Abstract
Cysteinyl leukotrienes (cys-LTs), LTC4, LTD4, LTE4 are potent inflammatory lipid mediators that act through two distinct G-protein-coupled receptors, CysLT1R and CysLT2R. Although cys-LTs are shown to induce vascular leakage and atherosclerosis, the molecular mechanism by which cys-LTs modulate endothelial function is not known. Here, we show that cys-LTs (LTC4 and LTD4) induce robust calcium influx in human umbilical vein endothelial cells (HUVECs) through CysLT2R, but not CysLT1R. Further, cys-LT treatment induced endothelial cell (EC) contraction leading to monolayer disruption via CysLT2R/Rho kinase dependent pathway. Furthermore, stimulation with cys-LTs potentiated TNFα-induced VCAM-1 expression and leukocyte recruitment to ECs through CysLT2R. In contrast, we found that both LTC4 and LTD4 stimulated EC proliferation through CysLT1R. Taken together, these results suggest that cys-LTs induce endothelial inflammation and proliferation via CysLT2R/Rho kinase and CysLT1R/Erk dependent pathways, respectively, which play critical role in the etiology of cardiovascular diseases such as atherosclerosis and myocardial infarction.
Similar content being viewed by others
Introduction
Inflammation and endothelial dysfunction are the major contributors for the initiation and progression of atherosclerosis and its associated cardiovascular risks1,2,3. The initial activation of endothelium subsequently results in the production of pro-inflammatory molecules that interact with leukocytes and further propagates the inflammatory process leading to change in endothelial cell (EC) constitutive properties and abnormal state of the endothelium with compromised function1,2,3. Although research has shown that atherosclerosis is an inflammatory disease, there is incomplete understanding of the role of inflammatory lipid mediators in its pathogenesis. Leukotrienes are pro- inflammatory mediators generated from arachidonic acid cascade and have been implicated in atherosclerosis3. Cysteinyl leukotrienes (cys-LTs), comprising of LTC4, LTD4 and LTE4 are implicated in inflammatory diseases such as asthma, rheumatoid arthritis and inflammatory bowel disease3,4. Cys-LTs exert their effects through two different G-protein-coupled receptors, CysLT1R and CysLT2R5,6. Several cys-LT receptor antagonists have been approved by FDA and are in the market for the treatment of asthma and allergic rhinitis7,8.
Although inflammatory cells were identified as the main source as well as target of cys-LTs, these lipids were also shown to be produced during vascular injury and affect vascular cell function. In the past, cys-LTs were shown to exert a broad variety of effects on cardiovascular system such as constriction of microvasculature, enhancement of permeability of post-capillary venules and reduction in coronary blood flow9,10. However, not much attention was given to cys-LTs in cardiovascular system until recently. The identification and characterization of G-protein coupled CysLTRs, CysLT1R and CysLT2R renewed the interest on cys-LTs. Specifically, CysLT2R has been recently shown to be involved in atherosclerosis and vascular leakage during myocardial injury and pathological retinal angiogenesis11,12,13,14. Since, cys-LTs are secreted by inflammatory cells in vascular wall during vascular injury it is conceivable that cys-LTs exert their effect on ECs. Endothelial CysLT2R overexpression was found to up-regulate the expression of genes including ICAM-1 and VCAM-115. However, the molecular mechanisms by which cys-LTs regulate EC function are not known. Endothelial function is often de-regulated during atherosclerosis contributing to endothelial dysfunction which includes enhanced EC proliferation, contraction of EC monolayer and increased permeability, expression of adhesion molecules and subsequent attachment of immune cells. In the current study, we analyzed the effects of cys-LTs on the modulation of above mentioned EC phenotypes as well as elucidate the mechanism of action behind.
Results
LTC4 and LTD4 induce calcium influx in HUVECs through CysLT2R but not CysLT1R
In order to determine the role of cys-LTs in regulating endothelial function, first we measured the expression of their receptors, CysLT1R and CysLT2R in HUVECs. Quantitative RT-PCR analysis revealed that the expression of CysLT2R is higher in HUVECs compared to that of CysLT1R (Fig. 1A). Western blot analysis showed that these cells express both CysLT1 and CysLT2 receptors (Suppl Fig. 1). To determine the functional significance of these receptors, we measured cys-LT induced calcium flux in HUVECs loaded with Fluo-4 AM. We found that both LTC4 and LTD4 induced rapid calcium flux in these cells (Fig. 1B,C). Interestingly, cys-LT-induced calcium influx was significantly abolished in the presence of a specific CysLT2R antagonist14, BayCysLT2 (1 μM) (Fig. 1B,C). In contrast, a specific CysLT1R antagonist MK571 (1 μM) failed to inhibit calcium influx by either LTC4 or LTD4 (Fig. 1B, C). These results clearly suggest that cys-LTs induce calcium influx through the activation of CysLT2R in endothelial cells.
Human endothelial cells (HUVECs) express both CysLT1R and CysLT2R and Cys-LTs induce calcium signaling through CysLT2R but not CysLT1R.
(A) RT-PCR analysis showing the expression of CysLT1R and CysLT2R in human endothelial cells. (B) HUVECs were loaded with Fluo-4 (4 μM) and stimulated with 500 nM of LTC4 or LTD4 and changes in fluorescence intensity was measured on confocal microscope in presence or absence of 1 μM MK571 or BayCysLT2 (Bay). (B) Calcium transient showing cys-LT-induced calcium influx. (C) Quantitative analysis showing the Δ calcium changes. The results shown are mean ± SEM from 3 independent experiments. The significance was analyzed using student's t-test and set at p ≤ 0.05.
Leukotrienes induce endothelial contraction and endothelial barrier disruption through a Rho kinase-dependent mechanism
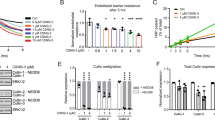
Cys-LTs were previously implicated in vascular leakage14. However, the molecular mechanism through which cys-LTs regulate EC contraction and permeability is not known. As a next step in understanding the role of cys-LTs in regulating EC function, we sought to determine if cys-LTs can induce contraction and barrier disruption in EC monolayer. EC were grown to a confluent monolayer, incubated with cys-LTs for 5 h followed by fixation and staining for F-actin. Treatment of monolayer with LTC4 or LTD4 induced EC contraction as evidenced by gap formation between the F-actin stained cells (Fig. 2A, B). In contrast, the EC monolayer was found intact in control non-treated cells. Thrombin stimulation induced robust gap formation in EC monolayer and served as a positive control. Quantitative analysis revealed that both LTC4 and LTD4 induced significant EC contraction compared to thrombin (80% and 90% of thrombin, respectively).
cys-LTs induce EC contraction and gap formation via CysLT2R/Rho kinase dependent pathway.
Confluent monolayer (>95%) of HUVECs were serum starved overnight and treated for 5 h with 500 nM LTC4 or LTD4 or 0.1 U/mL Thrombin. Cells were stained for F-actin (Texas Red-X phalloidin) and nucleus (DAPI) and images were taken under fluorescence microscope. (A) Fluorescence micrographs showing increase in gap formation after treatment with 500 nM LTC4 or LTD4 or thrombin and as evidenced by F-actin (red) and nucleus (blue) staining. (B) Quantitative analysis showing the gap formation by LTC4 and LTD4 and (C) by LTD4 in the presence and absence of a Rho kinase inhibitor, Y27632 (10 μM) or CysLT1R antagonist, MK571 (1 μM) or CysLT2R antagonist, BayCysLT2 (Bay, 1 μM). The results shown are mean ± SEM from 3 independent experiments. The data was analyzed using one way ANOVA followed by Tukey post-hoc analysis. The significance was set at p ≤ 0.05. NS = non significant.
We then investigated which CysLTR was involved in the EC contraction. We found that LTD4-induced EC contraction was significantly inhibited by CysLT2R antagonist, BayCysLT2, but not CysLT1R antagonist, MK571 (Fig. 2C, Suppl Fig. 2). Rho kinase has been shown to modulate histamine and thrombin-induced barrier dysfunction16. To explore if Rho kinase has a role in mediating cys-LT-induced contraction in HUVECs, we employed a pharmacological Rho Kinase inhibitor, Y27632, in EC contraction assays. Pre-treatment of cells with Rho kinase inhibitor, Y27632, significantly attenuated both LTC4 and LTD4-induced EC contraction and gap formation (Fig. 2C, Suppl Fig. 2).
Cys-LTs potentiate TNFα-induced responses in EC
Attachment of immune cells to endothelium, upon activation, is a distinct EC function which is a critical event in atherosclerosis. Hence, we explored if these potent pro-inflammatory cys-LTs can influence leukocyte attachment to EC. We found that LTC4 or LTD4 failed to induce expression of adhesion molecules or attachment of leukocytes to endothelium (Fig. 3). However, cys-LTs significantly potentiated expression of adhesion molecule VCAM-1 (Fig. 3C) and attachment of leukocytes in the presence of sub-physiological concentration of TNFα (Fig. 3A, B, Suppl Fig. 3). Notably, we found that this recruitment of leukocytes was significantly attenuated by BayCysLT2, but not MK571 (Fig. 3A, B, Suppl Fig. 3). These findings suggest that cys-LTs potentiate inflammatory signals elicited by other distinguished inflammatory mediators such as TNFα and enhance recruitment of leukocytes to endothelium further steering the endothelial dysfunction via CysLT2R.
LTC4 and LTD4 potentiate TNFα induced attachment of THP-1 cells to endothelial cells.
Confluent HUVECs treated with 500 nM of LTC4, LTD4 or TNFα (0.25 ng/mL) for 4 h were incubated with THP-1 cells prior stained with DilC12(3) fluorescent dye for 1 h.(A) Attachment of THP-1 cells to HUVECS after 5 times washing. Representative experiment is illustrated out of three performed. (B) Quantitative analysis showing the TNFα/LTD4-induced attachment of THP-1 cells to EC monolayer in the presence or absence of MK571 or BayCysLT2 (Bay). The results shown are mean ± SEM from 3 independent experiments. The data was analyzed using one way ANOVA followed by Tukey post-hoc analysis. The significance was set at p ≤ 0.05. (C) A representative Western blot showing the expression of VCAM-1 by TNFα or LTD4 alone or in combination. GAPDH served as a loading control.
Cys-LTs activate Erk and enhance proliferation through CysLT1R in HUVECs
We next asked whether cys-LTs regulate endothelial cell proliferation. First, we measured cell proliferation using XTT assay in serum starved HUVECs stimulated with cys-LTs. Both LTC4 and LTD4 stimulation resulted in significant increase in EC proliferation (Fig. 4A). To validate this result, we also measured proliferation by assessing BrdU incorporation into the cells by ELISA in response to LTD4 (Fig. 4B). We found that LTD4- induced EC proliferation was significantly inhibited by MK571, but not by BayCysLT2, indicating a role for CysLT1R in endothelial proliferation (Fig. 4B). Basal as well as cys-LT-induced proliferation was completely blocked by PD98059, a MEK inhibitor (Fig. 4B) suggesting that proliferative signal in HUVECs is mediated through Erk. Along these lines, earlier reports also suggest that cys-LTs enhance cell proliferation through Erk in a number of cell types earlier17,18. This suggests that cys-LTs might induce cell proliferation via Erk phosphorylation in endothelial cells. In order to find out if ERK is the signaling intermediate in cys-LT-induced EC proliferation, we measured phosphorylation of Erk. As shown in Fig. 4C, LTD4 significantly increased phosphorylation of Erk and this phosphorylation was transient, maximum at 5 min and declined.
Cys-LTs induce proliferation and Erk activation in HUVECs.
(A) HUVECs were cultured in 96 well plate, serum starved overnight, then treated with or without 500 nM LTC4 or LTD4 for 48 h and proliferation was assayed by XTT assay. (B) HUVECs were cultured in 96 well plate, serum starved overnight, then treated with or without 500 nM LTD4 for 48 h in the presence or absence of 1 μM of MK571 or BayCysLT2 (Bay) or PD98059 (50 μM). BrdU was added during the last 24 h and its incorporation into the cells was assayed by ELISA. The results shown are mean ± SEM from 2 independent experiments. The data was analyzed using one way ANOVA followed by Tukey post-hoc analysis. The significance was set at p ≤ 0.05. (C) HUVECs were stimulated with 500 nM of LTD4 for indicated points of time, lysed, proteins were separated and Erk phosphorylation was measured using phospho-specific antibodies. Total Erk was used as a loading control.
Discussion
In the present study, we delineated the molecular mechanism by which cys-LTs regulate endothelial function and demonstrated that the cys-LTs induce inflammatory signals through CysLT2R and proliferation through CysLT1R. We further demonstrate that CysLT2R activation results in EC contraction and barrier disruption through Rho kinase pathway and potentiate TNFα-induced attachment of leukocytes to endothelial monolayer via up-regulation of VCAM-1. To our knowledge, this is the first study to report the involvement of Rho kinase down-stream of CysLT2R in ECs. Finally, we demonstrated that EC proliferation is mediated through the activation of CysLT1R.
Cys-LTs mediate their effects through the activation of CysLT1R and CysLT2R in different cell types4,18,19,20,21. CysLT2R was reported to be dominantly expressed in endothelial cells (HUVECs) and has been shown to mediate cys-LT-mediated calcium signaling22. Also, initial studies on HUVECs demonstrated that EC predominantly express CysLT2R and stimulation with LTD4 induces early inflammatory genes23 such as EGR (Early growth response) and NRS4A (nuclear receptor subfamily 4 group A) transcription factors, IL-8 (interleukin-8), DSCR1(Down syndrome critical region gene 1), E-selectin, CXCL2 (CXC ligand 2), ADAMTS1(a disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motif 1), TF (tissue factor) and COX2 (cyclooxygenase 2)23. Using pharmacological inhibitors, both CysLT1R and CysLT2R are implicated in vascular permeability and ischemia-induced cerebral, renal and myocardial injury13,24,25,26. CysLT1R expression appears to be up-regulated in renal endothelial cells and correlated with ischemia-reperfusion injury in rat kidney24. Interestingly, CysLT1R was shown to translocate to nucleus in response to oxygen-glucose deprivation-induced damage in brain endothelial cells27. On the other hand, CysLT2R was demonstrated to play critical role in vascular leakage during myocardial injury and pathological retinal angiogenesis13,25,26. This vascular permeability seems to be mediated through trans-endothelial vesicle transport regulated by CysLT2R-induced calcium signaling26. Despite the clear demonstration that CysLTRs are critical mediators of vascular leakage, the molecular mechanism by which cys-LTs/CysLTRs mediate these effects are not known.
The present study identified three important mechanisms of cys-LT signaling that include a) Rho kinase as a down-stream regulator of CysLT2R-induced endothelial contraction b) potentiation of TNFα-induced leukocyte recruitment by CysLT2R signaling through the up-regulation of VCAM-1 expression and c) modulation of EC proliferation by CysLT1R. Notably, these mechanisms provide molecular basis for leukotriene-induced inflammatory EC phenotype in atherosclerosis as well as ischemia-induced vascular injury. Previous studies demonstrated that LTD4 induces expression of early inflammatory genes via activation of CysLT2R in HUVECs that are implicated in atherosclerosis. Interestingly, a well-known pro-thrombotic substance, thrombin also induced expression of similar genes in endothelial cells23. Moreover, LTD4 together with thrombin increased the fold expression of these genes suggesting that cys-LTs act in concert with other mediators and also activate similar signaling mechanism23. The observations in the present study that both cys-LTs induce EC contraction and gap formation through Rho kinase coupled with the fact that thrombin induces EC permeability through Rho kinase28,29 support the notion that both thrombin and LTD4 activate similar mechanisms, although they stimulate different G-proteins. Our study also presents an evidence that cys-LTs potentiate effects of an inflammatory cytokine, TNFα on endothelial cells via increased expression of VCAM-1 and leukocyte recruitment through CysLT2R. Thus, our results provide strong evidence for a role for CysLT2R signaling in the mediation of inflammatory EC phenotype.
Interestingly, calcium influx which is an immediate response of GPCR activation is exclusively mediated by CysLT2R but not CysLT1R in ECs. On the other hand, CysLT1R seems to be the preferred receptor for calcium influx in epithelial and inflammatory cells18,30. Calcium can activate a plethora of signaling events that modulate proliferation, migration, contraction and gene expression31. Although we do not know the exact role calcium plays in cys-LT-induced signaling in EC, previous studies have demonstrated that it acts up-stream of CysLT2R-induced trans-endothelial vesicle transport and vascular leakage26.
In addition to CysLT2R signaling, our study demonstrates that cys-LTs mediate EC proliferation through the activation of CysLT1R. We also found that cys-LTs activate Erk in endothelial cells. In contrast, LTD4 failed to induce proliferation in EA.hy926 (a HUVEC line), but promoted migration via CysLT1R mediated Erk activation32. Previous studies have shown that LTD4 induce proliferation of intestinal epithelial cells17,20 and mast cells18,21 through CysLT1R but has no direct effect on fibroblast proliferation33 suggesting cell specific effects which may explain the difference in proliferation in HUVECs and EA.hy926 cell line. Although we did not measure migration, LTD4 was shown to mediate migration in intestinal epithelial cells through CysLT1R34. Taken, together these findings suggest that cys-LTs regulate endothelial function via both CysLT1R and CysLT2R. However, it is not known how and whether particular CysLTR is activated by cys-LTs.
In conclusion, our study presents the molecular mechanism for the regulation of endothelial phenotype by cys-LTs and provides evidence for the activation of both CysLT1R and CysLT2R which induce proliferative signals via probable activation of Erk and inflammatory signals via Rho kinase and VCAM-1 expression. Our study shows the possible involvement of these inflammatory signals in two important pathological conditions a) Rho kinase-dependent EC contraction and increased gap formation which increases EC permeability in ischemia-induced vascular leakage and b) potentiation of TNFα-mediated increased expression of VCAM-1 and leukocyte recruitment which are critical events in the initiation of atherosclerosis. Understanding these down-stream molecular mechanisms of cys-LTs in the regulation of endothelial function may provide new therapeutic targets for the treatment of cardiovascular diseases such as ischemic heart disease, oxygen-induced retinopathy and atherosclerosis.
Methods
Materials
LTC4, LTD4, MK571, BayCysLT2, CysLT1R and CysLT2R antibodies were from Cayman Chemicals, Ann Arbor, MI. Validated real time primers for CysLT1R and CysLT2R were purchased from SABiosciences (cat # PPH02507A and PPH15153A). Phospho and total Erk antibodies were from cell signaling technology (Danvers, MA). VCAM-1 (E-10) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All secondary antibodies were obtained from Jackson Immunoresearch laboratories (West Groove, PA). XTT proliferation assay kit was from Trevigen (Gaithersburg, MD) and BrdU proliferation assay kit was purchased from CalBiochem EMD Millipore (La Jolla, CA). Human TNFα was obtained from Peprotech, Inc. (Rocky Hill, NJ). Thrombin and Y27632 were obtained from Sigma (St. Louis, MO) and Tocris Bioscience (Mineapolis, MN), respectively. Fluo-4 AM and Texas Red-X phalloidin were purchased from Molecular Probes (Eugene, OR) and DilC12 (3) fluorescent dye was purchased from BD Bioscience (Bedford, MA).
Cell culture
Human umbilical vascular endothelial cells (HUVECs) were maintained in EBM2 medium with EGM2 Bullet supplements, 10% fetal bovine serum and maintained at 37°C in a humidified 5% CO2 environment.
Calcium imaging
HUVECs cultured on glass bottomed dishes (MatTek) were loaded with Fluo-4/AM (1–4 μM) for 30 min and washed in calcium medium (136 mM NaCl, 4.7 mM KCl,1.2 mM MgSO4, 1.1 mM CaCl2, 1.2 mM KH2PO4, 5 mM NaHCO3, 5.5 mM glucose and 20 mM Hepes. pH 7.4). Cells were stimulated with LTC4 or LTD4 (500 nM) in calcium medium in the presence or absence of CysLTR antagonists, MK571 (1 μM) and BayCysLT2 (1 μM) pre-incubated for 30 min. Calcium imaging was performed on Olympus Fluo View 300 confocal microscope and analyzed using Fluo View software and Microsoft Excel30,35,36,37,38.
Endothelial monolayer contraction, F-actin staining and microscopy
ECs were grown on cell culture plates, serum starved and stimulated with cys-LTs (LTC4 or LTD4; 500 nM) or thrombin (0.1 U/ml) for 5 h, rinsed with phosphate-buffered saline (PBS) and fixed for 20 min at room temperature in PBS containing 4% paraformaldehyde. Following fixation, cells were permeabilized with PBS containing 0.25% Triton-X100, blocked with BSA and incubated with Texas Red conjugated phalloidin to stain actin stress fibers. Images were obtained using EVOS fluorescence microscope using 20× objective. Images were processed using Image J (NIH) software. In some cases, cells were pre-treated with specified antagonists for 30 minutes before stimulation with the indicated agonists.
Attachment assay
ECs were cultured as described above, serum starved and stimulated with or without cys-LTs (LTC4 or LTD4; 500 nM) and or TNFα 0.25 ng/mL for 4 hr. In some cases, cells were pre-treated with specified antagonists before stimulation with the indicated agonists. After stimulations, cells were washed with RPMI medium and co-incubated with THP-1 cells for 1 hr at 4°C prior stained with DilC12(3) fluorescent dye as described by the manufacturer protocol. Unattached cells were washed 5 times with PBS and attached cells counted under a fluorescent microscope. Data were analyzed using NIH Image J and Microsoft Excel.
Cell lysates and Western blotting
After stimulation with the respective agonists, ECs were lysed with lysis buffer (BD Bioscience) supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Pierce). In some experiments, cells were pre-treated for 30 min with respective antagonists as specified. All the antagonists used in the current study were dissolved in DMSO. Control cells were stimulated with DMSO alone. In some experiments, cells were also treated with inhibitors alone without agonist treatment. Immuno-blotting was performed as described previously17. Briefly, lysates were subjected to 4–12% SDS-PAGE and transferred to PVDF membrane. Membranes were incubated with respective primary phospho- and total antibodies diluted in 1× TBS, 5% dry milk, 0.1% Tween-20 (1:1000) overnight at 4°C on shaker and then with secondary antibody (peroxidase-conjugated anti-rabbit or anti-mouse). Western blot was incubated with ECL and the bands were visualized using imager (Protein Simple) and quantified using Image J (NIH).
Real-time quantitative PCR
The expressions of CysLT1R, CysLT2R transcripts were determined with real-time PCR performed on Light cycler 480 (Roche). HUVECs were cultured as described above and total RNA was isolated with an RNAeasy minikit (Qiagen) and cDNA was synthesized using cDNA synthesis kit from Quanta Biosciences containing qScript Reverse Transcriptase. Real time PCR was performed using real time CysLT1R, CysLT2R and GAPDH Primers purchased from Superarray and SYBR green PCR master mix from Quanta Biosciences. The levels of CysLT1R and CysLT2R relative to the GAPDH levels was analyzed and the ΔΔCT values are expressed as fold change.
Cell proliferation
HUVECs were plated at the density of 1500 cells/well of 96 well plate, serum starved overnight and were treated with 500 nM of LTC4 and LTD4. After 48 h, the proliferation was assayed by XTT or BrdU ELISA according to the manufacturer's protocols. BrdU label was added 24 h before the assay. In some experiments, cells were pre-incubated for 30 minutes with 1 μM of MK571 or BayCysLT2 or 50 μM of PD98059.
Data analysis
All the data shown is mean ± SEM from at least three independent experiments. Significance was determined using Student's t test as well as one-way ANOVA followed by Tukey post-hoc analysis and was set at p < 0.05.
References
Fan, J. & Watanabe, T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb 10, 63–71 (2003).
Sima, A. V., Stancu, C. S. & Simionescu, M. Vascular endothelium in atherosclerosis. Cell Tissue Res 335, 191–203 (2009).
Back, M. Inflammatory signaling through leukotriene receptors in atherosclerosis. Curr Atheroscler Rep 10, 244–251 (2008).
Laidlaw, T. M. & Boyce, J. A. Cysteinyl leukotriene receptors, old and new; implications for asthma. Clin Exp Allergy 42, 1313–1320 (2012).
Heise, C. E. et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem 275, 30531–30536 (2000).
Lynch, K. R. et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 399, 789–793 (1999).
Capra, V., Ambrosio, M., Riccioni, G. & Rovati, G. E. Cysteinyl-leukotriene receptor antagonists: present situation and future opportunities. Curr Med Chem 13, 3213–3226 (2006).
Nayak, A. & Langdon, R. B. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs 67, 887–901 (2007).
Carnini, C. et al. Synthesis of cysteinyl leukotrienes in human endothelial cells: subcellular localization and autocrine signaling through the CysLT2 receptor. Faseb J 25, 3519–3528 (2011).
Folco, G. et al. Leukotrienes in cardiovascular diseases. Am J Respir Crit Care Med 161, S112–116 (2000).
Riccioni, G. & Back, M. Leukotrienes as modifiers of preclinical atherosclerosis? ScientificWorldJournal 2012, 490968 (2012).
Riccioni, G., Back, M. & Capra, V. Leukotrienes and atherosclerosis. Curr Drug Targets 11, 882–887 (2010).
Barajas-Espinosa, A. et al. The cysteinyl leukotriene 2 receptor mediates retinal edema and pathological neovascularization in a murine model of oxygen-induced retinopathy. Faseb J 26, 1100–1109 (2012).
Ni, N. C. et al. A selective cysteinyl leukotriene receptor 2 antagonist blocks myocardial ischemia/reperfusion injury and vascular permeability in mice. J Pharmacol Exp Ther 339, 768–778 (2012).
Jiang, W. et al. Endothelial cysteinyl leukotriene 2 receptor expression mediates myocardial ischemia-reperfusion injury. Am J Pathol 172, 592–602 (2008).
Breslin, J. W. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol 9, 3–11 (2011).
Paruchuri, S., Hallberg, B., Juhas, M., Larsson, C. & Sjolander, A. Leukotriene D(4) activates MAPK through a Ras-independent but PKCepsilon-dependent pathway in intestinal epithelial cells. J Cell Sci 115, 1883–1893 (2002).
Paruchuri, S. et al. Leukotriene E4 activates peroxisome proliferator-activated receptor gamma and induces prostaglandin D2 generation by human mast cells. J Biol Chem 283, 16477–16487 (2008).
Evans, J. F. Cysteinyl leukotriene receptors. Prostaglandins Other Lipid Mediat 68–69, 587–597 (2002).
Paruchuri, S. & Sjolander, A. Leukotriene D4 mediates survival and proliferation via separate but parallel pathways in the human intestinal epithelial cell line Int 407. J Biol Chem 278, 45577–45585 (2003).
Paruchuri, S. et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med 206, 2543–2555 (2009).
Sjostrom, M. et al. Dominant expression of the CysLT2 receptor accounts for calcium signaling by cysteinyl leukotrienes in human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol 23, e37–41 (2003).
Uzonyi, B. et al. Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells. Proc Natl Acad Sci U S A 103, 6326–6331 (2006).
Matsuyama, M. et al. Study of cysteinyl leukotriene-1 receptor in rat renal ischemia-reperfusion injury. Transplant Proc 40, 2149–2151 (2008).
Moos, M. P. & Funk, C. D. Endothelial cysteinyl leukotriene 2 receptor expression and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 18, 268–273 (2008).
Moos, M. P. et al. Cysteinyl leukotriene 2 receptor-mediated vascular permeability via transendothelial vesicle transport. Faseb J 22, 4352–4362 (2008).
Fang, S. H. et al. Nuclear translocation of cysteinyl leukotriene receptor 1 is involved in oxygen-glucose deprivation-induced damage to endothelial cells. Acta Pharmacol Sin 33, 1511–1517 (2012).
Birukova, A. A. et al. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67, 64–77 (2004).
Kawkitinarong, K., Linz-McGillem, L., Birukov, K. G. & Garcia, J. G. Differential regulation of human lung epithelial and endothelial barrier function by thrombin. Am J Respir Cell Mol Biol 31, 517–527 (2004).
Thodeti, C. K., Adolfsson, J., Juhas, M. & Sjolander, A. Leukotriene D(4) triggers an association between gbetagamma subunits and phospholipase C-gamma1 in intestinal epithelial cells. J Biol Chem 275, 9849–9853 (2000).
Cole, K. & Kohn, E. Calcium-mediated signal transduction: biology, biochemistry and therapy. Cancer Metastasis Rev 13, 31–44 (1994).
Yuan, Y. M. et al. Leukotriene D4 stimulates the migration but not proliferation of endothelial cells mediated by the cysteinyl leukotriene cyslt(1) receptor via the extracellular signal-regulated kinase pathway. J Pharmacol Sci 109, 285–292 (2009).
Yoshisue, H. et al. Cysteinyl leukotrienes synergize with growth factors to induce proliferation of human bronchial fibroblasts. J Allergy Clin Immunol 119, 132–140 (2007).
Paruchuri, S., Broom, O., Dib, K. & Sjolander, A. The pro-inflammatory mediator leukotriene D4 induces phosphatidylinositol 3-kinase and Rac-dependent migration of intestinal epithelial cells. J Biol Chem 280, 13538–13544 (2005).
Adapala, R. K. et al. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol 301, H757–765 (2012).
Adapala, R. K. et al. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol 54, 45–52 (2013).
Thodeti, C. K. et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104, 1123–1130 (2009).
Thodeti, C. K., Nielsen, C. K., Paruchuri, S., Larsson, C. & Sjolander, A. The epsilon isoform of protein kinase C is involved in regulation of the LTD(4)-induced calcium signal in human intestinal epithelial cells. Exp Cell Res 262, 95–103 (2001).
Acknowledgements
This study was supported by the start-up funds from University of Akron, Department of Chemistry, James Foght Assistant Professorship and University of Akron Summer Grant (S.P.) and start-up funds from NEOMED (C.K.T.).
Author information
Authors and Affiliations
Contributions
E.D., R.A., N.A., V.K. and F.G. performed experiments and analyzed the data. C.K.T. provided cells, designed some of the experiments and edited the manuscript. S.P. designed, performed research, interpreted, analyzed data and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Duah, E., Adapala, R., Al-Azzam, N. et al. Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT2 and CysLT1 receptors. Sci Rep 3, 3274 (2013). https://doi.org/10.1038/srep03274
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03274
This article is cited by
-
Eicosanoids Signals in SARS-CoV-2 Infection: A Foe or Friend
Molecular Biotechnology (2023)
-
Cysteinyl leukotriene D4 (LTD4) promotes airway epithelial cell inflammation and remodelling
Inflammation Research (2021)
-
Mechanistic insight on the role of leukotriene receptors in ischemic–reperfusion injury
Pharmacological Reports (2021)
-
The Role of Arachidonic Acid Metabolism in Myocardial Ischemia–Reperfusion Injury
Cell Biochemistry and Biophysics (2020)
-
The leukotriene receptor antagonist montelukast and its possible role in the cardiovascular field
European Journal of Clinical Pharmacology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.