Abstract
Resveratrol (3, 5, 4′-trihydroxy-trans-stilbene), a plant polyphenol, has important drug-like properties, but its pharmacological exploitation in vivo is hindered by its rapid transformation via phase II conjugative metabolism. One approach to bypass this problem relies on prodrugs. We report here the synthesis, characterization, stability and in vivo pharmacokinetic behaviour of prodrugs of resveratrol in which the OH groups are engaged in an N-monosubstituted carbamate ester (-OC(O)NHR) linkage with a natural amino acid (Leu, Ile, Phe, Thr) to prevent conjugation and modulate the physicochemical properties of the molecule. We also report a convenient, high-yield protocol to obtain derivatives of this type. The new carbamate ester derivatives are stable at pH 1, while they undergo slow hydrolysis at physiological pH and hydrolyse with kinetics suitable for use in prodrugs in whole blood. After administration to rats by oral gavage the isoleucine-containing prodrug was significantly absorbed and was present in the bloodstream as non-metabolized unaltered or partially deprotected species, demonstrating effective shielding from first-pass metabolism. We conclude that prodrugs based on the N-monosubstituted carbamate ester bond have the appropriate stability profile for the systemic delivery of phenolic compounds.
Similar content being viewed by others
Introduction
Resveratrol (trans-3,5,4′-trihydroxystilbene), is a naturally occurring phytoalexin produced by some plants (e.g. V. vinifera) in response to stresses1. It exhibits a range of activities of biomedical interest, including lifespan extension in model systems2, protection of the cardiovascular apparatus3,4,5,6, anti-inflammatory activity3, improvement of glucose handling in diabetes7,8, decrease of fat and cholesterol load9,10,11,12, improvements of functionality in aging2,13,14,15, neuroprotection16, cancer chemoprevention17,18 and potentiation of chemotherapy19,20.
The efficacy of orally administered resveratrol depends on its absorption, metabolism and tissue distribution. Clinical studies with humans reported mostly small effects and sometimes controversial results21,22. Beneficial effects in vivo are in fact limited by low bioavailability. Thus, for example, administration of even very large doses (2.5–5.0 g) to volunteers resulted in circulating levels of non-metabolized resveratrol so low as to be considered insufficient for any bioactivity23. Resveratrol, like other polyphenols, is rapidly converted to phase II metabolites (mainly glucuronides and sulfates) during absorption and first pass through the liver. These hydrophilic metabolites are re-exported to the intestinal lumen by enterocyte ABC transporters, and/or rapidly excreted with faeces and urine23,24. Nonetheless, given their preponderance, their bioactivities are now under investigation to verify whether they may account for resveratrol’s effects. The available data suggest that a level of activity may well be maintained in specific contexts (e.g.25,26,27,28), but conjugation often determines at least a partial loss of bioefficacy29,30,31,32,33,34. Retention of bioactivity in vivo may be due in part to the metabolites acting as a temporary reservoir from which the more active species may be regenerated through the activity of enzymes such as glucuronidases and sulfatases35,36. While investigation of these aspects continues, a reduction or delay of phase II metabolism remains a primary target for the biomedical exploitation of resveratrol.
One of the strategies used to prevent drug metabolism and enhance bioavailability and effectiveness is based on prodrugs. In a successful resveratrol prodrug, the natural active compound is protected against phase II metabolism by having the reactive hydroxyl groups masked as suitably reversible derivatives (-OH → -OXR). The capped hydroxyl functionalities (-OXR) should withstand the processes of absorption, first pass through the liver and distribution. Once in the bloodstream, the prodrug should then release the active natural compound, resveratrol (-OXR → -OH). Appropriate regeneration kinetics may provide a form of sustained delivery, a possibly convenient alternative to other approaches such as slow-release capsules. Optimal prodrug performance is sought by modulating its physicochemical properties using the best combination of X, the reversible protecting linkage (ester, carbamate, acetal, …) and of R, the promoiety. The type of linkage X is mostly relevant for prodrug persistence whereas the promoiety R may determine its solubility in aqueous media and partitioning between aqueous and lipidic (membrane) phases. One crucial additional requirement is that hydrolysis of the prodrug produce, besides the original active natural compound, safe by-products.
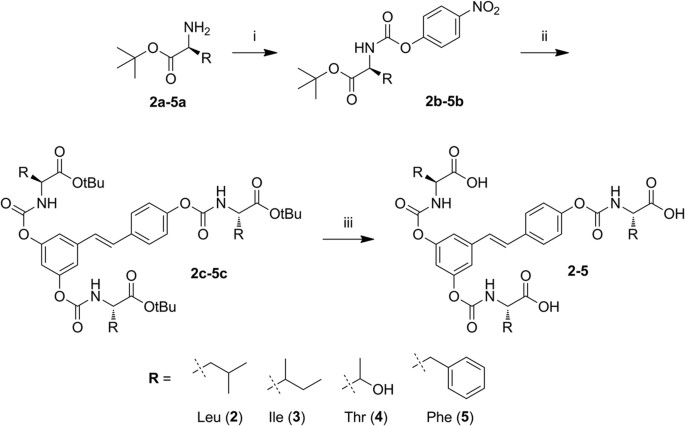
The carbamoyl moiety provides a versatile protecting group for phenols (-OH → -OC( = O)NR′R″)37,38, which allows fine-tuning of the properties of the molecule since the two substituents on nitrogen, R′ and R″, can be tailored to modulate the stability and physicochemical properties of the resulting prodrug. Several prodrugs have been developed to improve oral bioavailability of phenolic compounds39,40,41 but surprisingly there are only few examples of carbamate ester prodrugs of polyphenols. Mulholland et al.42 reported a water-soluble glycine N-monosubstituted carbamate ester prodrug (QC12) of the flavonoid quercetin. The advantage of QC12 was its high aqueous solubility. Quercetin-amino acid N-monosubstituted carbamate esters were also synthesized by Kim et al.43 and showed remarkable increases in water solubility, stability and cell permeability compared with the parent polyphenol. We have previously reported that N,N-disubstituted carbamoyl derivatives of resveratrol are too stable under physiological conditions to be of practical use as prodrugs44. We therefore turned our attention to N-monosubstituted carbamoyl derivatives -OC( = O)NHR and report here the synthesis and the properties of a family of new prodrugs in which three units of a natural amino acid are linked to resveratrol via carbamoyl derivatization of the three hydroxyl groups (Fig. 1).
Use of amino acids as promoieties in prodrug design has been recently reviewed45. Their presence increases water solubility and they are considered to be safe hydrolysis by-products. Moreover, we reasoned that prodrugs incorporating suitable amino acids in the promoieties might be recognized and transported by the various epithelial amino acid46 and peptide47,48,49 carrier systems. We selected Leu, Ile and Phe (Fig. 1) because intestinal epithelia are well provided with carriers of neutral/large amino acids. These are, in particular, known substrates of the LAT (Large-neutral Amino acid Transporters or L-type Amino acid Transporters)50 systems, with affinities in the μM range. Amino acid transporters LAT1 and LAT2 have been previously exploited for the transport/absorption of drugs; they are especially useful for the permeation of the blood brain barrier51,52,53. We also included threonine as a “control” amino acid because it is not efficiently transported by LAT (although it is a known substrate of the ASCT2 system)46.
The performance of the new derivatives 2–5 was assessed by kinetic studies of hydrolysis in aqueous media under different pH conditions of physiological relevance (strongly acidic, as in the stomach and near neutral, as in the intestine) as well as in blood. Pharmacokinetic experiments in rats were also performed in order to evaluate in vivo absorption, stability and metabolism of these new prodrugs.
Results & Discussion
Synthesis
Synthesis of N-monosubstituted carbamate esters is usually carried out in two steps: reaction of the desired primary amine with phosgene or its equivalent to give a reactive isocyanate derivative, followed by coupling of this intermediate with the phenolic function54. These procedures, however, led to the desired trisubstituted resveratrol derivatives in low yields, probably due to the high reactivity of the isocyanate group promoting side reactions of polymerization entraining the stilbene C-C double bond. In this study, therefore, we utilized and optimized an alternative approach via an activated 4-nitrophenyl carbamate intermediate43. By this route, outlined in Fig. 2, we prepared N-monosubstituted resveratrol carbamate esters 2–5 in fair overall yields.
The procedure involves conversion of the C-protected amino acids 2a–5a into the corresponding activated 4-nitrophenyl carbamates 2b–5b. After isolation, these intermediates are treated with resveratrol to afford the product of transesterification, the t-butyl protected resveratrol amino acid carbamate ester conjugates 2c–5c in good to excellent yields under mild conditions. The desired final products 2–5 are readily obtained by removal of the t-butyl protecting group by treatment with TFA.
Hydrolysis studies
The hydrolytic reactivity of the new derivatives 2–5 was tested in aqueous solutions mimicking gastric and intestinal pH and also in blood. All compounds turned out to be highly stable at pH values close to that of the human stomach, no reaction occurring over 24 hours at 37 °C in 0.1 N HCl and they underwent slow hydrolysis at near-neutral pH (pH 6.8, representing intestinal pH) thus ensuring protection of the phenolic moieties from first pass metabolism during absorption in the gastrointestinal tract. In contrast all the synthesized prodrugs hydrolyzed in murine whole blood, with kinetics suitable for use as prodrugs.
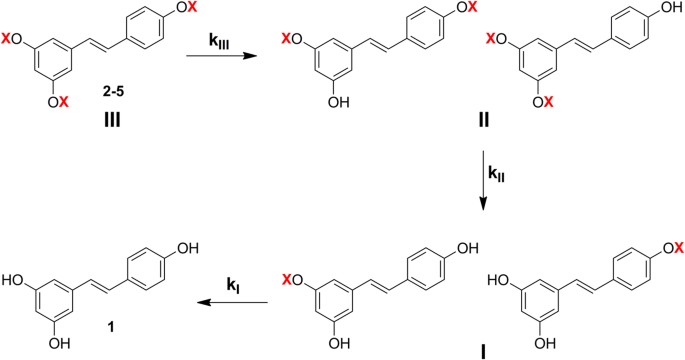
Hydrolysis of derivatives 2–5 to resveratrol proceeds stepwise through intermediates in which initially one and then two protecting groups have been sequentially removed, as shown in Fig. 3. There are two possible isomeric disubstituted intermediates and, likewise, two monosubstituted intermediates. By HPLC-UV and HPLC-MS analyses in most cases we were able to detect and separate all of them. However, to simplify the kinetic analysis of the data we considered each pair of isomeric intermediates, resulting from the first and second hydrolysis steps, as a single species, i.e. the two disubstituted and the two monosubstituted intermediates were handled as species II and I, respectively (Fig. 3). Fitting of the experimental data was then performed considering consecutive pseudo first order reactions and using a set of equations analogous to those described by Kozerski et al.55
Examples are shown in Fig. 4, which reports the results of kinetic and product analysis for hydrolysis of derivatives 3 (a, b) and 5 (c, d) in aqueous PBS 0.1 M, at pH 6.8 and 37 °C (a, c) and in rat blood (b, d).
Kinetics and products of hydrolysis of derivatives 3 (a,b) and 5 (c,d): (a) and (c): aqueous PBS 0.1 M at pH 6.8 and 37 °C; (b) and (d): rat blood.
The experimental data were processed and fitted as described in the text, with reference to Fig. 3. Please note that the abscissa scale varies among panels.
The full set of kinetic results obtained for hydrolysis of derivatives 2–5 according to the procedures described above is presented in Table 1 and, graphically, in Fig. 5.
The stability of our derivatives 2–5 in acidic solution and their reactivity at higher pH’s are consistent with a mechanism of base catalyzed hydrolysis of carbamates proceeding via deprotonation and elimination to give an isocyanate intermediate which rapidly adds water and decomposes releasing carbon dioxide and the amino acid (Fig. 6)56.
The data show that hydrolysis is much faster in blood, suggesting the involvement of enzymes. This notion is reinforced by the variability of the rates from one compound to the other. Hansen38 and co-workers have demonstrated that the predominant rate-accelerating component of plasma (human in that study) is albumin.
Pharmacokinetics studies
Derivatives 2–5 were then tested for their in vivo absorption and metabolism by performing pharmacokinetic studies after oral administration to rats. Each compound was administered as a single intragastric bolus, in an equimolar dose/kg body weight (88 μmol/kg). Blood samples were taken at different time points over a 24 h period, treated and analyzed as described in the Materials and Methods section. Each experiment was replicated at least 3 times.
Contrary to our expectations the linkage of resveratrol through the N-monosubstituted carbamate ester to amino acids resulted in poor absorption of the prodrugs after oral administration. We can only speculate that this result is probably due to the excessive hydrophilicity conferred by the three carboxylic ionisable groups present in the derivatives, which hinder permeation of lipophilic biomembranes. It is furthermore evident that these trisubstituted prodrugs were not readily recognized and/or transported by aminoacid carriers. The best results were obtained with the isoleucine derivative, 3, as summarized in Fig. 7. About one hour after oral administration the concentration of this prodrug in blood was higher than 0.5 μM and remained around this level for several hours (Fig. 7). Disubstituted hydrolysis products (II, Fig. 3) were also present, indicating the bioreversibility of the carbamate linker also in vivo and, interestingly, their level was about as high as that of their precursor, the trisubstituted derivative 3. Notably, neither sulfated nor glucuronidated species appeared in the bloodstream confirming the protection by the N-monosubstituted carbamate linker from first pass metabolism during absorption.
The concentration of tri- and di- substituted carbamate-Ile resveratrol prodrugs was substantially maintained for at least 8 hours, i.e. ample time for equilibration with organs. This behaviour may be due to the fact that the derivative is constantly absorbed from the intestinal mucosa and/or that it is slowly cleared from the body. Resveratrol (1) and monosubstituted carbamate-Ile (I) were not detected in the bloodstream probably because the blood concentration of these species were too low for reliable measurement. Nature and levels of the species found in blood may not be representative of the nature and levels of stilbenoid species in other organs: the hydrophilicity of the amino acid-decorated compounds is expected to result in their enrichment in blood, while less hydrophilic molecules, e.g. resveratrol itself, are expected to associate with membrane-rich compartments57,58.
In any case, the prodrug appears to perform as a slow-action vehicle providing a sustained delivery of precursor. By comparison, blood concentration of resveratrol after oral administration of an equimolar amount of resveratrol itself peaks at approximately 1 μM after only about 10 minutes from administration and rapidly declines. Phase II metabolites peak (∼10 μM) at about 1 h and then decline by 80–90% over the next 7 hours59.
In contrast with the behaviour of 3, administration of compounds 4 and 5 did not result in the appearance in blood samples of detectable amounts of either resveratrol, products of partial hydrolysis (II and I) or any metabolites. Similar results were also obtained with 2, although in this case the presence of very small amounts of partially hydrolyzed derivatives (II and I) cannot be excluded due to interfering co-eluting peaks due to the matrix.
Conclusions
The N-monosubstituted carbamate bond is a convenient linker for prodrugs of resveratrol60 which has been coupled in this study with amino acid promoieties. All synthesized compounds have good solubility and stability properties in aqueous media and in blood for use as resveratrol prodrugs. In vivo pharmacokinetic studies revealed an interesting behaviour of the isoleucine derivative 3, which acts as a slow-action vehicle providing a sustained delivery of the prodrug and partially hydrolysed unconjugated species, indicating protection from first pass metabolism during absorption. However, the choice of amino acid as promoieties proved to be unsatisfactory in terms of absorption of the prodrug, probably due to the excessive hydrophilicity of the resulting prodrugs bearing three ionisable groups and because carrier-mediated uptake apparently did not take place to a significant extent. Possibly the prodrugs were too large and ramified to be handled by the transporters. As a future development of this work, we aim to seek a better performing promoiety in order to improve absorption.
Methods
Chemistry
Materials and instrumentation
Resveratrol was purchased from Waseta Int. Trading Co. (Shangai, P.R.China). Other starting materials and reagents were purchased from Aldrich, Fluka, Merck-Novabiochem, Riedel de Haen, J.T. Baker, Cambridge Isotope Laboratories Inc., Acros Organics, Carlo Erba and Prolabo and were used as received. TLCs were run on silica gel supported on plastic (Macherey-Nagel Polygram®SIL G/UV254, silica thickness 0.2 mm) and visualized by UV detection. Flash chromatography was performed on silica gel (Macherey-Nagel 60, 230–400 mesh granulometry (0.063–0.040 mm)) under air pressure. The solvents were analytical or synthetic grade and were used without further purification. 1H NMR spectra were recorded with a Bruker AC250F spectrometer operating at 250 MHz and a Bruker AVII500 spectrometer operating at 500 MHz. Chemical shifts (δ) are given in ppm relative to the signal of the solvent. HPLC-UV analyses were performed with an Agilent 1290 Infinity LC System (Agilent Technologies), equipped with binary pump and a diode array detector (190–500 nm). HPLC/ESI-MS analyses and mass spectra were performed with a 1100 Series Agilent Technologies system, equipped with binary pump (G1312A) and MSD SL Trap mass spectrometer (G2445D SL) with ESI source. ESI-MS positive spectra of reaction intermediates and final purified products were obtained from solutions in acetonitrile, eluting with a water:acetonitrile, 1:1 mixture containing 0.1% formic acid. High resolution mass measurements were obtained using a Mariner ESI-TOF spectrometer (PerSeptive Biosystems). HPLC-MS analysis was used to confirm purity (>95% in all cases).
Synthesis of derivatives 2–5
General procedure for the preparation of activated 4-nitrophenyl urethanes (2b-5b, Fig. 2)
A solution of amino acid t-butyl ester (2a–5a) (8.2 mmol, 1.0 eq.) and DMAP (2.00 g, 16.4 mmol, 2.0 eq.) in acetonitrile (15 mL) was added dropwise to a solution of bis(4-nitrophenyl) carbonate (2.74 g, 9.0 mmol, 1.1 eq.) in acetonitrile (15 mL) and the resulting solution was stirred at 50 °C for 3 hours. The reaction mixture was then diluted in DCM (150 mL) and washed with 0.5 N HCl (100 mL). The aqueous layer was washed with DCM (5 × 100 mL) and all the organic fractions were collected, dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure and the residue was purified by flash chromatography.
Tert-butyl 4-methyl-2-(((4-nitrophenoxy)carbonyl)amino)pentanoate (2b)
Purified by flash chromatography using DCM:Acetone:Hexane = 8:0.5:1.5 as eluent. The first two spots were collected and the solvent was evaporated under reduced pressure, then the residue was absorbed on silica and purified by flash chromatography using Hexane:Ethyl Ether = 6.5:3.5 as eluent. 65% yield as a pale yellow oil. 1H-NMR (250 MHz, CDCl3) δ (ppm): 0.97–1.01 (m, 6 H, 2 ×-CH-CH3), 1.49 (s, 1 H, -CH-CH3), 1.52–1.85 (m, 11 H, 3 ×-C-CH3and -CH-CH2-), 4.27–4.36 (m, 1 H, NH-CH-), 5.56 (d, 1 H, -CH-NH-, 3JH-H = 8.5 Hz), 7.33 (d, 2 H, Ar-H, 3JH-H = 9.25 Hz), 8.24 (d, 2 H, Ar-H, 3JH-H = 9.25 Hz); 13C-NMR (62.9 MHz, CDCl3) δ (ppm): 171.7, 155.8, 152.7, 125.1, 122.0, 82.5, 53.2, 42.0, 28.0, 27.9, 24.9, 22.8, 22.0. ESI-MS (ion trap): m/z 353 [M+H]+; HRMS (ESI+): m/z 353.1720 [M+H]+, calcd for C17H25N2O6: 353.1713.
Tert-butyl 3-methyl-2-(((4-nitrophenoxy)carbonyl)amino)pentanoate (3b)
Purified by flash chromatography using DCM:Acetone:Hexane = 8:0.5:1.5. 93% yield as a pale yellow oil. 1H-NMR (300 MHz, CDCl3) δ (ppm): 0.95–1.38 (m, 5 H, -CH2-CH3, CH3-CH2-), 1.52 (s, 9 H, 3 ×-C-CH3), 1.87–2.04 (m, 1 H, -CH-CH2-), 4.29 (dd, 1 H, -CH-NH-, 3JH-H = 8.7, 4.3 Hz), 5.76 (d, 1 H, -CH-NH-, 3JH-H = 8.6 Hz), 7.32–7.37 (d, 2 H, Ar-H, 3JH-H = 9.25 Hz), 8.22–8.28 (d, 2 H, Ar-H, 3JH-H = 9.25 Hz); 13C-NMR (300 MHz, CDCl3) δ(ppm): 172.2, 157.6, 154.6, 145.5, 126.8, 123.7, 84.3, 79.2, 78.8, 78.3, 60.5, 29.8, 26.9, 17.1, 13.4. ESI-MS (ion trap): m/z 353 [M+H]+. ESI-MS (ion trap): m/z 353 [M+H]+; HRMS (ESI+): m/z 353.1724 [M+H]+, calcd for C17H25N2O6: 353.1713.
Tert-butyl 3-(tert-butoxy)-2-(((4-nitrophenoxy)carbonyl)amino)butanoate (4b)
Purified by flash chromatography using DCM:Acetone:Hexane = 8:0.5:1.5 as eluent. 93% yield as a pale yellow oil. 1H-NMR (250 MHz, CDCl3) δ (ppm): 1.19 (s, 9 H, 3 × C-CH3), 1.28 (d, 3 H, -CH-CH3, 3JH-H = 6.3 Hz), 1.49 (s, 9 H, 3 ×-C-CH3), 4.05–4.17 (m, 1 H, -O-CH-), 4.22–4.33 (m, 1 H, NH-CH-), 5.94 (d, 1 H, CH-NH-, 3JH-H = 9.5 Hz), 7.35 (d, 2 H, Ar-H, 3JH-H = 9.25 Hz), 8.27 (d, 2 H, Ar-H, 3JH-H = 9.25 Hz); 13C-NMR (62.9 MHz, CDCl3) δ(ppm): 169.7, 156.2, 153.8, 144.9, 125.2, 122.2, 82.5, 74.2, 67.2, 60.8, 28.9, 28.3, 21.4. ESI-MS (ion trap): m/z 397 [M+H]+. HRMS (ESI+): m/z 397.1977 [M+H]+, calcd for C19H29N2O7: 397.1975.
Tert-butyl 2-(((4-nitrophenoxy)carbonyl)amino)-3-phenylpropanoate (5b)
Purified by flash chromatography using DCM:Acetone = 9:1 as eluent. 81% yield as a pale yellow oil. 1H-NMR (250 MHz, CDCl3) δ (ppm): 1.54 (s, 9 H, 3 ×-C-CH3), 3.05–3.35 (m, 2 H, 2 × C-CH2), 4.70 (dd, 1 H, -NH-CH-CH2- J = 14.7, 6.5 Hz), 6.33 (d, 1 H, -NH-, J = 8.3 Hz), 7.22–7.45 (m, 7 H, Ar-H), 8.23 (d, 2 H, Ar-H, 3JH-H = 15.5 Hz); 13C-NMR (62.9 MHz, CDCl3) δ (ppm): 170.4, 155.8, 152.6, 144.6, 136.0, 129.5, 128.1, 127.5, 124.9, 121.9, 82.7, 55.6, 38.2, 27.3. ESI-MS (ion trap): m/z 387 [M+H]+. HRMS (ESI+): m/z 387.1547 [M+H]+, calcd for C20H23N2O6: 387.1556.
General procedure for the preparation of 3,4′,5-N-monosubstituted-resveratrol carbamate esters (2c–5c)
A solution of resveratrol (0.24 g, 1.1 mmol, 1.0 eq.) and DMAP (0.52 g, 4.2 mmol, 4.0 eq.) in ACN (15 mL) was added to a solution of the activated 4-nitrophenyl urethane (2b–5b) (4.8 mmol, 4.5 eq) in ACN (5 mL) and the resulting mixture was allowed to react under vigorous stirring at 50 °C for 24 h. The reaction mixture was diluted with DCM (150 mL) and washed with 0.5 N HCl (100 mL). The aqueous layer was washed with DCM (5 × 75 mL) and all the organic fractions were collected, dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure and the residue was purified by flash chromatography.
(E)-di-tert-butyl 2,2′-((((5-(4-(((1-(tert-butoxy)-4-methyl-1-oxopentan-2-yl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(4-methylpentanoate) (2c)
Purified by flash chromatography using CHCl3 as eluent. 55% yield as a colourless oil. 1H-NMR (250 MHz, CDCl3) δ (ppm): 0.93 (m, 18 H, 6 × CH-CH3), 1.44 (s, 27 H, 9 ×-C-CH3), 1.48–1.53 (m, 9 H, 3 × CH3-CH and 3 ×-CH-CH2), 4.23–4.32 (m, 3 H, 3 ×-NH-CH), 5.64 (m, 3 H, 3 ×-NH-), 6.80–7.07 (m, 7 H, H-4, H-3′, H-5′, H-2, H-6, H-7, H-8), 7.47 (d, 2 H, 3JH-H = 8.5 Hz, H-2′, H-6′); 13C-NMR (62.9 MHz, CDCl3) δ (ppm): 172.1, 172.3, 154.0, 153.7, 151.4, 150.5, 139.0, 134.0, 129.2, 134.0, 129.2, 127.3, 127.0, 121.7, 116.4, 114.2, 82.0, 53.1, 41.9, 27.9, 24.8, 22.8, 21.9. ESI-MS (ion trap): m/z 868 [M+H]+. HRMS (ESI+): m/z 868.4966 [M+H]+, calcd for C47H70N3O12: 868.4959.
(E)-di-tert-butyl 2,2′-((((5-(4-(((1-(tert-butoxy)-3-methyl-1-oxopentan-2-yl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(3-methylpentanoate) (3c)
Purified by flash chromatography using Hexane: EtOAc = 8:2. The first spot was separated and the remaining spots were collected and the solvent was evaporated under reduced pressure, then purified by flash chromatography using DCM:Hexane:EtOAc = 7:2:1. 60% yield as a colourless oil. 1H-NMR (300 MHz, CDCl3) δ (ppm): 0.87–1.05 (m, 18 H, 3 × CH2-CH3, 3 × CH-CH3), 1.12–1.33 (m, 6 H, 3 × CH-CH2-CH3), 1.49 (s, 27 H, 9 × C-CH3), 1.87–2.00 (m, 3 H, 3 × CH-CH-CH3), 4.11 (q, 1 H, -NH-CH-CH-, 3JH-H = 7.1 Hz), 4.23–4.33 (m, 2 H, 2 ×-NH-CH-CH-), 5.79 (d, 3 H, 3 ×-NH-, 3JH-H = 8.7 Hz), 6.82–7.30 (m, 7 H, H-4, H-3′, H-5′, H-2, H-6, H-7, H-8), 7.43 (d, 2 H, 3JH-H = 8.5 Hz, H-2′, H-6′); 13C-NMR (300 MHz, CDCl3) δ(ppm): 170.7, 170.6, 154.1, 153.7, 151.4, 150.6, 139.1, 134.0, 129.2, 127.3, 116.3, 114.2, 82.1, 58.6, 38.2, 27.9, 25.1, 15.3, 11.6. ESI-MS (ion trap): m/z 868 [M+H]+. HRMS (ESI+): m/z 868.4966 [M+H]+, calcd for C47H70N3O12: 868.4959.
(E)-di-tert-butyl 2,2′-((((5-(4-(((1,3-di-tert-butoxy-1-oxobutan-2-yl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(3-(tert-butoxy)butanoate) (4c)
Purified by flash chromatography using DCM:ethyl ether = 95:5 as eluent. 58% yield as a colourless oil. 1H-NMR (250 MHz, CDCl3) δ (ppm): 1.15 (s, 27 H, 9 × C-CH3), 1.23 (d, 9 H, 3 × CH-CH3, 3JH-H = 6.2 Hz), 1.45 (s, 27 H, 9 × C-CH3), 4.04–4.25 (m, 6 H, 3 × CH3-CH and 3 × NH-CH), 5.78 (d, 3 H, 3 ×-NH-, 3JH-H = 9.5 Hz), 6.85–7.30 (m, 7 H, H-4, H-3′, H-5′, H-2, H-6, H-7, H-8), 7.43 (d, 2 H, 3JH-H = 8.5 Hz, H-2′, H-6′); 13C-NMR (62.9 MHz, CDCl3) δ (ppm): 169.6, 154.7, 154.3, 151.4, 150.5, 139.0, 133.9, 129.1, 127.3, 127.1, 121.6, 116.2, 114.1, 81.9, 81.8, 73.7, 66.9, 60.3, 28.5, 27.9, 20.8. ESI-MS (ion trap): m/z 1001 [M+H]+. HRMS (ESI+): m/z 1000.5754 [M+H]+, calcd for C53H82N3O15: 1000.5746.
(E)-di-tert-butyl 2,2′-((((5-(4-(((1-(tert-butoxy)-1-oxo-3-phenylpropan-2-yl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(3-phenylpropanoate) (5c)
Purified by flash chromatography using Hexane:Diethyl ether = 7:3 as eluent until the exit of 4-nitrophenol from the column and Hexane:Diethyl ether:EtOAc = 5:3.5:1.5 thereafter. 57% yield as a colourless oil. 1H-NMR (250 MHz, CDCl3) δ (ppm): 1.41 (s, 27 H, 3 ×-C-CH3), 3.02–3.30 (m, 6 H, 3 × Ph-CH2), 4.16 (q, 1 H, NH-CH-CH2, 3JH-H = 7.1 Hz), 4.64 (q, 2 H, 2 × NH-CH-CH2, 3JH-H = 6.2 Hz), 5.79 (m, 3 H, 3 ×-NH-), 6.81–7.56 (m, 24 H, Ar-H); 13C-NMR (62.9 MHz, CDCl3) δ (ppm): 170.6, 154.0, 153.7, 151.6, 150.8, 139.4, 136.2, 128.8, 128.7, 127.7, 127.3, 122.0, 116.7, 114.5, 82.8, 55.6, 38.6, 28.2. ESI-MS (ion trap): m/z 970 [M+H]+. HRMS (ESI+): m/z 970.4499 [M+H]+, calcd for C56H64N3O12: 970.4490
General procedure for the tert-butyl ester deprotection in resveratrol-amino acid carbamoyl conjugates (2–5)
The t-butyl protected resveratrol-amino acid carbamoyl conjugate (2c–5c) (1.12 mmol) was dissolved in 10 mL of DCM at 0 °C. 500 μL of TIPS and 10 mL of TFA were then added and the reaction mixture was stirred at room temperature under nitrogen overnight. The solvent and residual TFA were removed under reduced pressure. The residue was triturated with toluene (3 × 5 mL) and the solvent was removed under reduced pressure. The product was purified by preparative reverse phase HPLC.
(E)-2,2′-((((5-(4-(((1-carboxy-3-methylbutyl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(4-methylpentanoic acid) (2)
Purified using a reverse-phase preparative HPLC column (ACE 5AQ 150 mm×21.2 mm from 7.5% to 38% of ACN in water in 14.5 minutes). 89% yield as a white powder after freeze-drying. 1H-NMR (250 MHz, DMSO-d6) δ (ppm): 0.89–0.94 (m, 18 H, 3 ×-CH(CH3)2), 1.45–1.81 (m, 9 H, 3 ×-CH-CH2- and 3 × (CH3)2CH-), 3.98–4.07 (m, 3 H, -NH-CH-), 6.76 (t, 1 H, 4JH-H = 2 Hz, H-4), 7.10–7.38 (m, 6 H, H-3′, H-5′, H-2, H-6, H-7, H-8), 7.63 (d, 2 H, H-2′, H-6′, 3JH-H = 8.7 Hz), 8.12–8.21 (m, 3 H, 3 ×-NH-); 13C-NMR (250 MHz, DMSO-d6) δ(ppm): 174.1, 174.0, 154.4, 154.2, 151.6, 150.7, 139.1, 133.7, 129.3, 127.6, 126.8, 121.9, 116.3, 114.4, 52.5, 39.6, 24.4, 23.0, 21.2. ESI-MS (ion trap): m/z 700 [M+H]+. HRMS (ESI+): m/z 700.3092 [M+H]+, calcd for C35H46N3O12: 700.3081.
(E)-2,2′-((((5-(4-(((1-carboxy-2-methylbutyl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(3-methylpentanoic acid) (3)
Purified using a reverse-phase preparative HPLC column (ACE 5AQ 150 mm×21.2 mm from 40% to 70% of ACN in water in 11 minutes). 93% yield as a white powder after freeze-drying. 1H-NMR (300 MHz, DMSO) δ (ppm): 0.86–0.95 (m, 18H, 3 ×-CH2-CH3 and 3 ×-CH-CH3), 1.21–1.55 (m, 6H, 3 ×-CH-CH2), 1.80–1.91 (m, 3 H, -CH-CH-CH3), 3.89–4.13 (m, 3 H, 3 × NH-CH-CH), 6.77 (t, 1 H, 4JH-H = 2 Hz, H-4), 7.06–7.37 (m, 6 H, H-3′, H-5′, H-2, H-6, H-7, H-8), 7.63 (d, 2 H, H-2′, H-6′, 3JH-H = 8.7 Hz), 8.04 (d, 1 H, -NH-, 3JH-H = 8.4 Hz), 8.10 (d, 2 H, 2 ×-NH-, 3JH-H = 8.4 Hz); 13C-NMR (300 MHz, DMSO) δ(ppm): 175.1, 175.0, 156.7, 156.5, 153.8, 152.9, 141.3, 135.8, 131.5, 129.8, 124.1, 118.4, 97.3, 61.0, 38.3, 26.9, 17.8, 13.5. ESI-MS (ion trap): m/z 700 [M+H]+. HRMS (ESI+): m/z 700.3075 [M+H]+, calcd for C35H46N3O12: 700.3081.
(E)-2,2′-((((5-(4-(((1-carboxy-2-hydroxypropyl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(3-hydroxybutanoic acid) (4)
Purified using a reverse-phase preparative HPLC column (ACE 5AQ 150 mm×21.2 mm from 7.5% to 38% of ACN in water in 14.5 minutes). 91% yield as a white powder after freeze-drying. 1H-NMR (250 MHz, DMSO-d6) δ (ppm): 1.17–1.19 (m, 9 H, 3 ×-CH-CH3), 4.00–4.23 (m, 6 H, 3 × CH3-CH- and 3 × NH-CH-), 6.80 (t, 1 H, 4JH-H = 2 Hz, H-4), 7.01–7.39 (m, 6 H, H-3′, H-5′, H-2, H-6, H-7, H-8), 7.61–7.72 (m, 5 H, 3 ×-NH- and H-2′, H-6′); 13C-NMR (250 MHz, DMSO-d6) δ(ppm):172.1, 172.1, 154.8, 154.6, 151.7, 150.8, 139.2, 133.8, 129.4, 127.7, 126.9, 122.0, 116.4, 114.6, 66.5, 60.4, 48.7, 20.5. ESI-MS (ion trap): m/z 664 [M+H]+. HRMS (ESI+): m/z 664.1993 [M+H]+, calcd for C29H34N3O12: 664.1990.
(E)-2,2′-((((5-(4-(((1-carboxy-2-phenylethyl)carbamoyl)oxy)styryl)-1,3-phenylene)bis(oxy))bis(carbonyl))bis(azanediyl))bis(3-phenylpropanoic acid) (5)
Purified using a reverse-phase preparative HPLC column (ACE 5AQ 150 mm × 21.2 mm from 35% to 65% of ACN in water in 13 minutes). 91% yield as a white powder after freeze-drying. 1H-NMR (250 MHz, DMSO-d6) δ (ppm): 2.86–3.19 (m, 6 H, 3 × Ph-CH2), 4.21–4.30 (m, 3H, 3 ×-NH-CH-CH2), 6.47 (t, 1 H, 4JH-H = 2 Hz, H-4), 6.91–7.33 (m, 21 H, Ar-H and H-3′, H-5′, H-2, H-6, H-7, H-8), 7.58 (d, 2 H, H-2′, H-6′, 3JH-H = 8.7 Hz), 8.20–8.29 (m, 3H, 3 ×-NH-); 13C-NMR (62.9 MHz, DMSO-d6) δ (ppm): 173.0, 154.3, 154.0, 151.5, 150.7, 139.1, 137.8, 137.8, 133.6, 129.3, 128.3, 127.6, 126.6, 121.8, 116.1, 114.2, 55.8, 55.0, 36.6; ESI-MS (ion trap): m/z 802 [M+H]+. HRMS (ESI+): m/z 802.2604 [M+H]+, calcd for C44H40N3O12: 802.2612.
Stability under physiologically relevant conditions
The stability of all new compounds was tested in aqueous media imitating gastric (0.1 N HCl, NormaFix) and intestinal (0.1 M PBS buffer, pH 6.8) conditions. A 5 μM solution of the compound was prepared from a 5 mM stock solution in DMSO and incubated at 37 °C for 24 hours; samples withdrawn at different times were analysed by HPLC-UV. Hydrolysis products were identified by HPLC/ESI-MS analysis of selected samples. Non-linear curve fitting was performed using Origin 8.0 data analysis software, using the equations described in55,61.
Stability in rat whole blood
Rats were anesthetised and blood was withdrawn from the jugular vein and transferred into tubes containing heparin. Blood samples (1 mL) were spiked with 5 μM compound (dilution from a 5 mM stock solution in DMSO) and incubated at 37 °C for 4 hours (the maximum period allowed by blood stability). Aliquots were taken after 10 min, 30 min, 1 h, 2 h and 4 h and treated as described below (blood sample treatment and analysis). Cleared blood samples were finally subjected to HPLC-UV analysis.
Blood sample treatment and analysis
Before starting the treatment, 4,4′-dihydroxybiphenyl was added as internal standard to a carefully measured blood volume (25 μM final concentration). 0.1 vol of 0.6 M TEA (pH 8.0) and 4 vol of MeOH were then added to the blood samples, which were then sonicated (2 min) and centrifuged (12,000 g, 7 min, 4 °C). The supernatant was finally collected and stored at −20 °C. Before analysis, MeOH was evaporated off at room temperature using a Univapo 150H (UniEquip) vacuum concentrator centrifuge and up to 40 μL of ACN were added to precipitate residual proteins. After centrifugation (12,000 g, 5 min, 4 °C), cleared samples were directly subjected to HPLC-UV analysis. Metabolites and hydrolysis products were identified by comparison of chromatographic retention time with true samples or by HPLC/ESI-MS analysis.
Internal standard recovery from the treatment used to extract resveratrol-AA derivatives was 91.8 ± 10.7%. The recovery of 3, expressed as ratio to the recovery of internal standard, was 0.633 ± 0.088. Recoveries of partially protected (disubstituted) derivatives (Fig. 7) were assumed to be the same as those of the corresponding fully substituted prodrug. Knowledge/assumption of these ratios allowed us to determine the unknown amount of analyte in a blood sample by measuring the recovery of internal standard57.
Since sample treatment includes an evaporation/concentration step, LOD and LOQ were determined relatively to the analytical part of the method (HPLC/UV analysis). The prodrugs have the same absorption coefficient of resveratrol itself within experimental error; LOD and LOQ were thus the same of resveratrol (i.e., 0.04 and 0.12 μM, respectively58) and quantification of the analytes in blood samples was done using the same calibration curve of resveratrol (y = 5.3085 x), taking into account the recovery ratio.57
Pharmacokinetics studies
Derivatives 2–5 were administered to overnight-fasted male Wistar rats from the facility of the Department of Biomedical Sciences, University of Padova, as a single intragastric dose (88 μmol/Kg, dissolved in 250 μl DMSO). Blood samples were obtained by the tail bleeding technique: before drug administration, rats were anesthetised with isoflurane and the tip of the tail was cut off; blood samples (80–100 μL each) were then taken from the tail tip at different time points after drug administration. Blood was collected in heparinised tubes, kept in ice and treated as described above within 10 min.
All experiments involving animals were approved by the University of Padova Ethical Committee for Experimentation on Animals (CEASA) and performed with the supervision of the University Central Veterinary Service, in compliance with Italian Law DL 116/92, embodying UE Directive 86/609.
Additional Information
How to cite this article: Mattarei, A. et al. Amino Acid Carbamates As Prodrugs Of Resveratrol. Sci. Rep. 5, 15216; doi: 10.1038/srep15216 (2015).
References
Jeandet, P. et al. Biosynthesis, metabolism, molecular engineering and biological functions of stilbene phytoalexins in plants. Biofactors 36, 331–341, 10.1002/biof.108 (2010).
Agarwal, B. & Baur, J. A. Resveratrol and life extension. Ann N Y Acad Sci 1215, 138–143, 10.1111/j.1749-6632.2010.05850.x (2011).
Csiszar, A. Anti-inflammatory effects of resveratrol: possible role in prevention of age-related cardiovascular disease. Ann N Y Acad Sci 1215, 117–122, 10.1111/j.1749-6632.2010.05848.x (2011).
Kroon, P. A., Iyer, A., Chunduri, P., Chan, V. & Brown, L. The cardiovascular nutrapharmacology of resveratrol: pharmacokinetics, molecular mechanisms and therapeutic potential. Curr Med Chem 17, 2442–2455, 10.2174/092986710791556032 (2010).
Khurana, S., Venkataraman, K., Hollingsworth, A., Piche, M. & Tai, T. C. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients 5, 3779–3827, 10.3390/nu5103779 (2013).
Raederstorff, D., Kunz, I. & Schwager, J. Resveratrol, from experimental data to nutritional evidence: the emergence of a new food ingredient. Ann N Y Acad Sci 1290, 136–141, 10.1111/nyas.12147 (2013).
Szkudelski, T. & Szkudelska, K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci 1215, 34–39, 10.1111/j.1749-6632.2010.05844.x (2011).
Liu, K., Zhou, R., Wang, B. & Mi, M. T. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr 99, 1510–1519, 10.3945/ajcn.113.082024 (2014).
Baile, C. A. et al. Effect of resveratrol on fat mobilization. Ann N Y Acad Sci 1215, 40–47, 10.1111/j.1749-6632.2010.05845.x (2011).
Wang, S. et al. Novel insights of dietary polyphenols and obesity. J Nutr Biochem 25, 1–18, 10.1016/j.jnutbio.2013.09.001 (2014).
Andrade, J. M. et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr, 10.1007/s00394-014-0655-6 (2014).
Andrade, J. M. et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 30, 915–919, 10.1016/j.nut.2013.11.016 (2014).
Biasutto, L., Szabo, I. & Zoratti, M. Mitochondrial effects of plant-made compounds. Antioxid Redox Signal 15, 3039–3059, 10.1089/ars.2011.4021 (2011).
Park, S. J. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433, 10.1016/j.cell.2012.01.017 (2012).
Foti Cuzzola, V., Ciurleo, R., Giacoppo, S., Marino, S. & Bramanti, P. Role of resveratrol and its analogues in the treatment of neurodegenerative diseases: focus on recent discoveries. CNS Neurol Disord Drug Targets 10, 849–862, 10.2174/187152711798072310 (2011).
Quincozes-Santos, A. & Gottfried, C. Resveratrol modulates astroglial functions: neuroprotective hypothesis. Ann N Y Acad Sci 1215, 72–78, 10.1111/j.1749-6632.2010.05857.x (2011).
Aggarwal, B. B. et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24, 2783–2840 (2004).
Vanden Berghe, W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol Res 65, 565–576, 10.1016/j.phrs.2012.03.007 (2012).
Kaminski, B. M., Steinhilber, D., Stein, J. M. & Ulrich, S. Phytochemicals resveratrol and sulforaphane as potential agents for enhancing the anti-tumor activities of conventional cancer therapies. Curr Pharm Biotechnol 13, 137–146, 10.2174/138920112798868746 (2012).
Gupta, S. C., Kannappan, R., Reuter, S., Kim, J. H. & Aggarwal, B. B. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci 1215, 150–160, 10.1111/j.1749-6632.2010.05852.x (2011).
Cottart, C. H., Nivet-Antoine, V. & Beaudeux, J. L. Review of recent data on the metabolism, biological effects and toxicity of resveratrol in humans. Mol Nutr Food Res 58, 7–21, 10.1002/mnfr.201200589 (2014).
Subramanian, L. et al. Resveratrol: challenges in translation to the clinic–a critical discussion. Clin Cancer Res 16, 5942–5948, 10.1158/1078-0432.ccr-10-1486 (2010).
Walle, T. Bioavailability of resveratrol. Ann N Y Acad Sci 1215, 9–15, 10.1111/j.1749-6632.2010.05842.x (2011).
Yu, C. et al. Human, rat and mouse metabolism of resveratrol. Pharm Res 19, 1907–1914, 10.1023/A:1021414129280 (2002).
Bresciani, L. et al. Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats. Nutr Metab Cardiovasc Dis 24, 408–415, 10.1016/j.numecd.2013.09.008 (2014).
Walker, J. et al. Resveratrol and its metabolites inhibit pro-inflammatory effects of lipopolysaccharides in U-937 macrophages in plasma-representative concentrations. Food Funct 5, 74–84, 10.1039/c3fo60236b (2014).
Eseberri, I., Lasa, A., Churruca, I. & Portillo, M. P. Resveratrol metabolites modify adipokine expression and secretion in 3T3-L1 pre-adipocytes and mature adipocytes. PLoS One 8, e63918, 10.1371/journal.pone.0063918 (2013).
Lasa, A., Churruca, I., Eseberri, I., Andres-Lacueva, C. & Portillo, M. P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol Nutr Food Res 56, 1559–1568, 10.1002/mnfr.201100772 (2012).
Kenealey, J. D. et al. Resveratrol metabolites do not elicit early pro-apoptotic mechanisms in neuroblastoma cells. J Agric Food Chem 59, 4979–4986, 10.1021/jf104901g (2011).
Lu, D. L. et al. Influence of glucuronidation and reduction modifications of resveratrol on its biological activities. Chembiochem 14, 1094–1104, 10.1002/cbic.201300080 (2013).
Calamini, B. et al. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem J 429, 273–282, 10.1042/bj20091857 (2010).
Hoshino, J. et al. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem 53, 5033–5043, 10.1021/jm100274c (2010).
Herath, W., Khan, S. I. & Khan, I. A. Microbial metabolism. Part 14. Isolation and bioactivity evaluation of microbial metabolites of resveratrol. Nat Prod Res 27, 1437–1444, 10.1080/14786419.2012.722089 (2013).
Ruotolo, R. et al. Anti-estrogenic activity of a human resveratrol metabolite. Nutr Metab Cardiovasc Dis 23, 1086–1092, 10.1016/j.numecd.2013.01.002 (2013).
Patel, K. R. et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med 5, 205ra133, 10.1126/scitranslmed.3005870 (2013).
Andreadi, C., Britton, R. G., Patel, K. R. & Brown, K. Resveratrol-sulfates provide an intracellular reservoir for generation of parent resveratrol, which induces autophagy in cancer cells. Autophagy 10, 524–525, 10.4161/auto.27593 (2014).
Ghosh, A. K. & Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J Med Chem, 10.1021/jm501371s (2015).
Hansen, J., Mørk, N. & Bundgaard, H. Phenyl carbamates of amino acids as prodrug forms for protecting phenols against first-pass metabolism. Int J Pharm 81, 253–261, 10.1016/0378-5173(92)90017-V (1992).
Biasutto, L. & Zoratti, M. Prodrugs of quercetin and resveratrol: a strategy under development. Curr Drug Metab 15, 77–95, 10.2174/1389200214666131211160005 (2014).
Ferriz, J. M. & Vinsova, J. Prodrug design of phenolic drugs. Curr Pharm Des 16, 2033–2052, 10.2174/138161210791293042 (2010).
Dhareshwar, S. & Stella, V. In Prodrugs Vol. V Biotechnology: Pharmaceutical Aspects eds Valentino J Stella et al.) Ch. 21, 731–799 (Springer New York, 2007).
Mulholland, P. J. et al. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann Oncol 12, 245–248, 10.1023/A:1008372017097 (2001).
Kim, M. K., Park, K. S., Yeo, W. S., Choo, H. & Chong, Y. In vitro solubility, stability and permeability of novel quercetin-amino acid conjugates. Bioorg Med Chem 17, 1164–1171, 10.1016/j.bmc.2008.12.043 (2009).
Mattarei, A. et al. New water-soluble carbamate ester derivatives of resveratrol. Molecules (Basel, Switzerland) 19, 15900–15917, 10.3390/molecules191015900 (2014).
Vig, B. S., Huttunen, K. M., Laine, K. & Rautio, J. Amino acids as promoieties in prodrug design and development. Adv Drug Deliver Rev 65, 1370–1385, 10.1016/j.addr.2012.10.001 (2013).
Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88, 249–286, 10.1152/physrev.00018.2006 (2008).
Brandsch, M. Drug transport via the intestinal peptide transporter PepT1. Curr Opin Pharmacol 13, 881–887, 10.1016/j.coph.2013.08.004 (2013).
Estudante, M., Morais, J. G., Soveral, G. & Benet, L. Z. Intestinal drug transporters: an overview. Adv Drug Deliver Rev 65, 1340–1356, 10.1016/j.addr.2012.09.042 (2013).
Tamai, I. Oral drug delivery utilizing intestinal OATP transporters. Adv Drug Deliver Rev 64, 508–514, 10.1016/j.addr.2011.07.007 (2012).
del Amo, E. M., Urtti, A. & Yliperttula, M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci 35, 161–174, 10.1016/j.ejps.2008.06.015 (2008).
Gynther, M. et al. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J Med Chem 51, 932–936, 10.1021/jm701175d (2008).
Peura, L. et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol Pharm 8, 1857–1866, 10.1021/mp2001878 (2011).
Peura, L. et al. Design, synthesis and brain uptake of LAT1-targeted amino acid prodrugs of dopamine. Pharm Res 30, 2523–2537, 10.1007/s11095-012-0966-3 (2013).
Wu, X., Cheng, L., Xiang, D. & Wei, Y. Syntheses of Carbamate Derivatives of Quercetin by Reaction with Amino Acid Ester Isocyanates. Lett Org Chem 2, 535–538, 10.2174/1570178054640796 (2005).
Kozerski, G. E., Gallavan, R. H. & Ziemelis, M. J. Investigation of trialkoxysilane hydrolysis kinetics using liquid chromatography with inductively coupled plasma atomic emission spectrometric detection and non-linear regression modeling. Anal Chim Acta 489, 103–114, 10.1016/S0003-2670(03)00710-4 (2003).
Adams, P. & Baron, F. A. Esters of Carbamic Acid. Chem Rev 65, 567–602, 10.1021/cr60237a002 (1965).
Azzolini, M. et al. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol Nutr Food Res 58, 2122–2132, 10.1002/mnfr.201400244 (2014).
Biasutto, L., Marotta, E., Garbisa, S., Zoratti, M. & Paradisi, C. Determination of quercetin and resveratrol in whole blood–implications for bioavailability studies. Molecules (Basel, Switzerland) 15, 6570–6579, 10.3390/molecules15096570 (2010).
Biasutto, L. et al. Soluble polyphenols: synthesis and bioavailability of 3,4′,5-tri(alpha-D-glucose-3-O-succinyl) resveratrol. Bioorg Med Chem Lett 19, 6721–6724, 10.1016/j.bmcl.2009.09.114 (2009).
Mattarei, A. et al. New derivatives of resveratrol. (2014). European Patent Application EP2774915. Application Number EP20140157948. Filing date 03/05/2014. Publication Date 09/10/2014.
Mattarei, A. et al. Acetal derivatives as prodrugs of resveratrol. Mol Pharm 10, 2781–2792, 10.1021/mp400226p (2013).
Acknowledgements
This work was supported by grants from the Fondazione Cassa di Risparmio di Padova e Rovigo (CARIPARO) (“Developing a Pharmacology of Polyphenols”), from the Italian Ministry of the University and Research (PRIN n. 20107Z8XBW_004) and by the CNR Project of Special Interest on Aging.
Author information
Authors and Affiliations
Contributions
A.M., C.P., M.Z. and L.B. designed the study and wrote the manuscript; A.M. carried out the syntheses; M.A., L.B. and M.L.S. performed the hydrolysis studies and pharmacokinetics experiments. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors have applied for a patent covering the compounds described in this paper.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mattarei, A., Azzolini, M., La Spina, M. et al. Amino Acid Carbamates As Prodrugs Of Resveratrol. Sci Rep 5, 15216 (2015). https://doi.org/10.1038/srep15216
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15216
This article is cited by
-
Potential nephroprotective effects of resveratrol in drug induced nephrotoxicity: a narrative review of safety and efficacy data
Advances in Traditional Medicine (2020)
-
Combined effects of resveratrol and radiation in GH3 and TtT/GF pituitary adenoma cells
Journal of Neuro-Oncology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.