Abstract
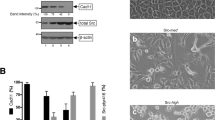
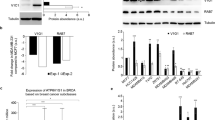
The chimeric oncogene Bcr-Abl is known to induce autonomous motility of leukemic cells. We show here that p210bcr-abl responsible for chronic myelogenous leukemia induces an amoeboid type of motility while p190bcr-abl, associated with acute lymphoid leukemia, induces a rolling type of motility. We previously reported that p210bcr-abl activates RhoA and Rac1, while p190bcr-abl although devoid of a Dbl-homology (DH) domain activates Rac1, but not RhoA. We investigated the regulation of GDP/GTP exchange factor (GEF) activities in the Bcr-Abl complex. For that purpose, different GEF activity mutants of Vav and of Bcr-Abl were constructed and stably transfected in Ba/F3 cells. Using these mutants, we demonstrate that RhoA is exclusively activated by the DH domain of p210bcr-abl, while Rac1 activation is mostly due to Vav. Inhibition of Rac1 by Vav GEF mutant leads to immobilization of cells. Vav depletion using shRNA also induces immobilization of cells and suppression of GTP-bound Rac1. RhoA inactivation induces the specific loss of amoeboid movements. These results suggest that Rac1 activation by Vav triggers the motility of Bcr-Abl-expressing Ba/F3 cells, while the specific amoeboid mode of motility induced by p210bcr-abl is a consequence of RhoA activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bassermann F, Jahn T, Miething C, Seipel P, Bai RY, Coutinho S et al. (2002). Association of Bcr-Abl with the proto-oncogene Vav is implicated in activation of the Rac-1 pathway. J Biol Chem 277: 12437–12445.
Bhatia R, Munthe HA, Verfaillie CM . (1999). Role of abnormal integrin-cytoskeletal interactions in impaired beta1 integrin function in chronic myelogenous leukemia hematopoietic progenitors. Exp Hematol 27: 1384–1396.
Bustelo XR . (2000). Regulatory and signaling properties of the Vav family. Mol Cell Biol 20: 1461–1477.
Chuang TH, Xu X, Kaartinen V, Heisterkamp N, Groffen J, Bokoch GM . (1995). Abr and Bcr are multifunctional regulators of the Rho GTP-binding protein family. Proc Natl Acad Sci USA 92: 10282–10286.
Clark SS, McLaughlin J, Timmons M, Pendergast AM, Ben-Neriah Y, Dow LW et al. (1988). Expression of a distinctive BCR-ABL oncogene in Ph1-positive acute lymphocytic leukemia (ALL). Science 239: 775–777.
Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR . (1997). Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385: 169–172.
Daley GQ, Baltimore D . (1988). Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci USA 85: 9312–9316.
Daley GQ, Van Etten RA, Baltimore D . (1990). Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247: 824–830.
Deininger MW, Goldman JM, Melo JV . (2000). The molecular biology of chronic myeloid leukemia. Blood 96: 3343–3356.
Friedl P, Wolf K . (2003). Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3: 362–374.
Harnois T, Constantin B, Rioux A, Grenioux E, Kitzis A, Bourmeyster N . (2003). Differential interaction and activation of Rho family GTPases by p210bcr-abl and p190bcr-abl. Oncogene 22: 6445–6454.
Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ . (2004). How do Abl family kinases regulate cell shape and movement? Trends Cell Biol 14: 36–44.
Ilaria Jr RL, Van Etten RA . (1996). p210 and p190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem 271: 31704–31710.
Lugo TG, Pendergast AM, Muller AJ, Witte ON . (1990). Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 247: 1079–1082.
Marvaud JC, Stiles BG, Chenal A, Gillet D, Gibert M, Smith LA et al. (2002). Clostridium perfringens iota toxin. Mapping of the Ia domain involved in docking with Ib and cellular internalization. J Biol Chem 277: 43659–43666.
Matsuguchi T, Inhorn RC, Carlesso N, Xu G, Druker B, Griffin JD . (1995). Tyrosine phosphorylation of p95Vav in myeloid cells is regulated by GM-CSF, IL-3 and steel factor and is constitutively increased by p210BCR/ABL. EMBO J 14: 257–265.
Nakashima S, Nozawa Y . (1999). Possible role of phospholipase D in cellular differentiation and apoptosis. Chem Phys Lipids 98: 153–164.
Raftopoulou M, Hall A . (2004). Cell migration: Rho GTPases lead the way. Dev Biol 265: 23–32.
Salgia R, Li JL, Ewaniuk DS, Pear W, Pisick E, Burky SA et al. (1997). BCR/ABL induces multiple abnormalities of cytoskeletal function. J Clin Invest 100: 46–57.
Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F et al. (1998). Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol 143: 1385–1398.
Sawyers CL . (1999). Chronic myeloid leukemia. N Engl J Med 340: 1330–1340.
Stasia MJ, Jouan A, Bourmeyster N, Boquet P, Vignais PV . (1991). ADP-ribosylation of a small size GTP-binding protein in bovine neutrophils by the C3 exoenzyme of Clostridium botulinum and effect on the cell motility. Biochem Biophys Res Commun 180: 615–622.
Unwin RD, Sternberg DW, Lu Y, Pierce A, Gilliland DG, Whetton AD . (2005). Global effects of BCR/ABL and TEL/PDGFRbeta expression on the proteome and phosphoproteome: identification of the Rho pathway as a target of BCR/ABL. J Biol Chem 280: 6316–6326.
Van Etten RA . (1999). Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol 9: 179–186.
Wertheim JA, Forsythe K, Druker BJ, Hammer D, Boettiger D, Pear WS . (2002). BCR-ABL-induced adhesion defects are tyrosine kinase-independent. Blood 99: 4122–4130.
Yamazaki D, Kurisu S, Takenawa T . (2005). Regulation of cancer cell motility through actin reorganization. Cancer Sci 96: 379–386.
Zheng J, Chen RH, Corblan-Garcia S, Cahill SM, Bar-Sagi D, Cowburn D . (1997). The solution structure of the pleckstrin homology domain of human SOS1. A possible structural role for the sequential association of diffuse B cell lymphoma and pleckstrin homology domains. J Biol Chem 272: 30340–30344.
Acknowledgements
We are grateful to Dr Michel Popoff (Unité des Toxines Microbiennes, Institut Pasteur, Paris) for providing us with Ia-C3/Ib. Many thanks to Dr Anne Cantereau for helpful and efficient confocal station technical assistance. We also thank Maria Barreira-Gonzalez and Mélanie Magnan for technical assistance, Pr Jean-Marc Gombert and Angélique Chavineau for FACS analysis and Dr Sylvie Chevalier (Inserm U564, Angers) for providing the Amaxa transfection device. This work was in part supported by grants provided by the ‘Ligue Nationale contre le Cancer, Charente-maritime, Charente and Vienne’. Thomas Daubon is recipient of a fellowship from MENRT. This manuscript is dedicated to the memory of Professor Pierre V Vignais.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Daubon, T., Chasseriau, J., Ali, A. et al. Differential motility of p190bcr-abl- and p210bcr-abl-expressing cells: respective roles of Vav and Bcr-Abl GEFs. Oncogene 27, 2673–2685 (2008). https://doi.org/10.1038/sj.onc.1210933
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210933
Keywords
This article is cited by
-
Leukaemia: a model metastatic disease
Nature Reviews Cancer (2021)
-
Phosphorylation of SOS1 on tyrosine 1196 promotes its RAC GEF activity and contributes to BCR-ABL leukemogenesis
Leukemia (2018)
-
An end-to-end software solution for the analysis of high-throughput single-cell migration data
Scientific Reports (2017)
-
Differential signaling through p190 and p210 BCR-ABL fusion proteins revealed by interactome and phosphoproteome analysis
Leukemia (2017)
-
Contributions of the RhoGEF activity of p210 BCR/ABL to disease progression
Leukemia (2013)