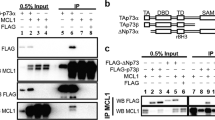
Abstract
Nutlin-3, a small molecule inhibitor, activates p53 by disrupting p53-HDM2 association. In this study, we found that Nutlin-3 suppressed cell growth and induced apoptosis in the absence of wild-type p53, suggesting a p53-independent mechanism for Nutlin-3-induced cell death. Like p53, its homolog p73 transactivates proapoptotic genes and induces cell death. Since HDM2, a key negative regulator of p53, also binds to and inhibits p73, we asked whether p73 could mediate Nutlin-3-induced apoptosis. We demonstrate that Nutlin-3 inhibits endogenous binding between the proapoptotic p73 isoform TAp73α and HDM2 in p53-null cells. Dissociation of p73 and HDM2 leads to increased p73 transcriptional activity with upregulation of p73 target genes noxa, puma and p21, as well as enhanced apoptosis. p73 knockdown by siRNA results in rescue of Nutlin-3-treated cells, indicating that Nutlin-3-induced apoptosis is, at least in part, p73 dependent. In addition, Nutlin-3 treatment increases TAp73α protein levels with prolongation of p73 half-life. These results provide the first evidence that Nutlin-3 disrupts endogenous p73–HDM2 interaction and enhances the stability and proapoptotic activities of p73 and thus, provides a rationale for the use of Nutlin-3 in the large number of human tumors in which p53 is inactivated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ambrosini G, Sambol EB, Carvajal D, Vassilev LT, Singer S, Schwartz GK . (2007). Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene 26: 3473–3481.
Balint E, Bates S, Vousden KH . (1999). Mdm2 binds p73 alpha without targeting degradation. Oncogene 18: 3923–3929.
Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X . (2004). ASPP1 and ASPP2: common activators of p53 family members. Mol Cell Biol 24: 1341–1350.
Bottger V, Bottger A, Garcia-Echeverria C, Ramos YF, van der Eb AJ, Jochemsen AG et al. (1999). Comparative study of the p53-mdm2 and p53-MDMX interfaces. Oncogene 18: 189–199.
Calabro V, Mansueto G, Parisi T, Vivo M, Calogero RA, La Mantia G . (2002). The human MDM2 oncoprotein increases the transcriptional activity and the protein level of the p53 homolog p63. J Biol Chem 277: 2674–2681.
Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de Frias M, Castano E et al. (2006). MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood 107: 4109–4114.
Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE et al. (2000). AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA 97: 5462–5467.
Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W et al. (2000). Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407: 645–648.
Irwin MS . (2004). Family feud in chemosensitivity: p73 and mutant p53. Cell Cycle 3: 319–323.
Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin Jr WG . (2003). Chemosensitivity linked to p73 function. Cancer Cell 3: 403–410.
Iwakuma T, Lozano G . (2003). MDM2, an introduction. Mol Cancer Res 1: 993–1000.
Kadakia M, Slader C, Berberich SJ . (2001). Regulation of p63 function by Mdm2 and MdmX. DNA Cell Biol 20: 321–330.
Kulikov R, Winter M, Blattner C . (2006). Binding of p53 to the central domain of Mdm2 is regulated by phosphorylation. J Biol Chem 281: 28575–28583.
Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ et al. (1996). Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274: 948–953.
Larusch GA, Jackson MW, Dunbar JD, Warren RS, Donner DB, Mayo LD . (2007). Nutlin3 blocks vascular endothelial growth factor induction by preventing the interaction b. Cancer Res 67: 450–454.
Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C et al. (2006). Inactivation of the p53 pathway in retinoblastoma. Nature 444: 61–66.
Ma J, Martin JD, Zhang H, Auger KR, Ho TF, Kirkpatrick RB et al. (2006). A second p53 binding site in the central domain of Mdm2 is essential for p53 ubiquitination. Biochemistry 45: 9238–9245.
Ongkeko WM, Wang XQ, Siu WY, Lau AW, Yamashita K, Harris AL et al. (1999). MDM2 and MDMX bind and stabilize the p53-related protein p73. Curr Biol 9: 829–832.
Patton JT, Mayo LD, Singhi AD, Gudkov AV, Stark GR, Jackson MW . (2006). Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3. Cancer Res 66: 3169–3176.
Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW . (2006). p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9: 45–56.
Shimizu H, Burch LR, Smith AJ, Dornan D, Wallace M, Ball KL et al. (2002). The conformationally flexible S9-S10 linker region in the core domain of p53 contains a novel MDM2 binding site whose mutation increases ubiquitination of p53 in vivo. J Biol Chem 277: 28446–28458.
Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H et al. (2006). Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA 103: 1888–1893.
Van Maerken T, Speleman F, Vermeulen J, Lambertz I, De Clercq S, De Smet E et al. (2006). Small-molecule MDM2 antagonists as a new therapy concept for neuroblastoma. Cancer Res 66: 9646–9655.
VanderBorght A, Valckx A, Van Dun J, Grand-Perret T, De Schepper S, Vialard J et al. (2006). Effect of an hdm-2 antagonist peptide inhibitor on cell cycle progression in p53-deficient H1299 human lung carcinoma cells. Oncogene 25: 6672–6677.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. (2004). In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848.
Wallace M, Worrall E, Pettersson S, Hupp TR, Ball KL . (2006). Dual-site regulation of MDM2 E3-ubiquitin ligase activity. Mol Cell 23: 251–263.
Wang X, Arooz T, Siu WY, Chiu CH, Lau A, Yamashita K et al. (2001). MDM2 and MDMX can interact differently with ARF and members of the p53 family. FEBS Lett 490: 202–208.
Watson IR, Blanch A, Lin DC, Ohh M, Irwin MS . (2006). Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem 281: 34096–34103.
Zeng X, Chen L, Jost CA, Maya R, Keller D, Wang X et al. (1999). MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol 19: 3257–3266.
Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R . (2004). MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem 279: 16000–16006.
Acknowledgements
We thank Lynn Cheng for technical assistance and Ian Watson and members of the Irwin Lab. This project is supported by The James Fund for Neuroblastoma Research at SickKids, the Terry Fox Foundation and the Canadian Cancer Society through the National Cancer Institute of Canada (NCIC). LL is a research fellow of the Terry Fox Foundation through the NCIC. MSI is Canada Research chair in Cancer Biology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Lau, L., Nugent, J., Zhao, X. et al. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene 27, 997–1003 (2008). https://doi.org/10.1038/sj.onc.1210707
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210707
Keywords
This article is cited by
-
Resistance mechanisms to inhibitors of p53-MDM2 interactions in cancer therapy: can we overcome them?
Cellular & Molecular Biology Letters (2021)
-
Reactivation of TAp73 tumor suppressor by protoporphyrin IX, a metabolite of aminolevulinic acid, induces apoptosis in TP53-deficient cancer cells
Cell Division (2018)
-
MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer
Journal of Hematology & Oncology (2017)
-
Nutlin-3 enhances the bortezomib sensitivity of p53-defective cancer cells by inducing paraptosis
Experimental & Molecular Medicine (2017)
-
Effect of low doses of actinomycin D on neuroblastoma cell lines
Molecular Cancer (2016)