokoe D et al. (2004). Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1-independent mechanism. Mol Biol Cell 15: 4841–4853.
Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M et al. (2003). Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci USA 100: 4281–4286.
Safe S, Abdelrahim M . (2005). Sp transcription factor family and its role in cancer. Eur J Cancer 41: 2438–2448.
Schrump DS, Nguyen DM . (2005). Targeting the epigenome for the treatment and prevention of lung cancer. Semin Oncol 32: 488–502.
Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC . (2006). Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res 66: 1277–1281.
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ . (2005). Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5: 615–625.
Suske G . (1999). The Sp-family of transcription factors. Gene 238: 291–300.
Suzuki T, Kimura A, Nagai R, Horikoshi M . (2000). Regulation of interaction of the acetyltransferase region of p300 and the DNA-binding domain of Sp1 on and through DNA binding. Genes Cells 5: 29–41.
Thomas K, Sung DY, Yang J, Johnson K, Thompson W, Millette C et al. (2005). Identification, characterization, and functional analysis of sp1 transcript variants expressed in germ cells during mouse spermatogenesis. Biol Reprod 72: 898–907.
Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI et al. (2005). Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res 65: 7751–7762.
Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT et al. (2004). Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res 10: 4109–4117.
Zendman AJ, Ruiter DJ, Van Muijen GN . (2003). Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol 194: 272–288.
Zhang Y, Fatima N, Dufau ML . (2005). Coordinated changes in DNA methylation and histone modifications regulate silencing/derepression of luteinizing hormone receptor gene transcription. Mol Cell Biol 25: 7929–7939.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Kang, Y., Hong, J., Chen, G. et al. Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene 26, 4394–4403 (2007). https://doi.org/10.1038/sj.onc.1210218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210218
Keywords
This article is cited by
-
The function of brother of the regulator of imprinted sites in cancer development
Cancer Gene Therapy (2023)
-
Defining the relative and combined contribution of CTCF and CTCFL to genomic regulation
Genome Biology (2020)
-
BORIS/CTCFL promotes a switch from a proliferative towards an invasive phenotype in melanoma cells
Cell Death Discovery (2020)
-
Differential regulation of MAGE-A1 promoter activity by BORIS and Sp1, both interacting with the TATA binding protein
BMC Cancer (2014)
-
BORIS/CTCFL is an RNA-binding protein that associates with polysomes
BMC Cell Biology (2013)