Abstract
Bladder cancer causes an estimated 150,000 deaths per year worldwide. Although 15% of the recurrent bladder cancer becomes an invasive type, currently used targeted therapy for malignant bladder cancer is still not efficient. We focused on the miR-130 family (miR-130b, miR-301a and miR-301b) that was significantly upregulated in bladder cancer specimens than that of the normal urothelial specimens. We analyzed the functional significance of miR-130 family using a 5637 bladder cancer cell line and revealed that miR-130 family of inhibitors suppressed cell migration and invasion by downregulating focal adhesion kinase (FAK) and Akt phosphorylation. Mechanistic analyses indicate that the miR-130 family directly targets phosphatase and tensin homolog deleted from chromosome 10 (PTEN), resulting in the upregulation of FAK and Akt phosphorylation. In clinical bladder cancer specimens, downregulation of PTEN was found to be closely correlated with miR-130 family expression levels. Overall, the miR-130 family has a crucial role in malignant progression of bladder cancer and thus the miR-130 family could be a promising therapeutic target for invasive bladder cancer.
Similar content being viewed by others
Introduction
Bladder cancer is a major malignancy worldwide with an estimated 380,000 new cases resulting in 150,000 deaths annually1. Although 55–60% of primary bladder cancer is superficial, about 50% cases relapse intravesically after surgical removal and approximately 15–40% of the recurrent bladder cancer becomes invasive and exhibits distant metastasis2,3. Radical cystectomy has been regarded as the first choice treatment for muscle-invasive bladder cancer, even though it lowers quality of life (QOL) for patients4. Even in those muscle-invasive bladder cancer patients who receive optimal treatment with chemotherapy and surgery, the 5-year survival rate is only 60% due to distant recurrence5,6. Limited therapeutic options for invasive bladder cancer have resulted in a median survival of 15 months for patients with metastatic disease7. Therefore, treatment modalities with improved clinical outcomes are urgently required, necessitating the search for novel therapeutic targets for advanced bladder cancer.
MicroRNA (miRNA) is a small noncoding RNA molecule of 20–25 nucleotides, which regulates gene expression through translational repression or mRNA degradation. In silico genome-wide analyses have predicted that >60% of all mammalian protein-coding genes can be regulated by miRNAs8,9. MiRNA can therefore modulate various cellular processes including cell growth, migration or invasion, consequently gaining attention as attractive therapeutic targets for cancer treatment10,11,12 Recently, several studies showed that targeting miRNAs known as miRNA cluster (e.g. miR-17–92 cluster, miRNA-23b/27b/24 cluster)13,14,15 or miRNA family sharing a common seed sequence (e.g. miR-34 family, miR-200 family)16,17, significantly affected tumour progression. Several reports have shown the impact of each miRNA of the miR-130 family in cancer progression18,19,20, but the comprehensive effect of the miR-130 family molecules on tumour progression, including bladder cancer has not been analyzed. The miR-130 family is composed of miR-130b, miR-301a and miR-301b, which share a common seed sequence.
Previous studies suggest that the development of noninvasive and invasive bladder cancer occurs through two separate pathways with distinct pathobiology. Early stage bladder cancer (pTa or pT1) is commonly linked to activating mutations of the FGFR3, HRAS or PI3K genes21,22,23, while advanced stage bladder cancer (≥pT2) is linked to mutations in the tumour suppressor genes TP53, RB and PTEN, which can act as a negative regulators of the PI3K/Akt signaling pathway24,25. Additionally, an integrated study of 131 high grade muscle-invasive bladder cancer samples has revealed dysregulation of the PI3K/Akt signaling pathway in 72% of cases26. Collectively, the data suggest that the PI3K/Akt pathway is critical to progression of bladder cancer.
We show here for the first time that the miR-130 family is upregulated in bladder cancer clinical specimens and coordinately promotes bladder cancer cell migration and invasion through the phosphorylation of focal adhesion kinase (FAK) as well as Akt by regulating PTEN expression or sub-cellular localization.
Results
The miR-130 family (miR-130b, miR-301a and miR-301b) was significantly upregulated in bladder cancer specimens
We recently found by miRNA microarray analysis, that the miR-130 family molecules including miR-130b, miR-301a and miR-301b, which have a common seed sequence (Fig. 1a,b) were significantly upregulated in invasive renal pelvis and ureter carcinoma, compared to normal upper tract urothelium and their non-invasive counterparts (unpublished data). Since the bladder is also lined with a layer of urothelium, we investigated the expression of the miR-130 family molecules in bladder cancer. We performed quantitative real-time PCR (qRT-PCR) using 19 normal upper tract urothelium tissue samples and 23 bladder cancer tissue samples (Table 1) and found that the miR-130 family was also upregulated in bladder cancer compared to normal upper tract urothelium (Fig. 1c). Although miR-301a showed no significant difference in expression among the different pathologic stages and grades, the expression of miR-130b and miR301b was higher in advanced bladder cancer (Fig. 1d,e).
The miR-130 family is significantly upregulated in bladder cancer specimens.
(a) Genomic localization of the miR-130 family. (b) The miR-130 family has a common seed sequence. The expression of miR-130 family was compared between normal and tumour tissues (c) among pathological tumour stages (d) and among pathological tumour grades (e). Data are mean ± S.D. *p < 0.05; **p < 0.01.
The miR-130 family promotes invasion and migration of bladder cancer cells
To investigate the function of the miR-130 family in bladder cancer cells, we first measured the expression level of the miR-130 family molecules in bladder cancer cell lines. In seven bladder cancer cell lines examined, the highest expression of the miR-130 family was found in 5637 cells (Fig. 2a). Therefore, we used 5637 cells for performing functional analysis of the miR-130 family using miR-130 family inhibitors. The inhibitory effect of the miR-130 family inhibitors was confirmed by luciferase vectors with a miR-130 family molecule-binding site (Supplementary Fig. 1a) in their 3′-UTR. Then, we examined the effect of miR-130 family inhibitors on cell growth, migration and invasion in 5637 cells. Although they did not significantly affect cell growth (Fig. 2b), the miR-130 family inhibitors reduced invasion (Fig. 2c) and significantly inhibited migration (Fig. 2d and lower panels) of 5637 cells. Next, we established stable cell lines expressing miR-130 family molecules using UM-UC-2 cells, which show a lower miR-130 family expression level (Fig. 2a). The expression levels of miR-130b, miR-301a and miR-301b were determined by qRT-PCR and observed to be 13.8- and 5.7- and 14.3-fold higher respectively, compared to a control cell line (mock vector transfection) (Supplementary Fig. 1b). A WST-1 assay showed that overexpression of miR-130 family had no significant effect on UM-UC-2 cell growth (Fig. 3a). However, overexpression of miR-301a and miR-301b induced mesenchymal-like cell morphology (Fig. 3b) and the cell invasion and migration activities were significantly upregulated compared to mock vector-transfected UM-UC-2 cells (Fig. 3c,d). Another clone of UM-UC-2 cells stably overexpressing miR-130 family molecules, also exhibited similar responses while growth was not affected, cell invasion and migration increased compared to mock-transfected cells (Supplementary Fig. 2). Collectively, these results suggest that the miR-130 family molecules regulate cell invasion and migration in bladder cancer cells.
Inhibition of the miR-130 family suppresses 5637 cell invasion and migration.
(a) Expression of the miR-130 family molecules in bladder cancer cell lines was measured by qRT-PCR. (b) The effect of miR-130 family inhibitors on cell growth was measured by a WST-1 assay. (c) Invasion assay was performed by xCELLigence real-time cell monitoring system 72 h after transfection. (d) Cell migration was estimated by a wound healing assay. The wound was formed by scraping 60 h after transfection and then relative cell migration was measured after 12 h. In all the experiments, 50 nM miRIDIAN hairpin miRNA inhibitors were transfected in 5637 cells. Data are mean ± S.D. of four (a) or three (c,d) independent experiments. *p < 0.05; **p < 0.01.
Stable expression of the miR-130 family promotes UM-UC-2 cell invasion and migration.
(a) Relative cell growth was measured by WST-1 assay (n = 4). (b) Characteristic morphologic features of UM-UC-2 cells stably miR-130 family are shown. (c) A cell invasion assay was examined. (d) Relative cell migration was examined by a wound healing assay. Data are mean ± S.D. of five (c) or three (d) experiments. **p < 0.01; ***p < 0.001.
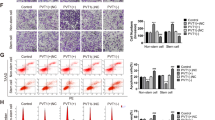
The miR-130 family promotes stress fiber formation via FAK in bladder cancer cells
The cell invasion and migration activities of bladder cancer cells are dependent on intracellular actin stress fiber formation27,28,29. Stress fibers are connected to the focal adhesion complex consisting of several integrin family molecules and FAK30, that plays an important role in cell migration and metastasis. We stained F-actin with fluorescent phalloidin and analyzed cells by fluorescent microscopy. The miR-130 family inhibitors markedly suppressed stress fiber formation in 5637 cells (Fig. 4a). It is known that the phosphorylation of FAK at Tyr576 upregulates stress fiber formation31. Therefore we analyzed FAK levels using Western blot. The levels of Tyr576-phosphorylated FAK, but not total FAK decreased upon miR-301a/b inhibition (Fig. 4b).
The effects of miR-130 family inhibitors on stress fiber formation, phosphorylation status of FAK and Akt and MMP-9 expression in 5637 cells.
(a) Stress fiber formation was observed by F-actin staining with Phalloidin. Phosphorylation status of FAK at Tyr576 (b) Akt at Ser473 (c) and MMP-9 expression (d) was examined by Western blot analysis. In all experiments, 50 nM miRIDIAN hairpin miRNA inhibitors were transfected in 5637 cells. Data show mean ± S.D. of three independent experiments. *p < 0.05.
We further examined the effect of miR-130 family inhibitors on Akt phosphorylation, which is known as a downstream signaling component of FAK32,33. The miR-130 family of inhibitors significantly suppressed Akt phosphorylation but not total Akt expression (Fig. 4c). Moreover, the expression of matrix metalloproteinase-9 (MMP-9) protein, a key factor of bladder cancer invasiveness regulated by the PI3K/Akt signaling pathway34, decreased in presence of the miR-130 family inhibitors (Fig. 4d). Conversely, stress fiber formation and phosphorylated FAK and Akt levels increased in UM-UC-2 cells stably overexpressing miR-130 family molecules (Fig. 5a–c). Moreover, MMP-9 protein expression also increased in these wells (Fig. 5d). These results suggest that the highly expressed miR-130 family increases migration and invasion activities of bladder cancer cells via FAK activation and subsequent upregulation of Akt signaling pathway.
Stress fiber formation, phosphorylation status of FAK and Akt and MMP-9 expression in the UM-UC-2 cells stably expressing miR-130 family.
(a) Stress fiber formation was observed by F-actin staining with Phalloidin. Phosphorylation status of FAK at Tyr576 (b) and Akt at Ser473 (c) and the protein expression of MMP-9 (d) were examined by Western blot analysis. Data show mean ± S.D. of triplicate (b and d) or sextuplicate independent experiments.
The miR-130 family downregulates the protein expression of PTEN in bladder cancer cells
To identify a target of the miR-130 family in bladder cancer cells, we utilized target prediction programs (miRBase and microRNA.org) and focused on PTEN as a potential target, since it is known as a tumor suppressor in various cancers and as a negative regulator of the PI3K/Akt signaling pathway35,36. The PTEN protein levels increased when miR-301a and miR-301b were inhibited (Fig. 6a). On the other hand, the effect of miR-130b inhibitor on the PTEN protein expression was weak due to the low expression of miR-130b in 5637 cells. On the other hand, overexpression of the miR-130 family tended to decrease PTEN expression in UM-UC-2 cells (Fig. 6b). To confirm whether PTEN was a direct target of the miR-130 family, we constructed luciferase reporter vectors carrying the human PTEN 3′-UTR containing either the predicted wild-type, or mutated binding sites for miR-130 family (Fig. 6c). The UM-UC-2 cells stably overexpressing miR-130 family molecules, were transfected with the modified luciferase reporter vectors and a dual-luciferase reporter assay was performed. Stable expression of the miR-130b significantly decreased the luciferase activity in UM-UC-2 cells transfected with the vector containing PTEN wild-type 3′-UTR, but no decrease was detectable when the cells were transfected with the mock vector, or the vector with mutant PTEN 3′-UTR (Fig. 6d). Weak but similar effects were detected in UM-UC-2 cells stably expressing miR-301a/b. From these results, we deduced that miR-130b directly targets PTEN, while miR-301a/b might suppress PTEN expression via an indirect mechanism in bladder cancer cells. To examine the relationship between the expression of miR-130 family and PTEN suppression in clinical samples, we performed immunohistochemistry with anti-PTEN antibody, on formalin-fixed, paraffin-embedded bladder cancer specimens. First, we validated the miR-130 family expression by qRT-PCR in twelve bladder cancer clinical samples and used the histopathological specimens derived from the samples showing the three highest or lowest expression levels of the miR-130 family for immunohistochemical analysis. As shown in Fig. 6e,f, PTEN was strongly expressed in the bladder cancer specimens with lower miR-130 family expression, whereas almost no expression was observed in specimens exhibiting higher miR-130 family expression. Collectively, the data strongly indicate that the miR-130 family negatively regulates PTEN protein expression in bladder cancer cells, promoting their migration and invasion properties.
The miR-130 family regulates PTEN protein expression.
Western blot analysis of PTEN in miRIDIAN hairpin inhibitor-transfected 5637 cells on days 3 and 5. (a), or UM-UC-2 cells stably expressing miR-130 family (b). (c) A schematic model of predicted miR-130 family binding sequences and its mutant sequence within the 3′-UTR of human PTEN gene. The number indicates the nucleotide position of the predicted miR-130 family binding site from the start of the PTEN 3′-UTR. (d) A dual luciferase reporter assay was performed with UM-UC-2 cells stably expressing miR-130 family. The cells were transfected with a reporter plasmid containing predicted miR-130 family binding site in the PTEN 3′-UTR. Results of MiR-130 family expression determined by qRT-PCR (e) and PTEN expression determined by PTEN-immunohistochemical staining (f), in bladder cancer clinical samples are shown. All staining images depict a magnified image in the upper left region of the images. Data show mean ± S.D. of three (a,b), or five independent (d) experiments. ***p < 0.001.
Discussion
Over the past two decades, there has been no significant improvement of therapeutic agents for locally advanced or metastatic bladder cancer37. Therefore, identification of a novel molecular target for bladder cancer treatment is urgently desired. In the present study, we have identified the miR-130 family members as potential molecular targets possessing a novel action mechanism, for bladder cancer treatment.
The integrated analysis of 131 muscle-invasive bladder cancer specimens reveals that dysregulation of PI3K/Akt pathway is detected in 72% of the tumours26, hence regulatory elements of the PI3K/Akt pathway have received much attention as potential therapeutic targets 38. PTEN is a negative regulator of the PI3K/Akt signaling pathway35,39 and shows loss of heterozygosity (LOH) in muscle-invasive bladder cancers40. However, the LOH in the PTEN region was detected in only 24.5% of the bladder cancer specimens. Therefore, the presence of alternative mechanisms of PTEN regulation is predicted in bladder cancer. We report here that the miR-130 family molecules are novel negative regulators of PTEN expression in bladder cancer. In the “Two-pathway model of bladder cancer”, inactivation of PTEN is considered a trigger for progression from non-invasive to invasive tumour25,41,42. We confirmed that the forced expression of PTEN reduced Akt phosphorylation as well as the migration of 5637 cells (Supplementary Fig. 3). Therefore, we conclude that the miR-130 family plays a role in bladder cancer progression via activation of PI3K/Akt signaling pathway by suppressing PTEN expression.
Although our data showed that miR-130 family molecules upregulated bladder cancer cell migration and invasion, the suppression mechanisms of PTEN expression seem to be different between miR-130b and miR-301a/b. It is reported that the membrane localization of PTEN is important for its phosphatase activity and protein stability35,43,44. We observed that the miR-130 family inhibited the membrane localization of PTEN in a bladder cancer cell line (Supplementary Fig. 4). Membrane localization of PTEN is mainly maintained by the interaction with membrane-associated proteins or the phosphorylation of PTEN C-terminal tail such as Ser380 44. A membrane-associated guanylate kinase family protein with multiple PDZ domains (MAGI2) is well known as a membrane-associated protein that can interact with PTEN to promote stability and subsequent phosphatase activity45. Although MAGI2 has a predicted miR-130 family target sequence in the 3′-UTR, the stable expression of the miR-130 family molecules had no significant effect on MAGI2 luciferase assay (Supplementary Fig. 5). The conformational changes induced by phosphorylation of PTEN C-terminal tail at Ser380 suppresses PTEN activation, by preventing its recruitment to the cell membrane46. As shown in Supplementary Fig. 4c, the stable expression of the miR-130 family molecules upregulated PTEN phosphorylation at Ser380 consistent with the increased Akt phosphorylation levels (Fig. 5c), indicating miR-130 family suppresses PTEN activation. The glioma tumour suppressor candidate region 2 (GLTSCR2, also known as PICT-1) and RhoA-associated kinase 1/2 (ROCK1/2) are shown to promote its phosphorylation at Ser380 35,47. ROCK1/2 are well-validated downstream signaling components of RhoA48 and pharmacological inhibition of tyrosine-protein phosphatase non-receptor type 11 (PTPN11) stimulates RhoA activity49. Importantly, PTPN11 harbors a predicted miR-130 family target site in its 3′-UTR and the dual-luciferase assay and western blot analysis revealed that miR-130b and miR-301b directly targeted PTPN11 (Supplementary Fig. 5b,c). Although the PTEN localization mechanism regulated by miR-301a is under investigation, miR-301b possibly regulates PTEN localization by targeting PTPN11 and subsequent RhoA-ROCK1/2 activation.
Although all the miR-130 family molecules were upregulated in bladder cancer specimens, it is very interesting that functional differences exist among the family members. The miR-130 family molecules possess slightly different sequences, in spite of their common seed region (Fig. 1b), resulting in regulation of different target genes. We observed that miR-130b had little effect on bladder cell migration and invasion. However, miR-130b is known to promote other oncogenic phenotypes such as stemness, drug resistance18,50 and angiogenesis51, by targeting tumor protein p53-inducible nuclear protein 1 (TP53INP) and Peroxisome Proliferator-Activated Receptor ɤ (PPAR ɤ), respectively. Therefore, miR-130b may contribute to bladder cancer progression by a distinct mechanism from miR-301a/b. As shown in Figs 3 and 5, UM-UC-2 cells overexpressing miR-130b, miR-301a and especially miR-301b exhibited strikingly high cell motility and invasiveness and phosphorylation of FAK. These findings further support that PTPN11 is possibly an additional direct target gene of the miR-130 family in bladder cancer cells. Both inhibition and overexpression of miR-301b were confirmed to regulate PTPN11 protein expression, suggesting PTPN11 as a direct target gene of miR-301b in bladder cancer. Importantly, PTPN11 dephosphorylates tyrosine residues in FAK52 and functional inhibition of PTPN11 leads to the formation of stress fiber and focal adhesion49,53. This finding is consistent with the distinct phenotype of the miR-301b-overexpressing UM-UC-2 cells. In addition to these genes, the miR-130 family may have other potential targets that act as tumour suppressors (e.g., TIMP254, TSC155, RUNX356, TP6320,41,42, Smad457 and CDKN1A58). Collectively, the miR-130 family might modulate cellular signaling pathways of bladder cancer cells by regulating various target genes, including PTEN and PTPN11.
Previous findings about the function of miRNA family in bladder cancer were related only to the tumour suppressor miR-200 family, whose inhibition induces epithelial-mesenchymal transition (EMT), resulting in the promotion of mesenchymal phenotype17. Therefore, our findings on the miR-130 family are the first report that clarifies the role of oncomiR family in bladder cancer. Given the clinical application of tumour suppressor miRNA such as the miR-200 family, restoring miRNA activity by using double-stranded miRNA mimic might be a possible therapeutic strategy. However, double-stranded miRNA mimics can potentially induce a non-specific interferon response though Toll-like receptors59. Therefore, inhibition of miRNA function might be a more appropriate therapeutic strategy. Each miRNA typically targets approximately 200 genes60,61, which in turn, enables modulation of entire pathways in a disease of interest, by targeting disease-associated miRNAs. However, the miRNA-mediated mechanism of translational repression has a relatively weak effect on target genes. Therefore, targeting a particular miRNA family that has overlapping roles in disease might enable wide and powerful inhibition of disease-related signaling pathways. Rottiers et al. have reported the effect of pharmacological inhibition of the miR-33 family using seed-targeting 8-mer locked nucleic acid (LNA) in an African green monkey metabolic disease model62. Treatment with miR-33 family-targeted LNA resulted in depression of direct miR-33 family target genes and increased the circulating high density lipoprotein (HDL) cholesterol levels more strongly than miR-33a or miR-33b inhibition alone. In case of the miR-130 family molecules, functional overlap in various biological processes is reported53,63,64,65, thus simultaneous inhibition of the miR-130 family can be expected to lead to effective therapeutic effects in bladder cancer.
Recently, the pan-cancer oncogenic miRNA superfamily has been identified using the Cancer Genome Atlas (TCGA) pan-cancer data set66. The miR-130 family is included in the miRNA superfamily and is upregulated in several types of cancer such as bladder, breast, lung, head and neck. Furthermore, the expression of miR-130 family members is significantly negatively correlated with the several tumour suppressor genes including PTEN, across 8 tumour types. These findings suggest that the miR-130 family members undergo coordinate regulation in tumours to mediate silencing of tumour suppressor genes in a synergistic manner. Generally, it is well known that bladder cancer shows extensive heterogeneity in clinical behavior. Now it is classified into five major subtypes based on various gene expression signatures42. Therefore, targeting the miR-130 family, which can regulate multiple cancer-related signaling pathways, would provide a most effective therapeutic strategy for the bladder cancer treatment.
Materials and Methods
Plasmid construction
To construct the miR-130 family or PTEN reporter plasmids, the following oligonucleotides for miR-130 family-putative targeted sequences and PTEN were used:
hsa-miR-130b sense 5′-CTAGCGGCCGCTAGTATGCCCTTTCATCATTGCACTGG-3′, antisense 5′-TCGACCAGTGCAATGATGAAAGGGCATACTAGCGGCCGCTAGAGCT-3′, hsa-miR-301a sense 5′-CTAGCGGCCGCTAGTGCTTTGACAATACTATTGCACTGG-3′, antisense 5′-TCGACCAGTGCAATAGTATTGTCAAAGCACTAGCGGCCGCTAGAGCT-3′, hsa-miR-301b sense 5′-CTAGCGGCCGCTAGTGCTTTGACAATATCATTGCACTGG-3′, antisense 5′-TCGACCAGTGCAATGATATTGTCAAAGCACTAGCGGCCGCTAGAGCT-3′, human PTEN 3′-UTR sense 5′-CTAGCGGCCGCTAGTTGGTTCACATCCTACCCCTTTGC-ACTTG-3′, antisense 5′-TCGACAAGTGCAAAGGGGTAGGATGTGAACCAACTAGCGGCCGCTAG-AGCT-3′, human MAGI-2 3′-UTR sense 5′-CTAGCGGCCGCTAGTTCTCCATGACTTCATTTGCACT-TG-3′, antisense 5′-TCGACAAGTGCAAATGAAGTCATGGAGAACTAGCGGCCGCTAGAGCT-3′. human PTPN11 3′-UTR sense 5′-TTGTGAGCTCTATTTTGCAGATTATGGGGA-3′, antisense 5′-TTGTGTCGACCATTTGGCGACCAAAAACAC-3′. human PTPN11 mut 3′-UTR sense 5′-AGTTGACCTAACGTGAGGCATTAAAGAGTC-3′, antisense 5′-GACTCTTTAATGCCTCACGTTAGGTCAACT-3′.
The annealed products were digested with Sac I and Sal I and inserted into the pmirGLO dual-luciferase miRNA target expression vector. The primary hsa-miR-130b, hsa-miR-301a and hsa-miR-301b were cloned from genomic DNA of 5637 cells by RT-PCR using KOD-FX and oligonucleotide primers as follows:
hsa-miR-130b sense 5′-TCGAAAGCTTTACCCAATTCGCTCCCTTCT-3′, antisense 5′-TCGAGGATCCCACCCACCTGATCCTCTGAT-3′, hsa-miR-301a sense 5′-GCGAATTCTCCAAATATGTAACAGAAAGCAACA-3′, antisense 5′-GCGGATCCTTCCTTTCTACATCTATGCATGTTT-3′, hsa-miR-301b sense 5′-GCAAGCTTGGTGTCCTGGGTTCTGAAGACC-3′, antisense 5′-GCGGATCCCAGGCCTGTCTAGAATCTCAAGTT-3′.
The PCR products were digested with HindIII and BamHI (hsa-miR-130b / hsa-miR-301b), or with EcoRI and BamHI (hsa-miR-301a) and inserted into the pmR-ZsGreen1 miRNA expression vector.
Clinical specimens
Bladder cancer tissue specimens were obtained from patients who had undergone transurethral resection of bladder tumour at the Osaka University Medical Hospital, Japan. Normal urothelial specimens were also obtained from resected tissues of renal pelvis and ureter. Tumours were staged according to the 6th AJCC TNM staging system and graded according to Fuhrman′s nuclear grading system. Prior written and informed consent was obtained from all patients and the study was approved by the institutional review board of the Osaka University Hospital and the methods were carried out in accordance with the approved guidelines. Clinical and histopathological data related to the clinical samples are presented in Table 1.
Quantitative real-time PCR (qRT-PCR)
Following operation, the tissue samples were immediately immersed in RNAlater, (Qiagen, Valencia, CA, USA) and stored at −20 °C until RNA extraction. miRNA was purified using the miRNeasy mini Kit (Qiagen). A qRT-PCR was conducted to determine the expression of miR-130 family molecules in normal upper tract urothelium tissue samples and 23 bladder cancer tissue samples by using MiR-X miRNA first-strand synthesis kit (Takara, Shiga, Japan) in duplicate. Thermal cycling conditions included an initial step at 98 °C for 30 seconds and 40 cycles at 95 °C for 2 seconds and at 63 °C for 5 seconds by using a miR-130 family specific primer as follows: miR-130b: 5′-CAGTGCAATGATGAAAGGGCAT-3′, miR-301a: 5′-CAGTGCAATAGTATTGTCAAAGC-3′, miR-301b: 5′-CAGTGCAATGATATTGTCAA-AGC-3′ and a U6 snRNA-specific primer (Takara) as an internal control.
Cell culture and transfection
Human bladder cancer cell lines 563767 and UM-UC-268 were cultured in RPMI 1640 medium (Wako, Osaka, Japan) and Dulbecco’s modified Eagle’s medium (DMEM: Wako), respectively, supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 100 mg/L kanamycin at 37 °C under a 5% CO2 atmosphere. 5637 and UM-UC-2 were each supplied from Department of Urology, Osaka University (Osaka, Japan) and Nara Medical University (Nara, Japan).
MiRIDIAN miRNA hairpin inhibitors for human hsa-miR-130b-3p (IH-300660-07-0005), hsa-miR-301a-3p (IH-300657-05-0005), hsa-miR-301b-3p (IH-301252-02-0005) and negative control (IN-001005-01-05) were purchased from GE Healthcare (Lafayette, CO, USA). The miRIDIAN miRNA hairpin inhibitors were transfected at a concentration of 50 nmol/L using Lipofectamine RNAiMAX reagent (Life Technologies, Carlsbad, CA, USA). For transfection with plasmid DNA (Supplementary Fig. 3), Lipofectamine 2000 reagent (Life Technologies) was used. These transfection experiments were performed according to the protocol supplied by the manufacturer.
Water-soluble tetrazolium salt-1 (WST-1) cell proliferation assay
Cell proliferation was examined by a WST-1 cell proliferation assay. MiRIDIAN hairpin inhibitor-transfected 5637 cells were reseeded in a 96-well plate (2 × 103 cells/well) 24 h after transfection and incubated for indicated times. After incubation for 1 h with WST-1 reagent (Dojindo Laboratories, Osaka, Japan) at 37 °C and 5% CO2, the optical density was read at a wavelength of 450/630 nm (measurement/reference) by Model 680 Microplate Reader (Bio-Rad, Hercules, CA, USA).
Construction of UM-UC-2 cells with stable miR-130 family overexpression
The pri-hsa-miR-130b, pri-hsa-miR-301a, or pri-hsa-miR-301b expression pmR-ZsGreen1 vectors were transfected into UM-UC-2 cells seeded at 5 × 104 cells/well in a 12-well plate using Lipofectamine 2000 as a transfection reagent. After 24 hours of transfection, culture medium was changed to new medium containing 800 μg/mL G418 (Wako) to select for transfectant cells. These cells were subjected to limited dilution cloning in a 96-well plate (0.7 cells/well) to form a single colony and ZsGreen1-positive colonies were subjected to miRNA purification and qRT-PCR to confirm expression of miR-130b family members.
Wound healing assay
5637 cells transfected with miRIDIAN hairpin inhibitor were seeded in a 12 well plate (7.5 × 104 cells/well) and incubated for 72 h. A wound was created in a monolayer of 5637 cells or the UM-UC-2 cells stably expressing miR-130 family at ~90% confluence, by using a sterile 1 mL pipette tip. Cell pictures were recorded at 0 and 12 h after wound creation by using OLYMPUS IX71 fluorescence microscope (Olympus, Tokyo, Japan).
Cell invasion assay
The Corning tumour invasion system with the 8.0 μm pore size FluoroBlok membrane (Corning, New York, NY, USA) was used to perform the cell invasion assay for the UM-UC-2 cells stably expressing miR-130 family. The cells were seeded in the insert of a 96-well plate (3 × 103 cells/well) in serum-free conditions and DMEM containing 10% FBS was used as a chemo-attractant in the base plate. Following incubation for 24 h, the cells were labeled with calcein AM (4 μg/mL) and the fluorescence of the invading cells was read at wavelengths of 494/517 nm (Ex/Em) by EnVision Multilabel Reader (PerkinElmer, Waltham, MA, USA). For miRIDIAN hairpin Inhibitor-transfected 5637 cells, the cell invasion was measured by xCELLigence system (Roche, Indianapolis, IN, USA) according to the manufactured protocol.
Western blotting analysis
The whole cell lysate was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then transferred to a polyvinylidene difluoride (PVDF: Millipore, Billerica, MA, USA) membrane by using the semidry transfer system (Bio-Rad). The membranes were probed with specific antibodies as indicated and then incubated with horseradish peroxidase (HRP)-conjugated antibody against mouse or rabbit immunoglobulin (1:5000, Cell Signaling Technology:CST, Beverly, MA, USA), followed by detection with enhanced chemiluminescence (ECL) Western blotting detection reagents (GE Healthcare). ImageQuant LAS4000 mini system (GE Healthcare) was used as a chemiluminescence detector.
The following antibodies were used for immunological analysis in this study: anti-FAK polyclonal (1:1000, Santa Cruz, sc-557, Santa Cruz, CA, USA), anti-p-FAK576 (1:1000, Sigma, SAB4503869, St Louis, MO, USA), anti-p-FAK576 (1:1000, Santa Cruz, sc-16563-R), anti-MMP9 (1:1000, CST, #3852S), anti-Akt (1:1000, CST, #C67E7), anti-p-Akt473 (1:1000, CST, #D9E), anti-PTEN polyclonal (1:1000, Sigma, SAB4300336: for western blot analysis and immunocytochemistry), anti-PTEN polyclonal (1:1000, CST, #9188: for western blot analysis and immunohistochemistry), anti-Actin polyclonal antibody (1:50000, Sigma, A5316).
Microscopic observations
For fluorescence microscopy observation of cultured cells, cells were grown on a micro coverglass, fixed by incubating in 4% formaldehyde and then permeabilized with blocking buffer containing 5% BSA, 0.1% Triton X-100 in phosphate buffered saline (PBS). The permeabilized cells were incubated with the first antibody at 4 °C overnight, followed by fluorochrome-conjugated secondary antibody for 1 h at room temperature. For F-actin staining, the permeabilized cells were incubated with 40 nmol/L Acti-stain 488 Fluorescent Phalloidin (Cytoskeleton, Inc., Denver, USA) at room temperature for 3 h. After that, coverslips were mounted into a slide glass using Dapi Fluoromount-G (SouthernBiotech, Birmingham, AL, USA). Fluorescent images were obtained with DP70 fluorescence microscope (Olympus).
Dual-luciferase reporter assay
A pmirGLO dual-luciferase miRNA target expression vector was used for the 3′-untranslated region (UTR) luciferase reporter assay (Promega, Madison, WI, USA). The sequences of the inserts are given in “plasmid construction” section. The UM-UC-2 cells stably expressing miR-130 family were transfected with the reporter construct containing the predicted miR-130 family binding site in the PTEN (Fig. 6c) or MAGI2 (Supplementary Fig. 5) 3′-UTR. After 48 h of transfection, the dual-luciferase reporter assay was performed according to the manufacturer′s protocol (Promega). Luciferase activity was determined using a luminometer (Turner Biosystems 20/20 luminometer; Promega).
Immunohistochemistry
The expression of PTEN was determined by immunohistochemical staining of the paraffin-embedded tissues obtained from bladder cancer patients. Formalin-fixed, paraffin-embedded tissue sections (5 μm in thickness) were deparaffinized and rehydrated. After the slides were steamed for 10 min in instant antigen activation solution H (LSI Medience Corporation, Tokyo, Japan) for antigen retrieval, endogenous peroxidase was blocked using 3% H2O2. Immunohistochemical staining for PTEN was performed using anti-PTEN antibody (CST, 1:100) and the Envision Detection System (Dako, Tokyo, Japan) was used according to the manufacturer′s instructions. Primary antibodies were incubated overnight at 4 °C and counter-stained with hematoxylin. Images were obtained with Bio-Zero fluorescence microscope (KEYENCE, Osaka, Japan).
Statistics
Results were expressed as the mean ± S.D. Differences between values were statistically analyzed using a Student′s t-test or one-way ANOVA with Bonferroni post-hoc tests (GraphPad Prism 5.0, GraphPad Software, San Diego, CA, USA). A p-value <0.05 was considered statistically significant.
Additional Information
How to cite this article: Egawa, H. et al. The miR-130 family promotes cell migration and invasion in bladder cancer through FAK and Akt phosphorylation by regulating PTEN. Sci. Rep. 6, 20574; doi: 10.1038/srep20574 (2016).
References
Ferlay, J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127, 2893–2917 (2010).
Hautmann, R. E., de Petriconi, R. C., Pfeiffer, C. & Volkmer, B. G. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 61, 1039–1047 (2012).
Sylvester, R. J. et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 3, discussion 475–477 (2006).
Nagele, U., Anastasiadis, A. G., Stenzl, A. & Kuczyk, M. Radical cystectomy with orthotopic neobladder for invasive bladder cancer: a critical analysis of long-term oncological, functional and quality of life results. World J Urol 30, 725–732 (2011).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349, 859–866 (2003).
Soloway, M. S. Bladder cancer: Lack of progress in bladder cancer–what are the obstacles? Nat Rev Urol 10, 5–6 (2013).
Von der Maase, H. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23, 4602–4608 (2005).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Friedman, R. C., Farh, K. K.-H., Burge, C. B. & Bartel, D. P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19, 92–105 (2009).
Valeri, N. et al. MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell 25, 469–483 (2014).
Gao, J. et al. miR-34a-5p suppresses colorectal cancer metastasis and predicts recurrence in patients with stage II/III colorectal cancer. Oncogene 31, 4142–4152 (2015).
Ma, Y. et al. Elevated oncofoetal miR-17-5p expression regulates colorectal cancer progression by repressing its target gene P130. Nat Commun 3, 1291 (2012).
Mogilyansky, E. & Rigoutsos, I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ 20, 1603–1614 (2013).
Murphy, B. L. et al. Silencing of the miR-17~92 cluster family inhibits medulloblastoma progression. Cancer Res 73, 7068–7078 (2013).
Ell, B. et al. The microRNA-23b/27b/24 cluster promotes breast cancer lung metastasis by targeting metastasis-suppressive gene prosaposin. J Biol Chem 289, 21888–21895 (2014).
He, L. et al. A microRNA component of the p53 tumour suppressor network. Nature 447, 1130–1134 (2007).
Adam, L. et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res 15, 5060–5072 (2009).
Ma, S. et al. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 7, 694–707 (2010).
Zhang, W. et al. MicroRNA-301a promotes migration and invasion by targeting TGFBR2 in human colorectal cancer. J Exp Clin Cancer Res 33, 113 (2014).
Funamizu, N. et al. MicroRNA-301b promotes cell invasiveness through targeting TP63 in pancreatic carcinoma cells. Int J Oncol 44, 725–734 (2014).
Czerniak, B. et al. Concurrent mutations of coding and regulatory sequences of the Ha-ras gene in urinary bladder carcinomas. Hum Pathol 23, 1199–1204 (1992).
Billerey, C. et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol 158, 1955–1959 (2001).
Lopez-Knowles, E. PIK3CA Mutations Are an Early Genetic Alteration Associated with FGFR3 Mutations in Superficial Papillary Bladder Tumors. Cancer Res 66, 7401–7404 (2006).
Sarkis, A. S. et al. Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: a marker for disease progression. J Natl Cancer Inst 85, 53–59 (1993).
Puzio-Kuter, A. M. et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev 23, 675–680 (2009).
The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Thomas, S. et al. Src and caveolin-1 reciprocally regulate metastasis via a common downstream signaling pathway in bladder cancer. Cancer Res 71, 832–841 (2011).
Wang, H. et al. Phosphorylation of RalB is important for bladder cancer cell growth and metastasis. Cancer Res 70, 8760–8769 (2010).
Yamasaki, M. et al. α-Lipoic acid suppresses migration and invasion via downregulation of cell surface β1-integrin expression in bladder cancer cells. J Clin Biochem Nutr 54, 18–25 (2014).
Tojkander, S., Gateva, G. & Lappalainen, P. Actin stress fibers–assembly, dynamics and biological roles. J Cell Sci 125, 1855–1864 (2012).
Nakamura, K., Yano, H., Schaefer, E. & Sabe H. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 during epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene 20, 2626–2635 (2001).
Xia, H. et al. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem 279, 33024–33034 (2004).
Tamura, M. et al. PTEN Interactions with Focal Adhesion Kinase and Suppression of the Extracellular Matrix-dependent Phosphatidylinositol 3-Kinase/Akt Cell Survival Pathway. J Biol Chem 274, 20693–20703 (1999).
Cheng, J. C.-H., Chou, C. H., Kuo, M. L. & Hsieh, C.-Y. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene 25, 7009–7018 (2006).
Tamguney, T. & Stokoe, D. New insights into PTEN. J Cell Sci 120, 4071–4079 (2007).
Tsuruta, H. et al. Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res 66, 8389–8396 (2006).
Siegel, R., Ma, J., Zou, Z. & Jemal, A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014).
Carneiro, B. A. et al. Emerging therapeutic targets in bladder cancer. Cancer Treat Rev 41, 170–178 (2014).
Hollander, M. C., Blumenthal, G. M. & Dennis, P. A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 11, 289–301 (2011).
Aveyard, J. S., Skilleter, A., Habuchi, T. & Knowles, M. A. Somatic mutation of PTEN in bladder carcinoma. Br J Cancer 80, 904–908 (1999).
Ho, P. L., Kurtova, A. & Chan, K. S. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol 9, 583–594 (2012).
Knowles, M. A. & Hurst, C. D. Molecular biology of bladder cancer : new insights into pathogenesis and clinical diversity. Nat Rev Cancer 15, 25–41 (2015).
Huang, J. et al. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun 3, 911 (2012).
Oinuma, I., Ito, Y., Katoh, H. & Negishi, M. Semaphorin 4D/Plexin-B1 stimulates PTEN activity through R-Ras GTPase-activating protein activity, inducing growth cone collapse in hippocampal neurons. J Biol Chem 285, 28200–28209 (2010).
Wu, X. et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci 97, 4233–4238 (2000).
Rahdar, M. et al. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA 106, 480–485 (2009).
Song, P. et al. Thromboxane A2 receptor activates a Rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling. J Biol Chem 284, 17120–17128 (2009).
Matsui, T. et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15, 2208–2016 (1996).
Schoenwaelder, S. M. et al. The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr Biol 10, 1523–1526 (2000).
Zong, C., Wang, J. & Shi, T.-M. MicroRNA 130b enhances drug resistance in human ovarian cancer cells. Tumour Biol 35, 12151–12156 (2014).
Colangelo, T. et al. MicroRNA-130b promotes tumor development and is associated with poor prognosis in colorectal cancer. Neoplasia 15, 1218–1231 (2013).
Tsutsumi, A. et al. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol 26, 261–276 (2006).
Kodama, A. et al. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol Biol Cell 11, 2565–2575 (2000).
Overall, C. M. et al. Domain interactions in the gelatinase A.TIMP-2.MT1-MMP activation complex. The ectodomain of the 44-kDa form of membrane type-1 matrix metalloproteinase does not modulate gelatinase A activation. J Biol Chem 275, 39497–39506 (2000).
Goebell, P. J. & Knowles, M. A. Bladder cancer or bladder cancers? Genetically distinct malignant conditions of the urothelium. Urol Oncol 28, 409–428 (2010).
Lai, K. W. et al. MicroRNA-130b regulates the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer 46, 1456–1463 (2010).
Liu, L. et al. The oncogenic role of microRNA-130a/301a/454 in human colorectal cancer via targeting Smad4 expression. PLoS One 8, e55532 (2013).
Hall, M. C. et al. The Growth Inhibitory Effect of p21 Adenovirus on Human Bladder Cancer Cells. J Urol 163, 1033–1038 (2000).
Peacock, H. et al. Nucleobase and Ribose Modifications Control Immunostimulation by a MicroRNA-122-mimetic RNA. J Am Chem Soc 133, 9200–9203 (2011).
Didiano, D. & Hobert, O. Perfect seed pairing is not a generally reliable predictor for miRNA-target interactions. Nat Struct Mol Biol 13, 849–851 (2006).
Esquela-Kerscher, A. & Slack, F. J. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 6, 259–269 (2006).
Rottiers, V. et al. Pharmacological Inhibition of a MicroRNA Family in Nonhuman Primates by a Seed-Targeting 8-Mer AntimiR. Sci Transl Med 5, 212ra162 (2013).
Bertero, T. et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest 124, 3514–3528 (2014).
Pfaff, N. et al. miRNA screening reveals a new miRNA family stimulating iPS cell generation via regulation of Meox2. EMBO Rep 12, 1153–1159 (2011).
Brock, M. et al. The hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell proliferation by directly targeting CDKN1A. Int J Biochem Cell Biol 61, 129–137 (2015).
Hamilton, M. P. et al. Identification of a pan-cancer oncogenic microRNA superfamily anchored by a central core seed motif. Nat Commun 4, 2730 (2013).
Fogh, J. Cultivation, characterization and identification of human tumor cells with emphasis on kidney, testis and bladder tumors. Natl Cancer Inst Monogr 49, 5–9 (1978).
Grossman, H. B., Wedemeyer, G. & Ren, L. UM-UC-1 and UM-UC-2: characterization of two new human transitional cell carcinoma lines. J Urol 132, 834–837 (1984).
Acknowledgements
The study was supported by a grant-in-aid for Scientific Research (25670025) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Translational Research Network Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Adaptable and Seamless Technology transfer Program through Target-driven R&D from the Japan Science and Technology Agency (JST) and Project MEET from Osaka University.
Author information
Authors and Affiliations
Contributions
Conception and design: H.E., K.F. and K.T. Development of methodology: H.E., Y.U., K.K. and K.T. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H.E., K.J., W.N., T.H., K.F., M.U., N.N. and K.T. Analysis and interpretation of data: H.E., T.H., W.N., K.F., M.U., N.N. and K.T. Writing, review, and/or revision of the manuscript: H.E., K.J., T.H., W.N., K.F., M.U., N.N. and K. T.
Ethics declarations
Competing interests
N. Nonomura and K. Tsujikawa report receiving a commercial research grant from Takeda Pharmaceutical, Novartis Pharma, Astra Zeneca and Daiichi Sankyo, Fujifilm, Tanabe Mitsubishi, respectively. No potential conflicts of interest were disclosed by the other authors.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Egawa, H., Jingushi, K., Hirono, T. et al. The miR-130 family promotes cell migration and invasion in bladder cancer through FAK and Akt phosphorylation by regulating PTEN. Sci Rep 6, 20574 (2016). https://doi.org/10.1038/srep20574
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20574
This article is cited by
-
Overexpression Pattern of miR-301b in Osteosarcoma and Its Relevance with Osteosarcoma Cellular Behaviors via Modulating SNX10
Biochemical Genetics (2023)
-
MicroRNA-130b functions as an oncogene and is a predictive marker of poor prognosis in lung adenocarcinoma
Laboratory Investigation (2021)
-
Exosomal miR-130b-3p targets SIK1 to inhibit medulloblastoma tumorigenesis
Cell Death & Disease (2020)
-
miR-301a promotes lung tumorigenesis by suppressing Runx3
Molecular Cancer (2019)
-
Gene Regulatory Networks in Peripheral Mononuclear Cells Reveals Critical Regulatory Modules and Regulators of Multiple Sclerosis
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.