Abstract
Mac-1 (CD11b) is expressed on bone marrow-derived immune cells. CD11b binds to ligands to regulate leukocyte adhesion and migration across the endothelium or epithelium. Here, we employed CD11b knockout mice and an ApcMin/+ spontaneous intestinal adenoma mouse model to clarify the function of CD11b in intestinal tumorigenesis. We showed that CD11b deficiency may contribute to the inhibition of myeloid cell trafficking to the tumor microenvironment and inactivated Wnt/β-catenin pathway to suppress tumor growth. This effect was partly mediated by inhibiting the myeloid cell-mediated decrease in TNF-α secretion, which inhibits the recruitment of myeloid-derived suppressor cells to the tumor microenvironment and subsequently induces IFN-γ and CXCL9 production. This work provides evidence for the mechanism by which CD11b may function as an important oncogene and highlights the potential of CD11b as a therapeutic target in CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the most common cause of cancer death world-wide. The accumulated molecular genetic alterations that underlie the development of CRC are well characterized1,2. However, the role of the tumor microenvironment during CRC tumorigenesis is not completely understood2,3. The development of CRC is a multistep process that involves interactions between the tumor cells and the host microenvironment. The tumor microenvironment consists of tumor cells, carcinoma-associated fibroblasts, leukocytes, blood, lymphatic vascular endothelial cells and the extracellular matrix, which contribute to tumor progression4,5. Moreover, leukocytes progressively accumulate in the tumor microenvironment and immune reactions accompany all stages of CRC6,7,8. Among the leukocytes that infiltrate the tumor microenvironment, bone marrow-derived myeloid cells predominantly accumulate in tumors and stimulate cancer initiation, progression and resistance to anti-cancer therapy4,7,9. However, the regulatory mechanism by which myeloid cell infiltration into the tumor microenvironment regulates tumor growth to be further clarified.
CD11b (Mac-1, αMβ2) is the α-subunit of the predominant β2 (CD18) integrin adhesion molecule, which is expressed on the surface of the myeloid cells10,11. On bone marrow-derived immune cells, CD11b is responsible for facilitating cell adhesion to and transmigration across the endothelium or epithelium and traffic to the inflammation sites to mediate the inflammatory response7,10,12,13,14,15. A recent report indicated that Mac-1 inhibition can reduce myeloid cell recruitment to the tumor environment and thereby enhance the tumor’s response to radiation11. Although CD11b has been widely implicated in myeloid cell adhesion and migration, the biological significance and molecular mechanisms of the role of CD11b in myeloid cell infiltration into the tumor microenvironment are largely undetermined.
Myeloid derived suppressor cells (MDSCs) are a population of immature myeloid cells that originate from the bone marrow. Several recent publications demonstrated that MDSCs are CD11b+Gr-1+ immunosuppressive cells that suppress T-cell activation and accumulate in the bone marrow, spleen and tumor sites in most patients and in tumor-bearing mice16,17. MDSCs consist of two major subsets of cells: cells with a granulocytic phenotype (Ly6G+Ly6Clow) and cells with a monocytic phenotype (Ly6G-Ly6Chigh)18,19. Only the Ly6G+Ly6Clow granulocytic MDSCs have been shown to expand in most tumor models and these cells may play an important role in the general process of angiogenesis and tumor progression19,20,21. Moreover, the number of circulating MDSCs was also significantly increased in cancer patients and correlated with the clinical cancer stage19. Recent studies demonstrated that myeloid cells in tumor hosts can be transported to the spleen, peripheral blood and tumor sites and produce cytokines and chemokines that activate MDSCs and allow them to infiltrate the tumor sites17. Therefore, we hypothesized that CD11b-deficient myeloid cells may likely regulate MDSCs infiltration into the tumor environment and thereby inhibit the angiogenesis and tumor growth of colorectal carcinoma.
Wnt/β-catenin signaling is constitutively activated in nearly all colorectal tumors and leads to a loss of E-cadherin and the nuclear translocation of β-catenin. This signaling pathway then modulates the expression of a broad spectrum of target genes to promote tumor cell proliferation. Inflammatory cells in CRC tumors have been shown to be associated with tumor progression. Moreover, cytokines that are secreted by the inflammatory cells may interact with the Wnt signaling pathway in the tumor microenvironment and lead to an accumulation of β-catenin in the nucleus22. However, the ability of MDSCs that infiltrate the tumor environment to activate Wnt/β-catenin signaling in CRC is unknown.
In this study, we used the ApcMin/+ CRC mouse model and Mac-1-deficient (CD11b−/−) mice to define the possible role and the underlying regulatory mechanisms of Mac-1 in intestinal tumorigenesis.
Materials and Methods
Reagents and antibodies
TNF-α was obtained from Peprotech (La Jolla, CA). Bromodeoxyuridine (BrdU) cell proliferation labeling reagent (RPN201, intraperitoneally injected with 0.1 mg/g mouse weight, 2 hours before killed) was obtained from GE Healthcare (Little Chalfond, UK). The following antibodies were used for Flow cytometry: anti-CD45-FITC (eBioscience, San Diego, CA), anti-CD11b-PC7 (BD Pharmingen, San Diego, CA), anti-Ly6C-BV421 (BioLegend, San Diego, CA), anti-Ly6G-PE-CF594 (BD Horizon, San Jose, CA). The primary antibodies were used for immunohistochemical (IHC), immunofluorescence (IF) analysis and Western blotting (WB) assays: and mouse anti-BrdU antibody (RPN202, diluted at 1:150 for IHC) was obtained from GE Healthcare (Little Chalfond, UK); rabbit anti-CyclinD1 (BM0771, diluted at 1:100 for IHC and 1:400 for WB), rabbit anti-CD34 (BA0532, diluted at 1:100 for IHC), rabbit anti-CD45 (BA3371, diluted at 1:100 for IHC) and rabbit anti-p65 (BA0610, diluted at 1:100 for IHC) were obtained from Boster (Wuhan, China); mouse anti-TNF-α (ab10863, diluted at 1:5000 for WB) was obtained from Abcam (Cambridge, UK); rabbit anti-IFN-γ (bs-0480R), rabbit anti-CXCL-9 (bs-2551R) (both diluted at 1:500 for WB), rabbit anti-CD11b (bs-1014R) and rabbit anti-Gr-1 (bs-2576R, diluted at 1:100 for IF) were obtained from Bioss (Beijing, China); mouse anti-Cytokeratin 8 (CK8, ZM-0310, diluted at 1:100 for IF) was purchased from ZSGB-BIO (Beijing, China); mouse anti-CD11b (Santa Cruz, USA, diluted at 1:100 for IF); mouse anti-E-cadherin (#610181, diluted at 1:100 for IHC and 1:5000 for WB) and mouse anti-β-catenin (#610154, diluted at 1:100 for IHC and 1:2000 for WB) were purchased from BD Transduction Laboratories (Franklin Lakes, NJ); rabbit anti-GAPDH (#2118s, diluted at 1:2000 for WB) was purchased from CST (USA); rabbit anti-pp65 (Ser276, sc-101749, diluted at 1:500 for IHC) was obtained from Santa Cruz Biotechnology Inc. (USA).
Patients and tissue samples
A total of 10 cases of colonic tumor tissues and their matched non-tumorous colonic tissues were collected from the Department of Pathology, The Third Affiliated Hospital of Sun Yat-Sen University. Written informed consent from each patient was obtained prior to the initiation of this study. Pathologic diagnosis was performed by two independently pathologists. All methods were performed in accordance with the guidelines approved by the Ethics Committee of Medicine, Guangdong Pharmaceutical University.
Animals and treatment
C57BL/6 (C57) mice were purchased from the Guangdong Medical Laboratory Animal Center. APCMin/+ mice (strain: C57BL/6J-ApcMin/J, stock number: 002020) and CD11b−/− mice (strain: B6.129S4-Itgamtm1Myd/J, stock number: 003991) on the C57BL/6J background were purchased from the Jackson Laboratory (Bar Harbor, ME). APCMin/+;CD11b−/− mice were obtained by crossbreeding the APCMin/+ mice and CD11b−/− mice to the F2 generation. The founder mice were viable and exhibited normal growth. Genotyping was performed by PCR using genomic DNA prepared from mouse tail according to the genotyping protocol. The primers for CD11b genotyping were 5′- TAG GCT ATC CAG AGG TAG AC-3′ (for the wild-type and targeted alleles); 5′- CAT ACC TGT GAC CAG AAG AGC-3′ (for the wild-type allele); and 5′- ATC GCC TTC TTG ACG AGT TC-3′ (for the targeted allele). The primers for APCMin/+ genotyping were 5′- GCC ATC CCT TCA CGT TAG-3′, 5′- TTC CAC TTT GGC ATA AGG C-3′ and 5′- TTC TGA GAA AGA CAG AAG TTA-3′ (for both the wild-type and targeted alleles). Eleven mice from each group were used for the studies, unless otherwise indicated. All mice (15-week old) that were used for analyses were maintained in a climate-controlled room at a temperature of 24 ± 2°C and a relative humidity of 60 ± 5% under a 12-h light/dark cycle. All surgical procedures were performed under diethylether anesthesia. BrdU (0.1 mg/g body weight) was intraperitoneally injected into the mice, which were sacrificed 2 hours later. All experiments were performed in accordance with the protocols approved by the Ethics Committee of the Center of Laboratory Animals at Guangdong Pharmaceutical University.
Cell Culture
The human HCT-116 and mouse CT-26 colorectal carcinoma cell lines were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin and incubated in a humidified chamber containing 5% CO2 at 37 °C. TNF-α was added to the cells at a dose of 50 ng/mL for 48 hours and the total proteins were prepared for an immunoblotting analysis.
Isolation of myeloid cell and co-culture
The single suspension of bone marrow derived all nucleated cells from femurs and tibias was collected and maintained in DMEM supplemented with 100 μg/mL PHA, 10% FBS, 100 U/mL penicillin and 200 μg/mL streptomycin and incubated in a humidified chamber containing 5% CO2 at 37 °C for 2 days.
CT-26 cells and HCT-116 cells were seeded into 24-well plates at a density of 8 × 104 per well and 2 × 105 per well respectively without FBS for 24 hours and then re-added FBS and co-cultured with myeloid cells for 24 hours. Myeloid cells were loaded into the upper compartment of the transwell chambers at a density of 1:10 (myeloid cell: tumor cell) with or without TNF-α antibody (mouse anti-TNF-α, 1:500, BA0131, Boster, China). Then the tumor cells were collected for further detection.
Analysis of intestinal tumors
After the APCMin/+ mice and APCMin/+;CD11b−/− mice were sacrificed, the entire gastrointestinal tract was excised and separated into the colon and 3 segments of the small intestine: proximal, medial and distal. All regions were opened longitudinally, flattened between sheets of filter paper, immersed in 10% phosphate-buffered formalin and then stained with 10% methylene blue. The tumor numbers and sizes were determined using dissecting microscope (OLYMPUS, Japan) and the tumor volume (V) was calculated according to the following equation: V = (L × W2) × 0.5236 (L: length; W: width). The intestinal neoplasias were classified using microscope as described previously23 (Supplementary Figure 1).
Histological and immunohistological staining
The intestinal tissues were fixed in neutral-buffered 10% formalin solution, embedded in paraffin and sectioned to a thickness of 3 μm. Hematoxylin & eosin (H&E), immunohistochemical (IHC) and immunofluorescent (IF) staining for BrdU, CD34, β-catenin, E-cadherin, Cyclin D1, CD45, CD11b, CK8 and Gr-1 were performed as previously described24,25. The sections were then observed under a scanning confocal microscope (Leica, Germany).
Microvessel Density
Microvessel density (MVD) was recorded as the number of point counts of endothelial cells with the specific antibody to CD34 per field at × 200 magnification. Ten fields were randomly selected in a section of tumors were examined. MVD counts were recorded independently by two observers in sections from three mice of each group.
Immunoblotting
The intestines were sliced longitudinally and the macroscopic tumors were cut off from the intestines. The total proteins from the tumors and cells were prepared using RIPA buffer and immunoblotting assays were performed as previously described26.
Flow cytometry (FACS)
A single cell suspension of blood cells, bone marrow cell, splenocytes or tumor digests that had been treated as described above was subjected to flow cytometry using the following MDSC surface markers: CD45, CD11b, Ly6C and Ly6G. To analyze the inflammatory cell infiltrates in the tumor tissue, the tumors were mechanically dissociated on a wire mesh by crushing with the plunger of a 10-mL syringe and then incubated in tissue-digestion buffer at 37 °C for 25 min. The cells were filtered through 70-μm nylon strainers (BD Biosciences, Bedford, MA), stained with specific antibodies and analyzed by flow cytometry. The FACS data were acquired using a Beckman Coulter Gallios flow cytometer and were analyzed using the FlowJo software package (Tree Star, Ashland, OR, USA).
To detect the cell cycle progression, the tumor cells in co-culture system were collected and fixed the cells with 75% ethanol for 40 min at 4 °C, centrifuged, washed twice in phosphate buffered saline and stained with PI solution (#550825, BD Biosciences, USA) at 37 °C for 15 min. The analysis was performed using a FACS Calibur flow cytometer (Becton Dickinson) and analyzed using the Modfit software, version 3.0 (Verity Software House).
Real-time quantitative PCR arrays
The total RNA was extracted from the blood or spleen of mice using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The total RNA (500 ng) was reverse transcribed using an PrimeScriptTM RT Reagent Kit (TaKaRa, Japan) and the real-time quantified PCR was performed on a LightCycler480 PCR machine (Roche) using the SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) PCR Kit (TaKaRa, Japan), to the manufacturer’s instructions. The data were analyzed using the 2−∆∆CT methodology as described27.
Enzyme-linked immunosorbent assay (ELISA)
Serum was collected from the APCMin/+ mice and APCMin/+;CD11b−/− mice and the levels of TNF-α were analyzed using a Mouse/Rat TNF-α Valukine ELISA Kit (1 KT) (VAL609, R&D Systems, USA), according to the manufacturer’s instructions. Each sample was measured in triplicate.
Statistical analysis
The data are presented as the mean ± standard deviation (SD) and the differences between groups were analyzed using a Student’s t test. Differences were considered statistically significant at P < 0.05. The protein expression levels in the IHC slices were determined by measuring the cumulated integrated optical density (IOD) using IPP software (Media Cybernetics, Inc., USA). The densitometric analysis of the immunoblotting bands was performed using Quantity One software (Bio-Rad, USA) and the protein band intensities were quantitated and normalized to those of GAPDH.
Results
Intestinal tumor development is accompanied by infiltrating myeloid cells
Infiltrating inflammatory myeloid cells often reportedly heavily infiltrate solid tumors7,28. To determine the level of myeloid cell infiltration, we stained the tumors with a CD11b antibody to detect myeloid cells using immunofluorescence analysis (IF) and further stained a single cell suspension of blood cells, bone marrow cell and splenocytes with CD11b antibody to detect myeloid cells (gated on CD45+ cells (all leukocytes)) using FACS assay11. To study the tumor microenvironment, we immunofluorenscently stained the human tumor tissues (Tumor) and their matched surrounding non-cancerous colonic tissues (Normal) with CD11b antibody. We observed that the levels of the CD11b+ myeloid cells were increased in the tumor tissues compared with the non-cancerous colonic tissues (Fig. 1a). The ApcMin/+ mouse is a model for the development of CRC. This transgenic mouse model has been widely used to study the development of intestinal tumorigenesis29. We examined the infiltration of myeloid cells in the spontaneous adenomatous tissues of ApcMin/+ mice by IHC staining. Many CD11b+ myeloid cells had infiltrated the mesenchyme of the tumor region (Fig. 1b). In addition, we detected the number of CD11b+ myeloid cells (gated on CD45+ cells) in the bone marrow, spleen and peripheral blood by FACS to determine the myeloid cell contribution to intestinal tumorigenesis. With the exception of the peripheral blood, the CD11b+ myeloid cell populations were substantially enriched in the bone marrow and spleen of the ApcMin/+ mice compared with the C57 mice (Fig. 1c–e). These results suggest that CD11b+ myeloid cells are activated during CRC development.
Intestinal tumor development is accompanied by infiltrating CD11b+ myeloid cells.
The number of CD11b+ myeloid cells increased in tumor tissues compared with the non-cancerous colonic tissues in CRC patients (a). The morphology of the colonic tissues was examined by H&E staining. The myeloid cell infiltration was detected by IF in the tumor tissues of CRC patients. CD11b+ myeloid cells were increased in tumor tissues compared with the normal small intestine tissues of ApcMin/+ mice (b). The morphology of the colonic tissues was examined by H&E staining. The myeloid cell infiltration was detected by IF in the tumor tissues of ApcMin/+ mice. The numbers of CD11b+ myeloid cells in the bone marrow (c), spleen (d) and peripheral blood (e) were analyzed by FACS gated on CD45+ leukocytes. The results of H&E and IF staining are representative of 11 independent CRC patients or ApcMin/+ mice (all mice were 16-weeks-old). The statistical data are expressed as the means ± S.D. **P <0.01 and ns: P > 0.05. Scale bars: 50 μm.
CD11b deficiency inhibits intestinal tumor growth in vivo
β2-integrin and CD11b expression on bone marrow-derived immune cells regulates leukocyte adhesion and migration to the tumor microenvironment to mediate the inflammatory response7,15,30. The ApcMin/+ mice were crossed with CD11b−/− mice to generate ApcMin/+;CD11b−/− mice (Supplementary Figure 2a–d), which were used to further confirm the effect of CD11b + myeloid cells infiltration into the tumor microenvironment on the intestinal tumorigenesis. As shown in Fig. 2a, the ApcMin/+;CD11b−/− mice were viable and survived significantly longer than the ApcMin/+ mice. A macroscopic examination showed that the ApcMin/+; CD11b−/−mice exhibited a significantly decreased the number of tumors and average tumor volume in the small intestine but not the colon compared with the ApcMin/+ mice (Fig. 2b–d and Supplementary Figure 2e). A holistic view of the intestines indicated fewer highly condensed nests of neoplastic cells in the ApcMin/+;CD11b−/− mice compared with the ApcMin/+ mice (Fig. 2e, left panel). Moreover, we also observed the development of invasive carcinoma in the intestines of 15-week-old ApcMin/+ mice, albeit less frequently. However, invasive carcinoma was not observed in the ApcMin/+;CD11b−/− mice at the same age (Fig. 2e, right panel).
CD11b deficiency inhibits intestinal tumor growth.
The small intestines from the ApcMin/+ and ApcMin/+; CD11b−/− mice were examined macroscopically after methylene blue staining (a). CD11b deficiency significantly inhibited the number (b) and size (c) of the tumors and prolonged survival (d). ApcMin/+: n = 11, ApcMin/+;CD11b−/−: n = 16 (b–d). A holistic view of the histological lesions in the intestines of the ApcMin/+ mice and ApcMin/+;CD11b−/− mice as observed on the “whole intestines” under a microscope (e). CD11b deficiency resulted in a marked decrease in the infiltration of CD45+ leukocytes (f); in cell proliferation, as indicated by attenuated BrdU uptake in the tumor tissues of ApcMin/+;CD11b−/− mice (g); and in angiogenesis, as indicated by the decreased expression of CD34 (h) and VEGF (i). The IHC results were quantitated using the IPP software (f–i, right panel). The results of the methylene blue staining and IHC staining are representative of 11 independent mice (all mice were 16–weeks-old). The statistical data are expressed as the mean ± S.D. **P < 0.01 and ***P < 0.001. Scale bars: 100 μm (e), 50 μm (f,h) and 20 μm (g,i).
Reports indicated that myeloid cells infiltrate the tumor microenvironment can support tumor growth and promote tumor angiogenesis4,17. The results showed that depletion of CD11b+ cells in the ApcMin/+ mice abrogated leukocyte cell infiltration in the tumor microenvironment but not in the adjacent normal villus tissue (Fig. 2f and Supplementary Figure 2g). We further detected tumor cell proliferation and angiogenesis by IHC staining. Most tumors in the experimental CD11b-deficient mice displayed significantly fewer BrdU-positive cells (Fig. 2g) and CD34 point counts as well as significantly reduced VEGF expression (Fig. 2h,i). These data suggest that the CD11b-deficient myeloid cells significantly inhibited the growth of intestinal tumors.
CD11b deficiency inactivates the Wnt/β-catenin pathway during intestinal tumorigenesis
The canonical Wnt/β-catenin signaling pathway is critical for the homeostasis and neoplastic transformation of the intestinal tract31,32. APC mutation results in the activation of the Wnt pathway. Constitutively active Wnt/β-catenin signaling is associated with CRC initiation and leads to an accumulation of β-catenin in the nucleus and a loss of E-cadherin. Whether CD11b-deficient myeloid cells that infiltrate the tumor microenvironment inhibit intestinal tumorigenesis by inactivating the Wnt/β-catenin signaling has not yet been determined. Compared with the ApcMin/+ mice, IHC staining (Fig. 3a–c and supplementary Figure 3) and immunoblotting assay (Fig. 3d) indicated a significant increase in the expression of E-cadherin and decreased expression levels of β-catenin and cyclin D1, the downstream target of the Wnt/β-catenin pathway, in the tumor tissues but not in the adjacent normal tissue of the ApcMin/+;CD11b−/− mice. Decreased nuclear translocation of β-catenin was observed in the tumor cells of the ApcMin/+;CD11b−/− mice by IF staining (Fig. 3e). These results indicate that activated Wnt/β-catenin signaling was partially inhibited by deficient CD11b+ myeloid cells infiltration into the tumor microenvironment.
CD11b deficiency inactivated the Wnt/β-catenin pathway.
The expression of E-cadherin, β-catenin and cyclin D1 was examined in the tumor tissues of the ApcMin/+ and ApcMin/+;CD11b−/− mice by IHC (a) and immunoblotting (b) and the IHC results were quantitatively determined using IPP the software and expressed as the means ± S.D. The subcellular location of β-catenin was examined in the tumor tissues of the ApcMin/+ and ApcMin/+;CD11b−/− mice by IF (c). All IHC and IF staining in the mouse tissues represent 11 independent mice (all mice were 16-week-old). The IHC results were quantified using the IPP software and are expressed as the means ± S.D. **P < 0.01, ***P < 0.001. Scale bars, 20 μm (a–c) and 25 μm (e).
CD11b deficiency inhibits intestinal tumor growth by reducing TNF-α release
Myeloid cells that infiltrate the tumor microenvironment stimulate cancer initiation, malignant progression and angiogenesis by releasing a number of potent pro-tumorigenic cytokines. Therefore, the Inflammatory Cytokines & Receptors PCR Array (#APM-011, SUPERARRAY, USA) was used to identify the cytokines that might affected by CD11b+ myeloid cells in CRC using a quantitative RT-PCR assay (supplementary Figure 4a). The results indicated that the CD11b-deficent tumor tissue exhibited a robust 1.36-fold decrease in TNF-α expression. We detected the concentration of TNF-α in the peripheral blood with an ELISA assay and found that the TNF-α level in peripheral blood was up-regulated in the ApcMin/+ mice compared with the C57 mice. However, the increase in the TNF-α level was reversed in CD11b-deficient ApcMin/+ mice (Fig. 4a). The total RNAs of the peripheral blood and spleen were extracted and the quantitative RT-PCR assay showed the same effect as the ELISA (Fig. 4b). TNF-α expression was also decreased in the tumor tissues of the ApcMin/+;CD11b−/− mice compared with ApcMin/+ mice, as analyzed by immunoblotting (Fig. 4c). We further detected the effect of TNF-α on tumor growth and Wnt/β-catenin signaling activity in HCT-116 cells (this cell line shows low Wnt/β-catenin signaling activity) and found that TNF-α significantly induced cell proliferation (Fig. 4d) and promoted the activation of Wnt/β-catenin signaling by inhibiting the expression of E-cadherin and up-regulating the expression of β-catenin and cyclin D1 (Fig. 4e). Further confocal microscopy studies also confirmed that TNF-α significantly induced the nuclear translocation of β-catenin in HCT-116 cells (Fig. 4f). Meanwhile, we observed the expression of NF-κB, TNF-α downstream targets and found that the expression of p65 showed no difference in the tumor tissues between the ApcMin/+;CD11b−/−mice and ApcMin/+ mice. However, the expression of pp65 (Ser276) was significantly inhibited in the tumor tissues of the ApcMin/+;CD11b−/− mice compared with ApcMin/+ mice (Supplementary Figure 4b). Moreover, the myeloid cells were isolated from the bone marrow of C57 mice and co-culture with CT-26 and HCT-116 cells. Compared with the negative control (NC) group, CT-26 (Fig. 4g) and HCT-116 (Fig. 4h) cells significantly accumulated in the G0/G1 peak and arrested the cell cycle at the G1/S transition in TNF-α antibody treated co-culture system. These results demonstrated that CD11b deficiency suppressed tumor growth by reducing the levels of TNF-α secreted by myeloid cells.
CD11b deficiency inhibits tumor growth by suppressing TNF-α release.
The concentration of TNF-α in the peripheral blood of the C57, ApcMin/+ and ApcMin/+;CD11b−/− mice was examined by ELISA (a). The mRNA level of TNF-α in the peripheral blood and spleen of the C57, ApcMin/+ and ApcMin/+;CD11b−/− mice was examined by qRT-PCR (b). The proteins expression of TNF-α in the tumor tissues from the ApcMin/+ and ApcMin/+; CD11b−/− mice was examined by immunoblotting (c). The protein band intensities were quantitated and normalized to those of GAPDH. TNF-α can significantly promoted cell proliferation (d) and Wnt/β-catenin activation (e) in HCT-116 cells (this cell line shows low Wnt/β-catenin signaling activity). TNF-α can also induce the nuclear translocation of β-catenin in HCT-116 cells (f), Scale bar: 25 μm. All results represent at least three separate experiments (d–f). Inhibit TNF-α arrested CT-26 cells (g) and HCT-116 cells (h) at the G1/S transition in myeloid cell and tumor cell co-culture system. The results are expressed as the means ± S.D. ns: P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
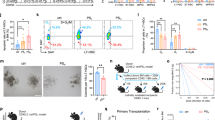
CD11b deficiency reduces MDSC recruitment in the tumor environment
The Ly6G+Ly6Clow subset of MDSCs exhibits pro-inflammatory activity and often enriched in tumor models16,19. Previous reports indicated that TNF-α is secreted by myeloid cells and enriched in the tumor microenvironment, which can drive MDSC accumulation33,34,35. Moreover, MDSCs can contribute to angiogenesis and facilitate tumor growth17. The Ly6G+Ly6Clow subset of MDSCs was significantly larger during CD11b+ myeloid cell-mediated tumor growth. We demonstrated that Gr-1+CD11b+ MDSCs accumulated in the tumor cells in the tumor microenvironment of the ApcMin/+ mice, but the number of Gr-1+ cells decreased in the tumor microenvironment of CD11b-deficient tumor-bearing mice (Fig. 5a). We further detected the difference in production of the Ly6G+Ly6Clow subset of MDSCs (gated on CD45+ cells) in the bone marrow of CD11b−/− mice and CD11b−/− tumor-bearing mice (wild type mice were used as controls) and found that the CD45+ leukocyte and granulocytic Ly6G+Ly6Clow MDSC populations did not differ in the bone marrow of the C57, CD11b−/−, ApcMin/+ and ApcMin/+;CD11b−/− mice (Fig. 5b). However, CD11b deficiency significantly inhibited the number of granulocytic Ly6G+Ly6Clow MDSCs that were recruited to peripheral blood, spleen and tumor microenvironment in the ApcMin/+;CD11b−/− mice compared with the ApcMin/+ mice (Fig. 5c).
CD11b deficiency reduced myeloid-derived suppressor cells (MDSC) recruitment in the tumor environment.
The accumulation of Gr-1+CD11b+ MDSC cells in the tumor microenvironment of the ApcMin/+ mice compared with the normal intestine tissues from the C57 mice; the Gr-1+ cells were decreased in the tumor microenvironment of the ApcMin/+;CD11b−/− mice (a). The number of the Ly6G+Ly6Clow subset of MDSCs (gated on CD45+ cells) in the bone marrow of C57, CD11b−/−, ApcMin/+ and ApcMin/+;CD11b−/− mice (b) and in the peripheral blood, spleen and tumor tissues of the ApcMin/+ and ApcMin/+;CD11b−/− mice (c) was measured by FACS. The results of the IF staining and FACS represent of 11 independent mice (all mice were 16–weeks-old). The proteins expression of IFN-γ and CXCL9 in the tumor tissues from the ApcMin/+ and ApcMin/+;CD11b−/− mice was examined by immunoblotting (d). The protein bands intensities were quantitated and normalized to those of GAPDH. The statistical data are expressed as the means ± S.D. *P<0.05, **P < 0.01 and ***P < 0.001. Scale bars: 50 μm (a).
MDSCs inhibit IFN-γ production by specifically inhibiting the CD8-mediated Ag-specific T cell response36,37. Moreover, IFN-γ can induce CXCL9 production38,39. Reports indicated that IFN-γ and CXCL9 can attenuate angiogenesis and tumor growth40,41,42. We further detected the expression of IFN-γ and CXCL9 in tumor site and found that IFN-γ and CXCL9 were significantly up-regulated in the tumor tissues of the ApcMin/+;CD11b−/− mice compared with the ApcMin/+ mice (Fig. 5d). These results demonstrate that CD11b deficiency suppressed tumor growth by reducing the amount of TNF-α secreted by myeloid cells and inhibiting MDSCs recruitment to the tumor microenvironment, which further prevented the inhibition of IFN-γ production and promoted the production of CXCL9 in CRC.
Discussion
In this study, we demonstrate an oncogenic role for CD11b during CRC tumorigenesis. Our findings demonstrated that CD11b expression on the myeloid cells promotes myeloid cell migration to the peripheral blood, spleen and tumor microenvironment. The myeloid cells also secrete cytokines, which promote MDSC recruitment to the tumor sites, further induce IFN-γ and CXCL9 production and activate Wnt/β-catenin signaling in CRC (Fig. 6).
Tumorigenesis is a complex process that involves many factors. A large number of reports indicated that chronic inflammation is an important factor for tumor development43. The tumor microenvironment is characterized by the chronic overexpression of inflammatory mediators that are produced by tumor-infiltrating immune cells, particularly bone marrow-derived cells. Therefore, the importance of the inflammatory tumor microenvironment cannot be overlooked. Recently, cytokines in the tumor microenvironment have been shown to contribute to angiogenesis, tumor cell proliferation, invasion and resistant to chemotherapy and radiotherapy. In CRC, tumor cells interact with cytokines in the tumor environment to activate the Wnt pathway22. Our work shows that the inhibition of myeloid cells infiltration into the CRC tumor environment can reduce the release of inflammatory factors and consequently activate the Wnt/β-catenin signaling pathway. The data generated in our current study further demonstrated the significance of the myeloid cells that infiltrate the tumor environment during tumorigenesis.
We found that the number of CD11b+ myeloid cells increased substantially in the bone marrow and spleens of the ApcMin/+ mice compared with the C57 mice. However, we did not observe a difference in the CD11b+ myeloid cells in the peripheral blood. The bone marrow and spleen are the important immune organs in which myeloid cells are generated and localized to activate an immune program. However, the peripheral blood is a part of the circulation system by which myeloid cells translocate from the central immune organs to the peripheral immune organ. Therefore, the differences in the CD11b+ myeloid cells may not be detected in the mobile phase of the peripheral blood. However, the peripheral blood of the ApcMin/+ mice tended to contain more CD11b+ myeloid cells than that of the C57 mice.
Tumor necrosis factor-α (TNF-α) is a key inflammatory cytokine that is primarily produced by myeloid cells44. However, it plays paradoxical roles in carcinogenesis. Reports indicated that TNF-α possesses both anti-tumor and pro-tumor activities45. It was reported that TNF-α can inhibit tumor growth in a breast cancer xenograft model46. Recently, a large number of studies demonstrated that TNF-α produced in tumor microenvironment may promote cancer development47,48,49,50. TNF-α produced by leukocyte was up-regulated in the colorectal carcinoma and blocking the expression of TNF-α in mice can reduces colorectal carcinogenesis51. TNF-α is produced primarily by activated macrophages and also by other cell types including monocytes and lymphoid cells. We observed no differences between the macrophages from the ApcMin/+ mice and those in the ApcMin/+;CD11b−/− mice (Supplementary Figure 5). However, the inhibition of TNF-α secretion in myeloid cells can significantly suppress HCT-116 cell proliferation. It is suggested that CD11b deficiency could inhibit the secretion of TNF-α by other type of myeloid cells, but not macrophage. Our results demonstrated that TNF-α, produced by myeloid cells, acts as a tumor-promoting factor that can significantly promote tumor growth in the ApcMin/+ mice.
CD11b, which is expressed on the surface of myeloid cells, has been widely implicated in mediating leukocyte adhesion and transendothelial migration10,12,13. CD11b inhibition can reduce myeloid cell recruitment in the tumor environment11. MDSCs are a population of bone marrow-derived immature myeloid cells, which constitute approximately 5% of the total cell population within CRC tumors20. However, granulocytic MDSC (Ly6G+Ly6Clow) are often expanded in the tumor sites16,19. Our study has confirmed that CD11b deficiency cannot affect the production of CD45+ leukocytes and granulocytic Ly6G+Ly6Clow MDSCs in the bone marrow. However, the number of the Ly6G+Ly6Clow subset of MDSCs was dramatically decreased in the peripheral blood, spleen and tumor sites. Our work suggests that CD11b may not mediate the production of these cells, but only regulates the transendothelial migration of the Ly6G+Ly6Clow subset MDSCs. These data are consistent with previous reports showing that CD11b deficiency is characterized by defects in leukocyte adhesion and migration across the endothelium52.
In the tumor, CD11b inhibition led to a significant enhancement of the tumor response to irradiation by suppressing vasculogenesis, with no effect on non-irradiated tumors from hypopharyngeal carcinoma cells that were transplanted into immunodeficient mice11. However, our data showed that CD11b deficiency can decrease the number of myeloid cells in tumor environment and thereby inhibit angiogenesis and tumor growth over the protracted course (15 weeks) of tumor development in ApcMin/+ mice under normal conditions, which were not consistent with previously published results11. MDSCs suppress T cell function, which contributes to tumor growth. The defective T cells evoke the functional disruption of myeloid cells in the immunodeficient mice with transplanted tumor. Our work was performed in mice with a normal immune system, which may not affect MDSC suppression of T cell function. In support of our data, previous report indicated that myeloid cells promote tumor growth by stimulating tumor angiogenesis and suppressing tumor immunity17. The data generated in our current study not only support the published data that CD11b deficiency can reduce myeloid cell recruitment in tumor environment, but also indicate that CD11b deficiency is likely to inhibit angiogenesis and tumor growth in CRC tumor-bearing mice.
In summary, our data have demonstrated that CD11b is critically involved in the transendothelial migration of bone marrow-derived immune cells to the tumor sites, resulting in intestinal tumorigenesis. Moreover, CD11b may serve as a potential biomarker for therapy of CRC treatment.
Additional Information
How to cite this article: Zhang, Q.-Q. et al. CD11b deficiency suppresses intestinal tumor growth by reducing myeloid cell recruitment. Sci. Rep. 5, 15948; doi: 10.1038/srep15948 (2015).
References
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Cammarota, R. et al. The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. J Transl Med 8, 112 (2010).
Witz, I. P. Tumor-microenvironment interactions: dangerous liaisons. Adv Cancer Res 100, 203–229 (2008).
Schouppe, E., De Baetselier, P., Van Ginderachter, J. A. & Sarukhan, A. Instruction of myeloid cells by the tumor microenvironment: Open questions on the dynamics and plasticity of different tumor-associated myeloid cell populations. Oncoimmunology 1, 1135–1145 (2012).
Lorusso, G. & Ruegg, C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 130, 1091–1103 (2008).
Formica, V., Cereda, V., Nardecchia, A., Tesauro, M. & Roselli, M. Immune reaction and colorectal cancer: friends or foes? World J Gastroenterol 20, 12407–12419 (2014).
Schmid, M. C. & Varner, J. A. Myeloid cells in tumor inflammation. Vasc Cell 4, 14 (2012).
Gueron, G., Cotignola, J. & Vazquez, E. Inflammatory Microenvironment in Prostate Carcinogenesis, Advances in Prostate Cancer, Dr. Gerhard Hamilton (Ed.), ISBN: 978-953-51-0932-7, InTech, 10.5772/52636, pp 423–461 (2013). Available from: http://www.intechopen.com/books/advances-in-prostate-cancer/inflammatory-microenvironment-in-prostate-carcinogenesis.
Zhao, L. et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57, 829–839 (2013).
Diamond, M. S., Garcia-Aguilar, J., Bickford, J. K., Corbi, A. L. & Springer, T. A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol 120, 1031–1043 (1993).
Ahn, G. O. et al. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA 107, 8363–8368 (2010).
Smith, C. W., Marlin, S. D., Rothlein, R., Toman, C. & Anderson, D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest 83, 2008–2017 (1989).
Parkos, C. A., Delp, C., Arnaout, M. A. & Madara, J. L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest 88, 1605–1612 (1991).
Anderson, D. C., Rothlein, R., Marlin, S. D., Krater, S. S. & Smith, C. W. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood 76, 2613–2621 (1990).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7, 678–689 (2007).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9, 162–174 (2009).
Schmid, M. C. & Varner, J. A. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol 2010, 201026 (2010).
Jayaraman, P. et al. Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. J Immunol 188, 5365–5376 (2012).
Youn, J. I., Nagaraj, S., Collazo, M. & Gabrilovich, D. I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181, 5791–5802 (2008).
Yang, L. et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421 (2004).
Finke, J. et al. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol 11, 856–861 (2011).
Huang, D. & Du, X. Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World J Gastroenterol 14, 1823–1827 (2008).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Wang, L. J. et al. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci 99, 510–517 (2008).
Zhou, W. J. et al. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res 21, 609–626 (2011).
Xu, H. et al. Liver-Enriched Transcription Factors Regulate MicroRNA-122 That Targets CUTL1 During Liver Development. Hepatology 52, 1431–1442 (2010).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3, 1101–1108 (2008).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation and cancer. Cell 140, 883–899 (2010).
Kettunen, H. L., Kettunen, A. S. & Rautonen, N. E. Intestinal immune responses in wild-type and Apcmin/+ mouse, a model for colon cancer. Cancer Res 63, 5136–5142 (2003).
Schmid, M. C. et al. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res 71, 6965–6975 (2011).
Sancho, E., Batlle, E. & Clevers, H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol 20, 695–723 (2004).
Konsavage, W. M., Jr., Kyler, S. L., Rennoll, S. A., Jin, G. & Yochum, G. S. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem 287, 11730–11739 (2012).
Zhao, X. et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest 122, 4094–4104 (2012).
Kim, S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 (2009).
Cox, G. W. et al. Tumor necrosis factor-alpha-dependent production of reactive nitrogen intermediates mediates IFN-gamma plus IL-2-induced murine macrophage tumoricidal activity. J Immunol 149, 3290–3296 (1992).
Nagaraj, S. & Gabrilovich, D. I. Myeloid-derived suppressor cells. Adv Exp Med Biol 601, 213–223 (2007).
Gabrilovich, D. I., Velders, M. P., Sotomayor, E. M. & Kast, W. M. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol 166, 5398–5406 (2001).
Ellis, S. L. et al. The cell-specific induction of CXC chemokine ligand 9 mediated by IFN-gamma in microglia of the central nervous system is determined by the myeloid transcription factor PU.1. J Immunol 185, 1864–1877 (2010).
Kanda, N., Shimizu, T., Tada, Y. & Watanabe, S. IL-18 enhances IFN-gamma-induced production of CXCL9, CXCL10 and CXCL11 in human keratinocytes. Eur J Immunol 37, 338–350 (2007).
Keane, M. P. et al. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol 163, 5686–5692 (1999).
Sahin, H. et al. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology 55, 1610–1619 (2012).
Alshaker, H. A. & Matalka, K. Z. IFN-gamma, IL-17 and TGF-beta involvement in shaping the tumor microenvironment: The significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int 11, 33 (2011).
Bremnes, R. M. et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 6, 824–833 (2011).
Salazar-Onfray, F., Lopez, M. N. & Mendoza-Naranjo, A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev 18, 171–182 (2007).
Balkwill, F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 13, 135–141 (2002).
Balkwill, F. R. et al. Human tumor xenografts treated with recombinant human tumor necrosis factor alone or in combination with interferons. Cancer Res 46, 3990–3993 (1986).
Negus, R. P., Turner, L., Burke, F. & Balkwill, F. R. Hypoxia down-regulates MCP-1 expression: implications for macrophage distribution in tumors. J Leukoc Biol 63, 758–765 (1998).
Leber, T. M. & Balkwill, F. R. Regulation of monocyte MMP-9 production by TNF-alpha and a tumour-derived soluble factor (MMPSF). Br J Cancer 78, 724–732 (1998).
Brest, P. et al. Autophagy and Crohn’s disease: at the crossroads of infection, inflammation, immunity and cancer. Curr Mol Med 10, 486–502 (2010).
Leek, R. D. et al. Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. Br J Cancer 77, 2246–2251 (1998).
Popivanova, B. K. et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118, 560–570 (2008).
Anderson, D. C. & Springer, T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1 and p150,95 glycoproteins. Annu Rev Med 38, 175–194 (1987).
Acknowledgements
The authors thank Hui Chen and Qiao-Bing Yuan for technical assistance. This research was supported by the National Natural Science Foundation of China (No. 31200896, 31271455 and 31471290), the Natural Science Foundation of Guangdong Province (No. 2014A030313582 and s2013010014905), the Science and Technology Planning Project of Guangdong Province (No. 2013B021800271 and 2015A030302083), the Educational Commission of Guangdong Province (No. 2013KJCX0155) and the Science and Technology Planning Project of Guangzhou (No. 201510010076).
Author information
Authors and Affiliations
Contributions
Q.Q.Z., H.W. and L.J.W. wrote the main manuscript text, L.J.W., Q.Q.Z. and H.W. conceived and designed experiments, Q.Q.Z., X.W.H., Y.L.L., Z.J.Y., Y.H.G. and D.L.Z. carried out experiments, Q.Q.Z., X.W.H., C.L.Q. and X.D.H. managed animals, acquired and managed patients, Q.Q.Z. and X.W.H. analyzed data (e.g., statistical analysis, biostatistics, computational analysis), H.W. helped interpret the results, Q.Q.Z., X.W.H. and L.J.W. searched literature and generated figures. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, QQ., Hu, XW., Liu, YL. et al. CD11b deficiency suppresses intestinal tumor growth by reducing myeloid cell recruitment. Sci Rep 5, 15948 (2015). https://doi.org/10.1038/srep15948
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15948
This article is cited by
-
Role of adhesion molecules in cancer and targeted therapy
Science China Life Sciences (2024)
-
Regulatory cells and the effect of cancer immunotherapy
Molecular Cancer (2023)
-
Wnt signaling in colorectal cancer: pathogenic role and therapeutic target
Molecular Cancer (2022)
-
Prognostic significance and targeting tumor-associated macrophages in cancer: new insights and future perspectives
Breast Cancer (2021)
-
Comparison of Immune Microenvironment Between Colon and Liver Metastatic Tissue in Colon Cancer Patients with Liver Metastasis
Digestive Diseases and Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.