Abstract
Study design: Randomized controlled trial of exercise training in persons with spinal cord injury.
Objective: The purpose of this study was to examine the effects of 9 months of twice-weekly exercise training on strength, arm ergometry performance, and indices of psychological well-being and quality of life.
Setting: Centre for Health Promotion and Rehabilitation, McMaster University, Hamilton, Ontario, Canada.
Methods: Thirty-four men and women (aged 19–65 years) with traumatic spinal cord injury (C4–L1; ASIA A–D) of 1–24 years duration volunteered to participate, and were randomized into exercise (EX; n=21) and control (CON; n=13) groups. Twenty-three subjects (11 EX; 12 CON) successfully completed the 9-month study. Subjects were assessed for one repetition maximum (1RM) strength, arm ergometry performance, and several indices of quality of life and psychological well-being at baseline, 3, 6, and 9 months.
Results: At baseline, there were no significant differences between groups in age, submaximal arm ergometry performance, muscle strength, or psychological well-being. Following training, the EX group had significant increases in submaximal arm ergometry power output (81%; P<0.05), and significant increases in upper body muscle strength (19–34%; P<0.05); no significant changes occurred in CON. Participants in EX reported significantly less pain, stress and depression after training, and scored higher than CON in indices of satisfaction with physical function, level of perceived health and overall quality of life (P<0.05). Exercise adherence (per cent of prescribed sessions attended) in those subjects who completed the 9 months of training was 82.5%.
Conclusions: These results demonstrate that long-term twice-weekly exercise training in this population is feasible, and results in significant gains in both physical and psychological well-being.
Similar content being viewed by others
Introduction
Now that life expectancy for individuals after spinal cord injury (SCI) is approaching that of the able-bodied population,1 the ultimate goal of rehabilitation for this group has shifted from extension of life expectancy to enhancement of independence and quality of life. The importance of regular physical activity on the course and success of rehabilitation after SCI has been increasingly recognized, especially with respect to the physical benefits of exercise for promoting functional independence. Furthermore, with coronary artery disease (CAD) emerging as a major cause of morbidity and mortality in the SCI population,2,3 modifiable risk factors, such as physical inactivity, are attracting increased attention from health care professionals.
There is good evidence from both cross-sectional4,5,6 and longitudinal7,8,9,10 studies that regular physical activity is effective in improving fitness in the SCI population. Unfortunately, the vast majority of longitudinal studies have been relatively short in duration (<24 weeks), restricted to either persons with paraplegia or persons with tetraplegia, and have been rather narrow in their focus on outcome measures. For example, studies have examined the effects of exercise training on either cardiorespiratory function7,9 or muscle strength,12,13 but relatively few have included both strength and cardiovascular endurance measures in their outcomes, and fewer still have included measures of quality of life (QOL). One study, more than two decades ago, attempted to include multiple outcome measures,14 and they reported significant improvements in exercise capacity (31%), maximum oxygen uptake (12%), and strength (19%) after 7 weeks of thrice-weekly training in persons with incomplete paraplegia. Further, the authors reported increased confidence and sense of well-being in the exercising participants after the training, but as there was no mention of the tools used to assess these QOL parameters, the reliability and validity of these results are questionable. More recently, Jacobs et al11 reported significant increases in maximum oxygen uptake (30%) and strength (12% to 30%) following 12 weeks of thrice-weekly circuit training in persons with complete paraplegia, but no indices of QOL were included as outcome measures.
The potential role of exercise training in improving quality of life in persons with SCI cannot be underestimated. Indeed, research in the last 10 years has demonstrated the positive effects of regular exercise on a wide variety of subjective outcomes (eg pain, depression, self-concept, quality of life) among several different impairment groups, including people with osteoarthritis,15 fibromyalgia,16 and HIV/AIDS.17 Despite these positive findings, there have been no systematic studies of the effects of long-term exercise training on psychological well-being and other aspects of QOL in persons with SCI. This is an unfortunate omission, since the increased lifespan in this population means that individuals could be facing many years of below average quality of life if we do not develop and evaluate interventions to improve the various factors that constitute one's QOL (eg psychological wellbeing, pain, mobility, ability to perform activities of daily living).
Given the potential role of regular exercise training in improving both physical fitness and multiple QOL components in persons with SCI, the present randomized controlled trial was designed. Previous exercise studies in this population have been plagued with poor adherence, and it has been suggested that the requirement of attending the standard three exercise sessions per week may be too rigid.7 In response to these observations, the training frequency in the present study was set at two-, rather than three-times per week. We hypothesized that 9 months of twice-weekly exercise training in persons with traumatic SCI would result in increased strength, improved exercise capacity, and positive changes in the QOL dimensions of psychological well-being, pain, perceived health status and overall life satisfaction.
Methods
Subjects
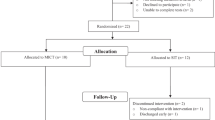
Thirty-four men and women (aged 19–65 years) with traumatic SCI of greater than 1 year duration (1–24 years) volunteered to participate in the study. All patients with acquired SCI at C4 or below (ASIA A-D) were eligible for participation. Exclusion criteria included ischemic heart disease, unstable angina, dysrhythmia, or autonomic dysreflexia, recent osteoporotic fracture, and tracheostomy. Individuals over the age of 45 yearts were required to pass a Stage 1 exercise tolerance test18 in order to be considered eligible for safe participation in the study. The study carried the approval of the McMaster Research Ethics Board (MREB), and subjects provided written informed consent in accordance with MREB guidelines. Following initial testing of all outcome measures, subjects were matched according to age, years post injury (⩽or >10 years) and relative mortality risk (using a scale developed by Coll et al19). Within each matching, participants were randomly assigned to either an exercise or a control condition at a 2 : 1 ratio. This ratio of exercise to control assignment was used as we anticipated more dropouts in the exercise condition than the control condition, and we were concerned that by the end of the study the sample of exercisers might not be sufficiently large to have adequate power for our statistical analyses. To guard against this possibility, 21 participants were assigned to the exercise condition (EX) and 13 served in the wait-list control (CON) condition. All subjects underwent outcome assessment at baseline, 3, 6 and 9 months. Complete subject characteristics are illustrated in Table 1.
Testing of arm ergometry performance
Following baseline measurements of resting heart rate and blood pressure, the discontinuous University of Toronto Arm Crank Protocol was used to assess heart rate/power output relationships at three submaximal wordloads.20 Subjects performed three 5–7 min steady-state workloads on Monark™ arm ergometers at power outputs approximating 40%, 60% and 80% of predicted age-adjusted maximal heart rate. In those subjects without a normal heart rate response to exercise (due to sympathetic decentralization8), Borg ratings of perceived exertion (RPE) of 1, 2, and 4 (10-pt scale21) were used to determine the intensity of the three workloads, an acceptable alternative for exercise prescription in this population.22
Testing of muscle strength
Training and testing of muscle strength took place on a multi-station wheelchair accessible weight training system (Equalizer Exercise Machines, Red Deer, Alberta, Canada) or unilateral wall pulleys (Sammons Preston, Mississauga, Ontario, Canada). In all subjects, the maximum load that could be lifted in one repetition (1RM) was assessed in both limbs for chest press, elbow flexion, and shoulder flexion maneuvers.
Testing of quality of life components
Stress
The 14-item scale Perceived Stress Scale (PSS23) measured the frequency of stressful encounters experienced within a participant's life over the past 4 weeks. Items were rated on a 6-point frequency scale ranging from 1 (all of the time) to 6 (none of the time). The internal consistency of the PSS was adequate at all measurement points as indicated by the Cronbach alpha coefficients (α>0.70).
Depression
The Centre for Epidemiological Studies Depression Scale (CES-D24), was used to measure depressive symptomatology. Respondents indicated how often, over the past week, they experienced each of 20 symptoms. Responses were made on a 4-point likert scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time). Scores on the scale can range from 0–60, with scores ⩾16 generally indicating that one is at increased risk for clinical depression.25 The CES-D had adequate internal consistency at all measurement points (α>0.70).
Physical self-concept
Physical self-concept was assessed using Reboussin and colleagues' 9-item body satisfaction questionnaire,26 which has 2 subscales: satisfaction with physical function and satisfaction with physical appearance. An additional item was added to measure satisfaction with arm muscle strength, as this is a very important aspect of physical functioning for this wheelchair-using population.27 Items were rated on a 7-point satisfaction scale ranging from −3 (very dissatisfied) to +3 (very satisfied). Reliability coefficients for the two subscales were acceptable at all measurement points (ie, α>0.70).
Pain
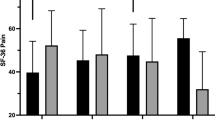
Pain perceptions were measured using the two pain items from the Short-Form 36-Item Health Survey (SF-3628). Using a 6-point scale, participants rated how much pain they experienced and how much pain interfered with normal work in the last 4 weeks (1=none/not at all, 6=very severe/extremely).
Perceived health
Using the single-item SF-36 Reported Health Transition subscale, participants rated their current health compared to their health 1 year ago. Ratings were made on a 5-point scale with verbal anchors on each item ranging from much better to much worse.
Quality of life
Overall satisfaction with fundamental needs of daily living were evaluated using the 11-item perceived quality of life scale (PQOL29) with four additional items: how often you get out of the house, the amount of walking/wheeling you do, your level of sexual activity or lack of sexual activity and the amount and kind of sleep you get. These additional items, often included in other measures of HRQL, reflect factors associated with quality of life for people with SCI,30 and were added to enhance the content validity of the PQOL for the SCI population. Participants indicated their responses to each item using a 7-point satisfaction scale with verbal labels ranging from very dissatisfied (1) to very satisfied (7). The PQOL had adequate internal consistency at all measurement points (α>0.70).
For each of the above-mentioned QOL components, a research assistant presented the questionnaire items verbally to each participant and recorded his/her responses. An interview format was used because not all of the participants were able to use pen and paper independently.
Training intervention
Subjects in the EX group participated in supervised progressive exercise training twice-weekly for 9 months. Exercise sessions were offered either on alternate afternoons or alternate evenings, each session lasting approximately 90–120 min. Subjects began each exercise session with a warm-up (wheeling around the indoor track or low-intensity arm ergometry) and gentle upper extremity stretching. This was followed by the aerobic portion of their training, which involved arm ergometry for 15–30 min, at an intensity of approximately 70% maximum heart rate (or a Borg rating of 3–4). Initially, subjects performed two arm ergometry bouts of 5–10 min; this was gradually increased to two bouts of 15–20 min as training progressed (the two bouts were generally interspersed with the resistance training portion of the exercise session). As the participants and trainers noted decreases in RPE and/or heart rate while performing the arm ergometry bouts, the workload and/or the duration of the bout was progressively increased.
Resistance training was carried out using wall pulley exercises, free weights, and the Equalizer weight machine. Subjects performed their resistance exercises in a circuit set system; training initially consisted of two sets of each exercise (50% of 1RM) and progressed to three sets (70% to 80% of 1RM) after the first 4 weeks. The resistance loads were reassessed approximately every 6 weeks, to ensure a constant training intensity. A wide variety of exercises were available for each of the following muscle groups: forearm/wrist, biceps, back, chest, abdominals, shoulder, triceps, and legs (appropriate subjects only). Subjects were instructed to select two exercises from each of the muscle groups on a given training day, and the training supervisors ensured that these exercises were varied across training sessions.
Subjects in the CON group were offered a bi-monthly education session (together with the EX group) on topics including exercise physiology for persons with SCI, osteoporosis after SCI, and relaxation techniques. CON subjects were also given the opportunity to join the exercise program once the 9-month study commitment was completed.
Statistical analyses
Baseline comparisons between EX and CON groups were performed with one-way (group) or two-way (group × lesion level) analysis of variance ANOVA. To determine if the EX and CON groups differed over time across the course of the study, the data were analyzed with a series of 2-way mixed analysis of covariance (ANCOVA), using a group (EX, CON) × time (3, 6 and 9 months) design with repeated measures on the time factor. For each analysis, the baseline values were used as a covariate to control between-subject and between-group differences at baseline. For some of the physiological outcome measures in which persons with tetraplegia were expected to differ significantly from those with paraplegia (eg HR, power output, strength), a third factor (lesion level: 1=tetra, 2=para) was included in the analyses. Tukey post-hoc tests were used as required to determine specific differences between means. To compare the amount of change experienced by the EX and CON groups at the study end-point, change scores from baseline to 9 months were also analyzed with an ANCOVA that controlled for baseline scores. Statistical significance was set at P<0.05, and throughout the text and figures, data are presented as means±standard deviation.
Results
Program adherence
Of the 21 subjects initially randomized to the EX group, two dropped out within the first 3 months due to illness. Six more dropped out between 3 and 6 months due to transportation difficulties or illness, and two dropped out between 6 and 9 months due to work/school conflicts. The 11 subjects who completed the 9 months of training had an attendance rate (percentage of available sessions attended) of 82.5%.
Only one of the 13 subjects in the CON group dropped out immediately after randomization (dissatisfied with group allocation).
Changes in resting measures of cardiovascular function and arm ergometry performance
There were no differences between groups in resting measures of heart rate, systolic or diastolic pressure at baseline, nor were there any changes in these variables in either group over the 9 months. Subjects with tetraplegia had similar resting HRs as those with paraplegia, but significantly lower systolic and diastolic blood pressures; there was no effect of time or group assignment on these measures. A summary of the resting cardiovascular measures is given in Table 2.
As expected, performance on the discontinuous 3-stage arm-crank protocol was different between individuals with tetraplegia versus paraplegia (main effect for lesion level; P<0.05), however, significant training-induced increases occurred in all subjects in EX (group × time interaction; P<0.05). After 9 months, power output in EX during stage 3 of the test increased by 15 and 20 W in the tetra- and paraplegic groups respectively, representing relative increases of 118% and 45%. The HR response during the three-stage test did not change significantly from baseline to 3 or 6 months, but at the 9 month time point there was a significant increase in HR during stage 3 of the test of almost 20 bpm. This coincided with a significant increase in RPE during stage 3 at the 9 month time point. There was no effect of lesion level on the HR or RPE response to the arm-crank test at any time point. Arm ergometry performance changes in the EX group (distinguished by lesion level) are presented in Figure 1 and Table 3.
The effect of training on the relationship between HR and power output on the arm ergometer (HR/W) was analyzed from data in all three stages of the multi-stage test. Results revealed significant group × time interactions at all three stages, such that the HR/W ratio declined significantly in the EX group only, with the majority of this decrease occurring in the first 3 months. Furthermore, significant group × lesion × time interactions were found in stages 2 (F(3.48)=4.64, P=0.006) and 3 (F(3.48)=3.55, P=0.02), revealing that subjects with tetraplegia had the greatest decrease in HR for a given power output after training. These results are depicted in Table 4.
Subjects in CON experienced no change in their performance on the discontinuous arm-crank test over time, nor was there any change in their heart rate, RPE response or HR/W relationship.
Changes in muscle strength
Although there was very large variability between subjects, there was no difference in baseline strength between EX and CON in any of the muscle groups measured. Furthermore, the only muscle groups in which subjects with tetraplegia were significantly weaker than those with paraplegia were the right and left chest press. Baseline chest press strength (averaged across right and left) was 30 kg in subjects with tetraplegia compared with 54.5 kg in those with paraplegia, which was not surprising given that the chest press exercise also uses the triceps muscle group which is often paretic in persons with tetraplegia. Subjects in EX experienced progressive increases in strength over the 9 months of training, ranging from 19–34%, whereas there was no significant change in strength in CON (Table 5). The group×time interactions were significant (P<0.05) in all muscle groups except for the anterior deltoid. Similarly, the change in strength from baseline to 9 months was significant in all muscle groups except for the left anterior deltoid. Figure 2 depicts the changes in strength observed in each of the muscle groups over the 9 months. There was no effect of lesion level on the magnitude of strength change over the 9 month period.
Changes in quality of life components
Repeated measure ANCOVAs (using baseline values as the covariate) on each of the HRQL variables revealed significant main effects for group. As predicted, across the study measurement points, exercisers reported less stress, fewer depressive symptoms and greater satisfaction with their physical functioning, than did controls (P<0.05). Similarly, there was a nonsignificant trend for exercisers to report greater satisfaction with physical appearance than controls (P=0.06). Also as predicted, the exercisers reported less pain (P<0.01), greater perceived improvements in their health and a better quality of life than did controls (P<0.05). Table 6 summarizes the QOL data, and while the group × time interactions did not reach statistical significance, one can see that many of the differences between EX and CON were already evident by 3 months into the study and were maintained for the study duration.
Discussion
This 9-month randomized controlled trial in persons with SCI has demonstrated that a twice-weekly program of progressive exercise training is effective in increasing strength, arm ergometry performance, as well as several components reflecting QOL and psychological well-being.
Program adherence
Of the 21 subjects that were initially randomized to the exercise group, 11 successfully completed 9 months of twice-weekly training. Two subjects were forced to drop out within the first 3 months due to illness, six more dropped out between 3 and 6 months due to transportation difficulties and/or illness, and a final two dropped out between 6 and 9 months due to work/school conflicts. In total, we experienced a 47.6% drop-out by 9 months of training, but the subjects that did complete had very high attendance rates (82.5% of available sessions attended).
There are no comparative compliance figures in the literature for a study of this duration, but Davis et al7 reported a drop-out of nine of 24 subjects (37.5%) by 4 months of thrice-weekly training in their study of persons with paraplegia, which increased to a 46% drop-out by 6 months. In comparison, the present study had a 38% drop-out by 6 months of training. Davis et al7 suggested that the thrice-weekly time commitment might have been too rigid for their study population to adhere to. Interestingly, both our study and that of Davis et al7 reported the largest decrement in compliance to occur between 3 and 6-months of training, which is similar to what one finds in the able-bodied population. Dishman31 observed that 50% of able-bodied exercisers typically drop-out within 6-months of initiating an exercise program.
Retention of our control participants remained high throughout the intervention (defined as the number of scheduled follow-up assessments attended). Only one control participant withdrew from the study immediately following randomization.
Submaximal exercise responses
Following 9 months of twice-weekly training, participants in the exercise group had increased their power output in stage 3 of the multi-stage arm ergometry protocol by an average of 81.5% (118% and 45% in subjects with tetra- and paraplegia, respectively). While it is true that submaximal HR (and RPE) also increased at the final testing point, the analysis of the HR/power output relationships revealed that the increase in HR was much smaller than the increase in power output, suggesting that following training, subjects in EX could perform significantly more work at a given HR than at baseline. This was true even at power outputs that elicited HR's less than 110 bpm, and was therefore not just a result of disrupted sympathetic outflow to the heart. Similar findings have been reported in previous, shorter duration studies,32,33,34 but none of these studies also incorporated strength training into the exercise program. While determining the mechanisms for the improved exercise capacity was not an objective of the present investigation, we can speculate that peripheral circulatory adaptations, together with increased muscle strength, likely contributed to the positive adaptations observed. The possible functional implications of these training adaptations were that subjects could perform certain tasks of daily living much easier after 9 months of training than at baseline, which may be predicted to result in greater independence.
We did not have very strong evidence of sympathetic dysfunction limiting exercise performance in the subjects with tetraplegia, as their HR response to the submaximal exercise protocol was not significantly different from the subjects with paraplegia. For example, in the exercising tetraplegics, HR during stage 3 of the arm-crank test was 123 bpm at baseline, and this increased to 139 bpm at 9 months (a 13% increase). In comparison, the exercising paraplegics had a stage 3 HR of 131 bpm at baseline, which increased to 152 bpm at 9 months (a 16% increase). It is noteworthy that all of the subjects with tetraplegia in the EX group had incomplete injuries (ASIA B–C), so perhaps they were better able to activate the sympathetic nervous system during exercise compared with those with complete cervical lesions. Furthermore, there is evidence that arm exercise can increase catecholamine release from sympathetic nerve endings in the arm muscles of persons with motor-complete tetraplegia,35 which may serve to compensate for any sympathetic decentraliziation effects.8
Changes in muscle strength
All subjects in EX experienced progressive increases in muscle strength in each of the muscle groups tested, and the change scores were significantly different from CON in all muscle groups except for the left anterior deltoid (increases were in the predicted direction, but did not reach statistical significance). As seen in Table 5, the increases in muscle strength were progressive across the four time points of measurement, and it is possible that subjects would have continued to get stronger had they continued training beyond 9 months. It is noteworthy that these strength increases were incurred with a training regimen of twice/week, which is less than the usual three times/week in most published studies in this population. Our results support a recent paper by Taafe et al,36 which demonstrated that once- or twice-weekly resistance training can be as effective as thrice-weekly training in improving strength in men and women aged 65–79 years. Thus, for the community-dwelling individual with SCI, a twice-weekly commitment may be as effective yet much easier to manage than three times weekly, and future training studies among this population may find that recruitment and subject retention is easier with this reduced training frequency.
Changes in quality of life components
The results of this study show that a 9-month program of twice-weekly exercise can decrease self-reported stress, pain, and depression, and can enhance physical self-concept and overall QOL in persons with SCI. It is evident that similar to other populations that experience chronic pain and disability (eg people with fibromyalgia and osteoarthritis), persons with SCI can significantly enhance their sense of subjective well-being by engaging in a structured exercise program. Thus, exercise can and should be used as a therapeutic modality for improving both physical fitness and psychological well-being among people with SCI. In addition, the finding that control subjects experienced decrements along some of the QOL indices suggests that exercise may also have a prophylactic effect in terms of preventing decline in QOL after SCI.
There are at least three possible mechanisms through which exercise might enhance or prevent decline in QOL. First, research suggests that among people with SCI–a population that experiences considerable pain37–exercise-induced changes in pain mediate changes in QOL.38 Second, among patients with chronic disability, exercise has been shown to improve QOL by improving the sense of control and mastery that people feel regarding their physical functioning.39 Given that our subjects experienced decreased pain and increased physical functioning, we suspect that these factors contributed, at least in part, to improvements along the QOL parameters. Of course, we can not rule out a third possibility, that the social interactions experienced by the exercise group led to changes in QOL. Logistical considerations prevented us from providing control subjects with social interactions equivalent to that experienced by the exercisers. However, other clinical studies in which the exercise and control groups received similar amounts of social contact have found the effects of exercise on psychological well-being and QOL to be greater than the effects of mere social interaction.40 As such, we are confident that the effects observed in the present study were largely due to the exercise.
In conclusion, to our knowledge this is the first randomized controlled trial of exercise training in persons with SCI to look at both physiological and psychological outcome measures, and our results revealed that 9 months of twice-weekly strength and endurance training results in significant increases in strength, arm ergometry performance and quality of life.
References
Gordon T & Mao J . Muscle atrophy and procedures for training after spinal cord injury. Phys Ther 1994; 74: 50–60.
DeVivo MJ, Black KJ & Stover SL . Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 1993; 74: 248–254.
Whiteneck GG et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia 1992; 30: 617–630.
Coutts KD & Strogryn JL . Aerobic and anaerobic power of Canadian wheelchair track athletes. Med Sci Sports Exerc 1987; 19: 62–65.
Davis GM & Shephard RJ . Cardiorespiratory fits in highly-active versus less-active paraplegics. Med Sci Sports Exerc 1988; 20: 463–468.
Davis GM, Tupling SJ & Shephard RJ . (ed.). Dynamic strength and physical activity in wheelchair users. In: Sherrill C (ed). Proc 1984 Olympic Scientific Congress: Sport for the Disabled, Champaign, Il, Human Kinetics, 1986, pp 139–146.
Davis GM, Plyley MJ & Shephard RJ . Gains of cardiorespiratory fitness with arm-crank training in spinally disabled men. Can J Sports Sci 1991; 16: 64–72.
Figoni SF . Exercise responses and quadriplegia. Med Sci Sports Exerc 1993; 25: 433–441.
DiCarlo SE . Effect of arm ergometry training on wheelchair propulsion endurance of individuals with quadriplegia. Phys Ther 1988; 68: 40–44.
Hooker SP & Wells CL . Effects of low- and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc 1989; 21: 18–22.
Jacobs PL, Nash MS & Rusinowski JW . Circuit training provides cardiorespiratory and strength benefits in persons with paraplegia. Med Sci Sports Exerc 2001; 33: 711–717.
Davis GM & Shephard RJ . Strength training for wheelchair users. Br J Sports Med 1990; 24: 25–30.
Rodgers MM et al. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation-induced knee extension exercise training. J Rehabil Res Dev 1991; 28: 19–26.
Nilsson S, Staff PH & Pruett EDR . Physical work capacity and the effect of training on subjects with long-standing paraplegia. Scand J Rehab Med 1975; 7: 51–56.
Ettinger WH et al. A randomized control trial comparing aerobic exercise and resistance exercise with health education program in older adults with knee osteoarthritis. The fitness arthritis and seniors trial (FAST). JAMA 1997; 277: 64–70.
Buckelew SP et al. Beiofeedback/relaxation training and exercise interventions for fibromyalgia: A prospective trial. Arthritis Care Res 1998; 11: 196–209.
Lox CL, McAuley E & Tucker RS . Exercise as an intervention for enhancing subjective well-being in an HIV-1 population. J Sport Exerc Psych 1997; 17: 345–362.
Jones NL Clinical exercise testing, 4th edn. Philadelphia: WB Saunders Co:, 1997, pp 104–109.
Coll JR, Frankel HL, Charlifue SW & Whiteneck GG . Evaluating neurological group homogeneity in assessing the mortality risk for people with spinal cord injuries. Spinal Cord 1998; 36: 275–279.
Davis GM . Exercise capacity of individuals with paraplegia. Med Sci Sports Exerc 1993; 25: 423–432.
Borg GAV . Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1970; 14: 377–381.
McLean KP, Jones PP & Skinner JS . Exercise prescription for sitting and supine exercise in subjects with quadriplegia. Med Sci Sports Exerc 1995; 27: 15–21.
Cohen S, Kamarch T & Mermelstein R . A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396.
Radloff LS . The CES-D scale: A self-report depression scale for research in the general population. App Psych Meas 1977; 1: 385–401.
Fuhrer MJ et al. Depressive symptomatology in persons with spinal cord injury who reside in the community. Arch Phys Med Rehabil 1993; 74: 255–260.
Reboussin BA et al. Correlates of satisfaction with body function and body appearance in middle- and older-aged adults: The Activity Counseling Trial (ACT). Psychology & Health 2000; 15: 239–254.
Curtis KA et al. Effects of standard exercise protocol on shoulder pain in long-term wheelchair users. Spinal Cord 1999; 37: 421–430.
Ware JE & Sherbourne CD . The MOS 36-item short form health survey (SF-36). Conceptual framework and item selection. Med Care 1992; 30: 473–483.
Patrick D, Danis M, Southerland LI & Hong G . Quality of life following intensive care. J Gen Int Med 1988; 3: 218–223.
Gerhart KK, Johnson RL & Whiteneck GG . Health and psychosocial issues of individuals with incomplete and resolving spinal cord injuries. Paraplegia 1992; 30: 282–287.
Dishman RK . Increasing and maintaining exercise and physical activity. Behaviour Ther 1989; 22: 345–378.
DiCarlo SE . Improved cardiopulmonary status after a two-month program of graded arm exercise in a patient with C6 quadriplegia: A case report. Phys Ther 1982; 62: 456–459.
Hjeltnes N . Capacity for physical work and training after spinal injuries and strokes. Scan J Soc Med 1982; 29 (Suppl): S245–S251.
Pollock ML et al. Arm pedaling as an endurance training regimen for the disabled. Arch Phys Med Rehabil 1974; 55: 418–424.
Bloomfield SA, Jackson RD & Mysiw WJ . Catecholamine response to exercise and training in individuals with spinal cord injury. Med Sci Sports Exerc 1994; 26: 1213–1219.
Taaffe DR, Duret C, Wheeler S & Marcus R . Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc 1999; 47: 1208–1214.
Rintala DH et al. Chronic pain in a community-based sample of men with spinal cord injury: prevalence, severity, and relationship with impairment, disability, handicap, and subjective well-being. Arch Phys Med Rehabil 1998; 79: 604–614.
Martin K et al. The effects of exercise training on HRQL among people with spinal cord injury and the mediating influence of pain. Rehab Psych 2001 (in press).
Rejeski WJ, Ettinger, Jr WH, Martin K & Morgan T . Treating disability in knee osteoarthritis with exercise therapy: a central role for self-efficacy and pain. Arthritis Care Res 1998; 11: 94–101.
McCain GA, Bell DA, Mai FM & Halliday PD . A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arth Rheumatism 1988; 31: 1135–1141.
Acknowledgements
This study was supported by a grant from the Ontario Neurotrauma Foundation. DS Ditor was the recipient of a studentship from the Ontario Neurotrauma Foundation. The authors gratefully acknowledge the training assistance provided by many volunteer undergraduate and graduate kinesiology students.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hicks, A., Martin, K., Ditor, D. et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord 41, 34–43 (2003). https://doi.org/10.1038/sj.sc.3101389
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101389
Keywords
This article is cited by
-
Acute changes in antioxidants and oxidative stress to vigorous arm exercise: an intervention trial in persons with spinal cord injury and healthy controls
Spinal Cord Series and Cases (2023)
-
Telehealth high-intensity interval exercise and cardiometabolic health in spinal cord injury
Trials (2022)
-
The effects of active upper-limb versus passive lower-limb exercise on quality of life among individuals with motor-complete spinal cord injury
Spinal Cord (2022)
-
Treatment of shoulder pain in people with spinal cord injury who use manual wheelchairs: a systematic review and meta-analysis
Spinal Cord (2022)
-
Rethinking aerobic exercise intensity prescription in adults with spinal cord injury: time to end the use of “moderate to vigorous” intensity?
Spinal Cord (2022)