Abstract
Several studies have investigated the association between polymorphisms in the Deleted in AZoospermia-Like (DAZL) gene and male infertility risk, but with inconsistent results. We aimed to derive a more precise estimation of the relationship, therefore a meta-analysis was performed. A total of 13 case-control studies, including 2556 cases and 1997 controls, were selected. Two polymorphisms in DAZL were investigated, namely T12A (Thr12 → Ala) and T54A (Thr54 → Ala). Our meta-analysis showed that A > G is a risk factor for male infertility (P = 0.047, OR = 1.262, 95%CI = 1.003–1.587). However, when using trial sequential analysis (TSA) to confirm, we found that A > G risk effect turned out to be false positive. In addition, significant association was found between the T54A polymorphism and male infertility under co-dominant model (AG vs. AA: OR = 4.364, 95%CI = 2.207–8.630, P < 0.001) and dominant model (OR = 4.584, 95%CI = 2.320–9.058, P < 0.001). Stratified analysis showed that significantly strong association between T54A polymorphism and male infertility was present only in Asians, but not in Caucasians. Further studies of T12A and T54A with their biological functions are needed to understand the role of these polymorphisms in the development of male infertility.
Similar content being viewed by others
Introduction
Globally, around 15% of heterosexual couples suffer from inability to conceive their own child without any assistance1. In half of the cases, males are the ones to blame. Many reasons can lead to male infertility, such as malformations of the reproductive tract2, infection3 and chemical exposures4. Still, 50–70% of male infertility is of unknown etiology and that much of this is likely genetic. To unveil the reason, intensive research for genetic causes of male infertility has been performed in recent years.
DAZL (deleted in azoospermia-like) is an autosomal homologue of the DAZ (deleted in azoospermia), a gene cluster which gets deletions in at least 10% of males with azoospermia or oligozoospermia5,6,7,8. As a result, DAZL has always been seen as a promising candidate for male infertility. Although varies in detail, studies aiming at DAZL expression share the same point: DAZL plays an important role in the human spermatogenic processes and might function as a translational activator through regulating mRNA expression9,10,11,12, though its mechanism is still largely unknown. In a study on human beings, DAZL protein is shown to be present in male germ cells in many stages during spermatogenesis6 and the nuclear localization of DAZL protein is also observed in gonocytes and spermatogonia. Another study has demonstrated that DAZL protein is located in the nuclei of gonocytes and relocalized to the cytoplasm in adults13. There is ever-growing evidence on animals that confirms similar points: in two experiments performed on transgenic mice with a Dazl null background, either carrying human DAZL or human DAZ shows a partial rescue of the Dazl knockout mice. Although the mice remain infertile, both transgenes enable prophase spermatocytes to be produced14.
Recently, a number of molecular epidemiological studies have been conducted to examine the association between DAZL polymorphisms and male infertility in diverse populations. Among them, two non-synonymous single nucleotide polymorphisms (SNPs) at nucleotide position 260 (exon 2) and 386 (exon 3), resulted by the amino acid exchange T12A (Thr12 → Ala) and T54A (Thr54 → Ala) respectively, are most frequently studied15,16,17,18,19,20,21,22,23,24,25,26,27. However, the results of these studies are inconsistent or even contradictory. Most studies till date have analyzed these polymorphisms in rather small sample size, leading to under-estimation of the association. To estimate the effect of polymorphisms and risk of male infertility, as well as to quantify the potential between-study heterogeneity, we conducted a meta-analysis on 13 eligible and published case-control studies.
Results
Study characteristics
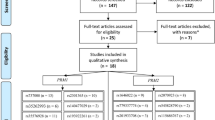
Through the literature search and selection based on inclusion criteria, 13 articles were identified by reviewing potentially relevant articles (Figure 1). The characteristics of the selected studies are shown in Table 1 and Table 2.
T12A polymorphism: A total of 10 studies were included in the meta-analysis with 2174 cases and 1594 controls. The number of cases included in the studies varied from 92 to 660, with a mean (± SD) of 217.40 (±163.16) and the number of controls varied from 40 to 350, with a mean (± SD) of 159.40 (±89.67).
T54A polymorphism: In total, twelve studies met the inclusion criteria and were selected for the meta-analysis including 2456 cases and 1897 controls. The number of cases included in the studies varied from 71 to 660, with a mean (± SD) of 204.67 (±151.70) and the number of controls varied from 40 to 350, with a mean (± SD) of 158.08 (±85.35).
Meta-analysis of T12A polymorphism and male infertility
The evaluation of the association between T12A polymorphism and male infertility risk is summarized in Table 3, Figure 2 and Figure S1. No significant association was observed between T12A polymorphism and male infertility under dominant model and recessive model (for AG + GG vs. AA: OR = 1.244, 95%CI = 0.994–1.557, P = 0.057; for GG vs. AA + AG: OR = 0.840, 95%CI = 0.384–1.834, P = 0.661). However, we found that when companied with AA genotype, AG is significantly associated with male infertility (P = 0.047, OR = 1.262, 95%CI = 1.003–1.587). Furthermore, in the subgroup analyses based on ethnicity and case types, no significant association was found between the T12A polymorphism and male infertility in the Caucasian group (P = 0.213, OR = 1.259, 95%CI = 0.876–1.808), Asian group (P = 0.122, OR = 1.264, 95%CI = 0.939–1.700), azoospermia group (P = 0.102, OR = 1.411, 95%CI = 0.934–2.129) and OAT group (P = 0.986, OR = 0.986, 95%CI = 0.616–1.579).
Forest plot of the T12A polymorphism and male infertility risk in the co-dominant model.
Studies are plotted according to the last name of the first author and followed by the publication year in parentheses. Horizontal lines represent 95% CI. Each square represents the OR point estimate and its size is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with confidence interval given by its width. The unbroken vertical line is at the null value (OR = 1.0). CI, confidence interval; OR, odds ratio.
Meta-analysis of T54A polymorphism and male infertility
The evaluation of the association between T54A polymorphism and male infertility risk is summarized in Table 4 and Figure 3. In the overall analysis, significant association was found between T54A polymorphism and male infertility under co-dominant model (for AG vs. AA: OR = 4.364, 95%CI = 2.207–8.630, P < 0.001) and dominant model (OR = 4.584, 95%CI = 2.320–9.058, P < 0.001). To clarify the potential ethnic difference, subgroup analysis by ethnicity of study population was also conducted. Significant association was found between T54A polymorphism and the risk of male infertility in the subgroups of Asians under co-dominant model (for AG vs. AA: OR = 4.842, 95%CI = 2.339–10.025, P < 0.001) and dominant model (OR = 5.097, 95%CI = 2.463–10.546, P < 0.001). While no such conclusion can be found in Caucasian group under co-dominant model (for AG vs. AA: OR = 1.195, 95%CI = 0.124–11.549, P = 0.878) and dominant model (OR = 1.195, 95%CI = 0.124–11.549, P = 0.878).
Forest plot of the T54A polymorphism and male infertility risk in the co-dominant model and dominant model.
Studies are plotted according to the last name of the first author and followed by the publication year in parentheses. Horizontal lines represent 95% CI. Each square represents the OR point estimate and its size is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with confidence interval given by its width. The unbroken vertical line is at the null value (OR = 1.0). CI, confidence interval; OR, odds ratio.
Publication Bias and Small-study Effects
Begg's funnel plot and Egger's test were performed to assess the publication bias of literatures. For T12A, the shape of the funnel plot did not reveal any evidence of obvious asymmetry (Figure S2). Moreover, the Egger's test was used to provide statistical evidence of funnel plot symmetry. The results did not suggest any evidence of publication bias or small-study effects (P = 0.678 for AG vs. AA; P = 0.388 for GG vs. AA; P = 0.308 under dominant model; and P = 0.356 under recessive model). For T54A, Egger's test revealed the existence of significant publication bias and small-study effects (P < 0.001 for AG vs. AA; P = 0.021 for GG vs. AA; P < 0.001 under dominant model; and P = 0.022 under recessive model).
Sensitive analysis
Sensitivity analyses were conducted to determine whether modification of the inclusion criteria of the meta-analysis affected the final results. We conducted the sensitive analyses on T12A under co-dominant model by omitting one study at a time in the calculation of the summary outcome. The results showed that no study fundamentally changed the relationship between T12A and risk of male infertility. As for T54A, although the sample size for cases and controls in all eligible studies varies, the corresponding pooled ORs were not qualitatively altered with or without any study (Figure S3).
Trial sequential analysis
With the settings mentioned in the Methods section, we calculated the required information size to 3883 patients. As the number of patients included in the meta-analysis did not exceed the required information size, we also applied futility boundaries to potentially facilitate a firm ‘negative’ conclusion. The resulting trial sequential analysis is shown in Figure 4. The trial sequential analysis showed that the cumulative Z-curve (blue line) did cross the conventional P = 0.05 boundary (red straight lines), but failed to cross the trial sequential monitoring boundaries for harm (inward sloping red lines). Neither did the cumulative Z-curve reach the trial sequential monitoring boundaries for futility. Within the set assumptions for confidence and effect size, we found that in T12A the A > G risk effect turned out to be false positive. As for T54A, the positive result was confirmed (Figure 5).
Trial sequential analysis of the T12A polymorphism and male infertility risk in the co-dominant model.
The diversity-adjusted required information size (3883 participants) was based on a relative risk reduction of 10%; an alpha of 5% and a beta of 5%. The blue line represents the cumulative Z-score of the meta-analysis. The red straight represent the conventional P = 0.05 statistical boundaries. The inward sloping red lines represent the truncated trial sequential monitoring boundaries.
Trial sequential analysis of the T54A polymorphism and male infertility risk in the co-dominant model.
The diversity-adjusted required information size (5705 participants) was based on a relative risk reduction of 10%; an alpha of 5% and a beta of 5%. The blue line represents the cumulative Z-score of the meta-analysis. The red straight represent the conventional P = 0.05 statistical boundaries. The inward sloping red lines represent the truncated trial sequential monitoring boundaries.
Discussion
Spermatogenesis is a complex process of mitotic and meiotic divisions of germ cells finally resulting in the formation of haploid spermatozoa28. A highly coordinated expression of genes and a subtle post-transcriptional regulation are therefore, crucial for normal germ cell development29. A most recent meta-analysis on T12A polymorphism included six studies30. Although the previous meta-analysis may be involved some parts of the relationship between T12A polymorphism and male infertility risk, its eligible studies are not quite comprehensive. The lack of four published studies17,24,25,26 may lead to the decrease of studies and may cause a deviation to the final result. Also it failed to involve explore the ethnicity background differences between studies. In our present study, we included 10 studies, dating back from 2002 to 2014. Moreover instead of comparing AA vs. GG/AG, we compared the distribution of AG vs. AA, GG vs. AA, AG/GG vs. AA (dominant model) and GG vs. AA/AG (recessive model) among case group and control group. Interestingly, we found that when companied with AA genotype, AG is a risk factor to male infertility (P = 0.047, OR = 1.262, 95%CI = 1.003–1.587). However, when using trial sequential analysis (TSA) to confirm, we found that A > G risk effect turned out to be false positive. Our analysis suggests that further exploration of the true association between DAZL polymorphism and male infertility is demanded.
The literature on the relationship between T54A polymorphism and male infertility risk is replete with small studies that report controversial findings. No clear consensus has been reached and there is no meta-analysis. Only in Taiwanese population T54A polymorphism do pose a significant difference between case and control group. We have the following assumptions: firstly, the studies from mainland China with a relatively small sample size (average of 171 cases and 89 controls) might lack of adequate power to draw a fair conclusion. Inconsistent associations in Caucasian data indicate that there may be differences in the magnitude of the contribution to male infertility susceptibility by ethnicity. Secondly, the progress of male infertility has long been seen as the outcome of the interaction between gene and environment31. Take the recent mice experiment as an example, DAZL was observed to be translocated to stress granules (SGs) upon heat stress. Furthermore, SG assembly activity was significantly diminished in the early male germ cells of Dazl-knockout mice. The findings suggest DAZL's interactions with environment is essential in protecting male germ cells from heat stress32. Taiwan is geographically far away from China mainland, this discrepancy may also be attributed to the different climate, diet, lifestyle and economic status. Hence, chances are high that the conflicting results of genetic studies of DAZL can be explained when taking interaction into account. Thirdly, apart from interactions, SNPs' joint effects and DAZL haplotypes should also be considered. An individual with a clinical disorder is not the product of the single gene that is disrupted, but that the genetic disruption is embedded within the context of that individual's entire genome33. In a study carried in Taiwan, protective haplotypes occurred in 72% of fertile controls and deleterious haplotypes occurred in 59.2% of infertile men, suggesting the association between autosomal DAZL haplotypes and human spermatogenic failure23,34,35. Last but not the least, novel missense mutations in DAZL and DAZL's role of epigenetic mechanisms in male infertility should be taken into future studies36. Teng et al.37 found that 792G > A was more prevalent in the infertile men and it affected sperm concentration and motility. In a recent study, abnormal DNA methylation of the DAZL promoter is found to be closely associated with oligozoospermia38.
Our meta-analysis suggests that DAZL has a relationship with male infertility, but the exact molecular mechanisms of how the variant T54A (located on exon 3) affects male infertility is unknown. In order to explore the probable mechanisms, a secondary structure of the DAZL mRNA sequence prediction was performed, using RNAfold (http://rna.tbi.univie.ac.at/cgi-bin/.RNAfold.cgi)39. Pointed areas in Figure 6 show significant changes of RNA structure both under MFE (minimum free energy) model and centroid secondary structure, suggesting the T54A polymorphism might affect the stability of the RNA or the interaction of the RNA with other macromolecules.
Prediction of the secondary structure of mRNA sequence containing the T54A variants.
All structures were predicted with RNAfold software (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi).
However, some limitations need to be addressed in our meta-analysis. Firstly, some studies with small sample size may not have enough statistical power to explore the real association and are thought to be more likely to report larger beneficial effects than large trials40. Secondly, our results were based on unadjusted estimates, while a more precise analysis should be conducted if all individual data were available, which would allow for the adjustment by other co-variants and even novel algorithms to limit the wide-spreading data fitting problems41. Thirdly, inclusion of zero-event trials can sometimes decrease the effect size estimate and narrow confidence intervals42.
In conclusion, our meta-analysis suggested that further exploration of the true association between T12A polymorphism and male infertility is demanded. At the same time, significant associations were found between the T54A polymorphism and male infertility under co-dominant model and dominant model. Concerning male infertility with multifactorial etiology, more studies with a large sample size and stratified by different ethnic background, environmental exposure or other risk factors are needed to be performed to clarify the possible roles of DAZL polymorphism in the pathogenesis of male infertility in the future.
Methods
Study selection
We systematically collected published studies from 2002 to 2014 by searching both the common English database (PubMed) and the Chinese literature databases [CNKI (http://www.cnki.net), VIP (http://www.cqvip.com) and WanFang (http://www.wanfangdata.com.cn)]. The following searching phrases were used: (DAZL or deleted-in-azoospermia-like) and (polymorphism or polymorphisms) and male infertility. Data from single reports were extracted and summarized in Table 1 and Table 2.
Two independent reviewers assessed the full text of eligible studies through the above databases. Additional studies were identified by a manually search of references of original or review articles on this topic. The inclusion criteria were: (i) evaluation of T12A, T54A polymorphism and male infertility risk; (ii) studied on human beings; (iii) case-control study design; (iv) had detailed genotype frequency of cases and controls or could be derived from the article text; and (v) the full text of the paper could be obtained.
Data extraction and verification
Firstly, two reviewers independently screened the citations using the inclusion criteria. Next, one reviewer extracted the data and the other cross-checked the data. Any disagreement was resolved by reviewing and discussing. The main elements of the extracted information included the first author's name, year of publication, country or region of origin, ethnicity, number of cases and controls. Different ethnicity was categorized as Asian and Caucasian.
Quality score assessment
The quality of the studies was evaluated using the Newcastle–Ottawa scale (NOS)43. The NOS ranges between zero (worst) and nine stars (best). Each study was assessed based on three broad perspectives: selection, comparability and exposure (Table S1). The ultimate score of six stars or more was regarded as high-quality.
Statistical analysis
All statistical analyses were carried out using STATA 12.0 (STATA Corp, LP) and P < 0.05 was considered to be significant. The strength of association between the polymorphisms and male infertility risk was assessed by ORs with 95% CIs. The combined ORs were respectively calculated for three genetic models (co-dominant model, dominant model and recessive model). Furthermore, we conducted subgroup analyses by stratifying ethnicity into Caucasians and Asians separately and case types [oligoasthenoteratozoospermia (OAT), azoospermia]. Taking consideration of possible between-study heterogeneity, a statistical test for heterogeneity was performed. If the P value for heterogeneity was >0.10 and I2 < 50%, indicating an absence of heterogeneity between studies and we used the fixed-effect model to evaluate the summary OR. In contrast, if the P value for heterogeneity was ≤0.10 or I2 ≥ 50%, indicating a high extent of heterogeneity between studies and we used the random-effect model to evaluate the summary OR. A fixed-effect model using the Mantel-Haenszel method and a random-effects model using the DerSimonian and Laird method were used to combine values from studies. Begg's and Egger's test and funnel plots were utilized to provide a diagnosis of publication bias and small-study effects (linear regression asymmetry test). Due to the lack of polymorphism, several trials report zero events in both infertility and control groups. Exclusion of these trials could inflate the size of pooled treatment. To compensate for this we applied a continuity correction of 0.5 in zero-event trials44. Furthermore, we conducted a sensitive analysis to investigate the influence of a single study on the overall effect estimate by omitting one study in each turn.
Trial sequential analysis
A novel statistical analysis software, TSA (The Copenhagen Trial Unit, Center for Clinical Intervention Research, Denmark), can adjust the threshold for statistical significance according to the quantified strength of evidence and the impact of multiplicity. A meta-analysis may result in type I errors and type II errors if data are sparse or if there is repeated testing for significance when new trials are added45,46.
To minimize the risk of type-I errors, TSA program was used. TSA combines conventional meta-analysis methodology with meta-analytic sample size considerations (i.e., required information size) and methods for repeated significance testing on accumulating data in trials. Recent studies show that TSA has the potential to make conclusions more reliable than those traditional meta-analyses45,47. The required information size was calculated according to an overall type-I error of 5%, a power of 95% and a relative risk reduction (RRR) assumption of 10%. A continuity correction of 0.5 was also applied in zero-event trials.
References
de Kretser, D. M. Male infertility. Lancet 349, 787–790, S0140673696083419 (1997).
Phillips, K. P. & Tanphaichitr, N. Human exposure to endocrine disrupters and semen quality. J. Toxicol. Environ. Health B Crit. Rev. 11, 188–220, 10.1080/10937400701873472 (2008).
Yang, Y., Jia, C. W., Ma, Y. M., Zhou, L. Y. & Wang, S. Y. Correlation between HPV sperm infection and male infertility. Asian J. Androl. 15, 529–532, 10.1038/aja.2013.36 (2013).
Zhang, X. F. et al. Bisphenol A exposure modifies DNA methylation of imprint genes in mouse fetal germ cells. Mol. Biol. Rep. 39, 8621–8628, 10.1007/s11033-012-1716-7 (2012).
Nagafuchi, S. et al. A minute deletion of the Y chromosome in men with azoospermia. J. Urol. 150, 1155–1157 (1993).
Reijo, R. et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat. Genet. 10, 383–393, 10.1038/ng0895-383 (1995).
Nakahori, Y. et al. The Y chromosome region essential for spermatogenesis. Horm. Res. 46 Suppl 1, 20–23 (1996).
Saxena, R. et al. The DAZ gene cluster on the human Y chromosome arose from an autosomal gene that was transposed, repeatedly amplified and pruned. Nat. Genet. 14, 292–299, 10.1038/ng1196-292 (1996).
Lee, K. H. et al. Dazl can bind to dynein motor complex and may play a role in transport of specific mRNAs. EMBO J. 25, 4263–4270, 10.1038/sj.emboj.7601304 (2006).
Reynolds, N. et al. Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum. Mol. Genet. 14, 3899–3909, 10.1093/hmg/ddi414 (2005).
Haston, K. M., Tung, J. Y. & Reijo Pera, R. A. Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS One 4, e5654, 10.1371/journal.pone.0005654 (2009).
Lin, Y. M. et al. Expression patterns and transcript concentrations of the autosomal DAZL gene in testes of azoospermic men. Mol. Hum. Reprod. 7, 1015–1022, 10.1093/molehr/7.11.1015 (2001).
Ruggiu, M., Saunders, P. T. & Cooke, H. J. Dynamic subcellular distribution of the DAZL protein is confined to primate male germ cells. J. Androl. 21, 470–477, 10.1002/j.1939-4640.2000.tb03403.x (2000).
Slee, R. et al. A human DAZ transgene confers partial rescue of the mouse Dazl null phenotype. Proc. Natl. Acad. Sci. U. S. A. 96, 8040–8045 (1999).
Singh, K. & Raman, R. A386G polymorphism of the DAZL gene is not associated with idiopathic male infertility in North India. J. Hum. Reprod. Sci. 2, 54–56, 10.4103/0974-1208.57222 (2009).
Tschanter, P. et al. No association of the A260G and A386G DAZL single nucleotide polymorphisms with male infertility in a Caucasian population. Hum. Reprod. 19, 2771–2776, 10.1093/humrep/deh522 (2004).
Wen, X. H., Zhang, J. Y., Zuo, W. J. & Wu, W. Autosomal DAZL single nucleotide polymorphisms not associated with male infertility in northeast China. Zhonghua Nan Ke Xue 13, 713–717 (2007).
Yang, X. J. et al. Survey of the two polymorphisms in DAZL, an autosomal candidate for the azoospermic factor, in Japanese infertile men and implications for male infertility. Mol. Hum. Reprod. 11, 513–515, 10.1093/molehr/gah202 (2005).
Thangaraj, K. et al. A to G transitions at 260, 386 and 437 in DAZL gene are not associated with spermatogenic failure in Indian population. Int. J. Androl. 29, 510–514, 10.1111/j.1365-2605.2006.00685.x (2006).
Teng, Y. N. et al. Association of a single-nucleotide polymorphism of the deleted-in-azoospermia-like gene with susceptibility to spermatogenic failure. J. Clin. Endocrinol. Metab. 87, 5258–5264, http://dx.doi.org/10.1210/jc.2002-020016 (2002).
Bartoloni, L., Cazzadore, C., Ferlin, A., Garolla, A. & Foresta, C. Lack of the T54A polymorphism of the DAZL gene in infertile Italian patients. Mol. Hum. Reprod. 10, 613–615, 10.1093/molehr/gah073 (2004).
Becherini, L. et al. DAZL polymorphisms and susceptibility to spermatogenic failure: an example of remarkable ethnic differences. Int. J. Androl. 27, 375–381, 10.1111/j.1365-2605.2004.00520.x (2004).
Teng, Y. N. et al. Association of DAZL haplotypes with spermatogenic failure in infertile men. Fertil. Steril. 86, 129–135, 10.1016/j.fertnstert.2005.12.027 (2006).
Kumar, K., Venkatesh, S., Sharma, P. R., Tiwari, P. K. & Dada, R. DAZL 260A > G and MTHFR 677C > T variants in sperm DNA of infertile Indian men. Indian J. Biochem. Biophys. 48, 422–426 (2011).
Ye, L. W. et al. DAZL gene polymorphisms and asthenoteratozoospermia. Zhonghua Nan Ke Xue 19, 311–314 (2013).
Wang huan, D. X. Association of a single-nucleotide polymorphism of the deleted-in-azoospermia-like gene with male infertility:study using liquid-chip technology. J. Sichuan Univ. (Med Sci Edi) 40, 1135–1138 (2009).
Poongothai, J., Gopenath, T. S. & Manonayaki, S. A386G transition in DAZL gene is not associated with spermatogenic failure in Tamil Nadu, South India. Indian J. Hum. Genet. 14, 16–19, 10.4103/0971-6866.42322 (2008).
Li, X., Mao, Z., Wu, M. & Xia, J. Rescuing infertility of Pick1 knockout mice by generating testis-specific transgenic mice via testicular infection. Sci. Rep. 3, 2842, 10.1038/srep02842 (2013).
Sutcliffe, M. J. & Burgoyne, P. S. Analysis of the testes of H-Y negative XOSxrb mice suggests that the spermatogenesis gene (Spy) acts during the differentiation of the A spermatogonia. Development 107, 373–380 (1989).
Tuttelmann, F., Rajpert-De Meyts, E., Nieschlag, E. & Simoni, M. Gene polymorphisms and male infertility--a meta-analysis and literature review. Reprod. Biomed. Online 15, 643–658 (2007).
Harland, S. J. Conundrum of the hereditary component of testicular cancer. Lancet 356, 1455–1456, 10.1016/S0140-6736(00)02863-4 (2000).
Kim, B., Cooke, H. J. & Rhee, K. DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development 139, 568–578, 10.1242/dev.075846 (2012).
Dipple, K. M., Phelan, J. K. & McCabe, E. R. Consequences of complexity within biological networks: robustness and health, or vulnerability and disease. Mol. Genet. Metab. 74, 45–50, 10.1006/mgme.2001.3227 (2001).
Tung, J. Y. et al. Variants in Deleted in AZoospermia-Like (DAZL) are correlated with reproductive parameters in men and women. Hum. Genet. 118, 730–740, 10.1007/s00439-005-0098-5 (2006).
Hsu, C. C. et al. Quantitative trait analysis suggests human DAZL may be involved in regulating sperm counts and motility. Reprod. Biomed. Online 21, 77–83, 10.1016/j.rbmo.2010.03.026 (2010).
Tung, J. Y. et al. Novel missense mutations of the Deleted-in-AZoospermia-Like (DAZL) gene in infertile women and men. Reprod. Biol. Endocrinol. 4, 40, 10.1186/1477-7827-4-40 (2006).
Teng, Y. N. et al. A single-nucleotide polymorphism of the DAZL gene promoter confers susceptibility to spermatogenic failure in the Taiwanese Han. Hum. Reprod. 27, 2857–2865, 10.1093/humrep/des227 (2012).
Navarro-Costa, P. et al. Incorrect DNA methylation of the DAZL promoter CpG island associates with defective human sperm. Hum. Reprod. 25, 2647–2654, 10.1093/humrep/deq200 (2010).
Gruber, A. R., Lorenz, R., Bernhart, S. H., Neubock, R. & Hofacker, I. L. The Vienna RNA websuite. Nucleic. Acids. Res. 36, W70–74, 10.1093/nar/gkn188 (2008).
Zhang, Z., Xu, X. & Ni, H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit. Care. 17, R2, 10.1186/cc11919 (2013).
Li, J. et al. Identification of high-quality cancer prognostic markers and metastasis network modules. Nat. Commun. 1, 34, 10.1038/ncomms1033 (2010).
Diamond, G. A., Bax, L. & Kaul, S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann. Intern. Med. 147, 578–581 (2007).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Proceedings of the 3rd symposium on systematic reviews: beyond the basics;. Oxford, UK. Oxford: Centre for Statistics in Medicine. 2000 Jul 3–5.
Friedrich, J. O., Adhikari, N. K. & Beyene, J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med. Res. Methodol. 7, 5, 10.1186/1471-2288-7-5 (2007).
Bjelakovic, G., Nikolova, D. & Gluud, C. Meta-regression analyses, meta-analyses and trial sequential analyses of the effects of supplementation with Beta-carotene, vitamin a and vitamin e singly or in different combinations on all-cause mortality: do we have evidence for lack of harm.? PLoS One 8, e74558, 10.1371/journal.pone.0074558 (2013).
Borm, G. F. & Donders, A. R. Updating meta-analyses leads to larger type I errors than publication bias. J. Clin. Epidemiol. 62, 825–830 e810, 10.1016/j.jclinepi.2008.08.010 (2009).
Moller, C. H., Penninga, L., Wetterslev, J., Steinbruchel, D. A. & Gluud, C. Clinical outcomes in randomized trials of off- vs. on-pump coronary artery bypass surgery: systematic review with meta-analyses and trial sequential analyses. Eur. Heart. J. 29, 2601–2616, 10.1093/eurheartj/ehn335 (2008).
Acknowledgements
We thank Prof. Jianming Wang and Dr. Shaowen Tang (Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, PR China) for their expert advice on meta-analysis and TSA. We also appreciate the valuable comments from other members of our laboratory. This work was supported by grants National Natural Science Foundation of China (No. 81302457), Jiangsu Natural Science Foundation (No. BK20130894), China Postdoctoral Science Foundation (No. 2013M531388), Jiangsu Postdoctoral Science Foundation (No. 1301053B), University Natural Science Research Project in Jiangsu Province (No. 13KJB330002), Practice and Innovation Training Programs for Jiangsu Province College Students (No. 201310312051X), Science and Technology Development Fund Key Project of Nanjing Medical University (No. 2012NJMU002) and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: W.W., S.Z., Q.T. Searched for and selected the publications: S.Z., Q.T. Analyzed the data: S.Z., Q.T., B.Y., L.C. Prepare figures: W.W., S.Z., Q.T. Contributed materials/analysis tools: W.W., Y.X., H.D., L.H., D.C. Wrote and revised the paper: W.W., J.S., X.W.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Zhang, S., Tang, Q., Wu, W. et al. Association between DAZL polymorphisms and susceptibility to male infertility: systematic review with meta-analysis and trial sequential analysis. Sci Rep 4, 4642 (2014). https://doi.org/10.1038/srep04642
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04642
This article is cited by
-
AZF deletions in Indian populations: original study and meta-analyses
Journal of Assisted Reproduction and Genetics (2020)
-
The susceptibility of FSHB -211G > T and FSHR G-29A, 919A > G, 2039A > G polymorphisms to men infertility: an association study and meta-analysis
BMC Medical Genetics (2017)
-
Idiopathic male infertility and polymorphisms in the DNA methyltransferase genes involved in epigenetic marking
Scientific Reports (2017)
-
Gr/gr deletions on Y-chromosome correlate with male infertility: an original study, meta-analyses and trial sequential analyses
Scientific Reports (2016)
-
Amniotic membrane mesenchymal stem cells can differentiate into germ cells in vitro
In Vitro Cellular & Developmental Biology - Animal (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.