Abstract
We aimed to evaluate the risk of atrial fibrillation (AF) development following retinal vein occlusion (RVO). We performed a nationwide propensity score-matched cohort study by retrospectively reviewing a database from the Korean National Health Insurance Service, comprising approximately 1 million random subjects. RVO and AF were diagnosed based on the Korean Classification of Disease codes. The RVO group was composed of patients with an initial diagnosis of RVO made between 2003 and 2007 (n = 1,801), excluding those who were diagnosed in 2002. The comparison group was composed of randomly selected patients (5 for each patient with RVO, n = 8,930) who were matched to the RVO group according to sociodemographic factors and the year of enrollment. Each sampled patient was tracked until 2013. The predictive value of RVO for AF was analyzed using Cox regression analysis with a hazard ratio (HR) and confidence interval (CI). AF developed in 6.5% of patients in the RVO group and 4.0% of those in the comparison group (p < 0.001). RVO was associated with a greater risk of AF development after adjusting for possible confounders (HR, 1.35; 95% CI, 1.09–1.67). An association between RVO and subsequent AF development was found after adjusting for possible confounding factors.
Similar content being viewed by others
Introduction
If the central retinal vein or one of its main four branches is occluded, retinal hemorrhage and retinal edema subsequently occur and frequently cause low vision or blindness in adults1. This is called retinal vein occlusion (RVO), and there are two types: branch RVO (BRVO) and central RVO (CRVO). The suggested mechanisms of CRVO are increasing intraocular pressure, an atherosclerotic central retinal artery, and deformation of the lamina. For BRVO, the suggested mechanisms are interrupted venous flow at the arteriovenous crossing by arterial stiffening due to atherosclerosis2. Several previous studies have shown that RVO increases the risk of cardiovascular/cerebrovascular disease and their associated mortalities3,4,5,6,7,8,9,10,11,12,13,14. Considering the data for the risks of subsequent stroke or myocardial infarction following RVO development, it seems likely that small-sized to medium-sized vascular disease of the eye is associated with a higher likelihood of cardiovascular disease.
Atrial fibrillation (AF) is common in the elderly, and it is a significant public health problem associated with increased mortality and high cardiovascular morbidities such as stroke and heart failure (HF)15. In the United States, AF is predicted to affect 6–12 million people by 2050 because of the aging population16. To the best of our knowledge, the association between RVO and AF has not been established previously in a longitudinal study with a large sample size. Therefore, in the current study, we investigated the risk of subsequent AF development following RVO using a representative nationwide sample of 1 million participants, which was provided by the National Health Insurance Service -National Sample Cohort from 2002–2013 (NHIS-NSC 2002–2013) database in South Korea.
Results
Patients’ baseline characteristics
Overall, 10,731 subjects, including 1,801 patients with RVO and 8,930 controls, were eligible for analysis (Table 1). During the entire study period (median, 7.7 years), AF occurred more frequently in subjects with RVO than in those without RVO (6.5% vs. 4.0%, p < 0.001). Comorbidities including HF, cerebrovascular disease, hypertension, diabetes mellitus (DM), chronic kidney disease (CKD), and liver disease were more common in the RVO group than in the sociodemographic-matched comparison group (p < 0.001, all). For sociodemographic variables, we found no difference in the proportion of patients according to the presence of RVO, which was used to perform matching between the two groups.
Factors associated with AF occurrence
In multivariable model, we found that RVO was associated with a subsequent diagnosis of AF (hazard ratio [HR], 1.35; 95% confidence interval [CI], 1.09–1.67). HF (HR, 3.26; 95% CI, 2.65–4.02), hyperthyroidism (HR, 1.87; 95% CI, 1.09–3.19), acute myocardial infarction (HR, 1.55; 95% CI, 1.00–2.41), cerebrovascular disease (HR, 1.30; 95% CI, 1.03–1.64), and liver disease (HR, 1.24; 95% CI, 1.01–1.54) were associated with AF development in our multivariable model (Table 2); however, other comorbidities were not associated with AF development. Compared to male sex, female sex was more strongly associated with a decreased risk of AF occurrence (HR, 0.70; 95% CI, 0.58–0.84).
RVO was associated with a higher incidence of AF in both men and women (men: HR, 1.65; 95% CI, 1.22–2.24 vs. women: HR, 1.14; 95% CI, 0.85–1.54, Table 3). However, the HR was greater in men than in women, and in women, the result was not statistically significant. HF was the strongest predictive factor in both men and women (men: HR, 3.51; 95% CI, 2.58–4.78 vs. women: HR, 3.00; 95% CI, 2.26–3.98). In men, patients with hyperthyroidism (HR, 2.37; 95% CI, 0.96–5.86) and acute myocardial infarction (HR, 1.73; 95% CI, 0.94–3.16) were more likely to have an episode of AF, albeit marginally. In women, patients with cerebrovascular disease were more likely to have an episode of AF (HR, 1.37; 95% CI, 1.01–1.87).
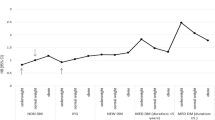
The follow-up period of 79,482 person-years, including 66,244 person-years for the comparison group and 13,238 person-years for the RVO group, was analyzed (Fig. 1). AF occurred in 8.8 per 1,000 person-years in the RVO group, and in 5.3 per 1,000 person-years in the comparison group. The incidence and risk of AF according to the presence of RVO were examined in each age and sex subgroups, and in the subgroups with or without comorbidities using multivariable Cox regression analysis. In the age subgroup analysis, subjects with RVO had a greater risk of subsequent AF in both age groups, albeit marginally in the older age group (age group <65 years: HR, 1.49; 95% CI, 1.06–2.12 vs. age group ≥65 years: HR, 1.27; 95% CI, 0.97–1.67). In men, RVO increased the risk of subsequent AF by an HR of 1.65 (95% CI, 1.22–2.24), whereas in women, the increased risk did not reach statistical significance (HR, 1.14; 95% CI, 0.85–1.54). RVO had an independent predictive value for AF development among subjects with HF (HR, 1.36; 95% CI, 1.05–1.76). However, in subjects without HF, the AF occurrence rate was relatively low (comparison group: 2.6 vs. RVO group: 3.8 per 1,000 person-years); thus, the difference was not statistically significant considering the large CI. In addition, in subjects with and without hypertension, DM, or chronic lung disease, RVO also increased the AF risk with HRs of >1; however, in the subgroups of subjects without hypertension or DM but with chronic lung disease, the results did not reach statistical significance due to the wide CIs.
Incidence and risk of atrial fibrillation (AF) in the retinal vein occlusion (RVO) and comparison groups.
Incidence rate per 1,000 person-years. CI, confidence interval; HR, hazard ratio. HRs were calculated based on multivariable Cox regression after being adjusted for sociodemographic factors and comorbidities. The P for interaction was calculated using the interaction term for RVO and each subgroup based on the multivariable Cox regression.
Survival curves for AF
Figure 2 displays the Kaplan-Meier survival curves for up to 11 years. Overall, compared to patients in the comparison group, those with RVO experienced AF more frequently (Fig. 2A). The AF-free survival rates were different between the two groups regardless of the age subgroup (Fig. 2B). In terms of sex, the AF-free survival rate decreased faster starting early in the study period in men with RVO compared to men without RVO; this trend was not found in women (Fig. 2C).
Discussion
We found an temporal relationship between newly developed RVO and subsequent AF development.
Recently, the retinal vasculature has gained more attention, because it is unique in that the retinal artery or vein and abnormal vasculature such as hypertensive retinopathy can be directly visualized using ophthalmoscopy. Therefore, RVO could be a surrogate marker for cardiovascular disease such as hypertension or stroke12. Although a new onset of RVO mainly causes decreasing vision, peripheral RVO does not affect vision in some cases. Therefore, the patient may not know about an RVO occurrence in his or her eyes. Underdiagnosed RVO could be detected by an ophthalmologist through the diagnosis of an eye complication such as sclerotic vessel change, optociliary shunt vessels (collaterals), sequelae of neovascularization, or thinning of the retinal nerve fiber layer17,18,19,20. The evaluation of these signs based on a retinal examination in vivo could be an easy, non-invasive way to stratify the risk of cardiovascular/cerebrovascular disease.
The characteristics of RVO are similar to those of stroke, because the structure of the retina is derived from the central nervous system21. Our previous study showed that RVO significantly increases the risk of stroke, with an HR of 1.48 (95% CI, 1.24–1.76) after adjusting for comorbidities12. AF has been the most common risk factor associated with stoke22. One case-control study showed that AF was more common in a CRVO group; however, cases of AF were excluded from the final model of stepwise logistic regression analysis23. According to a nationwide cohort study in Denmark of 605 patients with RVO retinal vascular (artery or vein) occlusion among 86,572 patients with non-valvular AF, RVO significantly increased the risk of stroke among patients with AF (HR, 1.26; 95% CI, 1.02–1.54)24. In this study, retinal vein or artery occlusion was suggested to be an independent risk factor for stroke in AF; therefore, it would merit at least 1 or 2 points in the CHA2DS2-VASc scoring system, fulfilling the stroke/thromboembolism criterion or vascular criterion24. In our study, RVO increased the likelihood of AF. Finally, RVO alone was independently associated with an increased risk of stroke and AF, and RVO increased the stroke risk in patients with AF. However, the effect size of the HRs could not be compared directly between studies because of the different ethnic populations and study designs.
The present study showed that the overall AF development was more common in men (Table 2), which is consistent with the results of previous reports25,26,27. Nevertheless, HF is one of the strongest predictors for incident AF in men (HR, 3.51) and women (HR, 3.00). However, other risk factors showed sex-related differences in terms of the AF risk. In a previous population-based study using a Danish cohort, hyperthyroidism was more common in women. However, male sex was associated with AF in those with hyperthyroidism28, and this significant result was consistent with our results. In a previous study using the Canadian Registry of AF, myocardial infarction was more prevalent in men, whereas stroke was more prevalent in women at the time of the initial presentation of AF29. In the present study, the HR for AF due to acute myocardial infarction was also greater in men than in women, and the HR for AF due to cerebrovascular disease was greater in women than in men. Results of previous studies and our study all indicate that there are sex-related differences in the clinical burden of AF. In particular, RVO also showed a sex-dependent association: RVO significantly increased the incidence AF in men, not in women (Table 3). Figure 2C also shows the different trends in the AF risk between men and women over time. The relatively large gap in the AF incidence between the two groups in men was sustained from the beginning to the end of the study period. However, the gap was relatively small in women. In our previous studies, RVO was a stronger predictor for acute myocardial infarction in younger men rather than in younger women14. In addition, RVO was a stronger predictor of HF in men than in women13. Sex-related differences have been recognized thus far, and RVO could be a possible cause of AF, acute myocardial infarction14, and HF13, particularly in men, as well as a cause of stroke in men and women12.
In accordance with previously published reports, we found that HF30, hyperthyroidism28, acute myocardial infarction, cerebrovascular disease, and liver disease31 were predictors of incident AF32. However, other comorbidities such as hypertension, DM, CKD, and chronic lung disease were not associated with AF based on our multivariable model. In the stratified analysis by HF (Fig. 1), RVO increased the risk of AF with an HR of approximately 1.3 in both subjects with or without HF. These results suggest an independent predictive value for RVO as well as HF for AF. Of note, RVO could be a better and earlier stage predictor for AF compared to other cardiovascular risk factors including hypertension, DM, or CKD.
Retinal microcirculation can be assessed non-invasively, directly, and safely. Retinopathy has been assumed to be a surrogate marker for microvascular dysfunction, which plays a pivotal role in the development of metabolic syndrome and cardiovascular disease33,34,35. Recently, metabolic syndrome has been assumed to be an important risk factor for AF development36,37. Autonomic neuropathy, oxidative stress, myocardial fibrosis, and microvascular dysfunction could be the underlying pathophysiologic mechanism for this association38,39. Further research to determine the causality and underlying mechanisms associated with the occurrence of AF and RVO is warranted. This epidemiological study does not provide evidence for the mechanism of RVO and AF.
The strengths of the present study include the large number of subjects and a long-term follow-up. Previous studies have shown an association between RVO and other cardio/cerebrovascular diseases such as stroke, hypertension, and DM. However, there have been no previous reports about the association between RVO and AF. This is the first report to examine AF development following RVO.
The most major limitation of the present study was the potential inaccuracy of the KCD code-diagnoses of RVO and AF. The accuracy of the health insurance service claims regarding RVO were mentioned in our previous report based on the NHIS-NSC 2002–2009 database12. The use of medical insurance claims dataset and the accuracy of these dataset are relevant issues to consider in the clinical setting. Unfortunately, there were no previous studies about the validity of national insurance claims for AF in South Korea. However, in Fig. 2A, the incidence of AF in the comparison group consistently increased for 11 years; this may partially represent the validity of AF diagnosis in our study. In addition, due to their asymptomatic nature, nasal occlusions are less likely to be diagnosed and reported. However, if patients with nasal-side RVO belong to the comparison cohort, the real HR may be greater than the current HR, as RVO increased the risk of AF.
Aside from the aforementioned possible inaccuracy of the KCD code-based diagnosis of RVO and AF, the following limitations of this study should be mentioned: (1) the possible misclassification of diagnoses for RVO, AF, or comorbidities; (2) possible underreporting of asymptomatic RVO or paroxysmal AF; (3) possibility of the delayed diagnosis of RVO or AF due to delayed visits to physicians; (4) possibility of inappropriately included patients with chronic RVO or AF; (5) potential that other health behavioral information such as alcohol use is missing; (6) higher possibility of bias among control patients from the medical claims than among control patients from the general population who have not received medical care; and (7) possible ethnic differences that may exist in ethnic groups other than the South Korean population that were not considered in this study.
In summary, RVO was associated with subsequent AF development in our multivariable Cox model. A retinal examination may be a safe method to evaluate one’s risk of AF. Therefore, ophthalmologists and cardiologists should be cautious of patients with RVO, as these patients, especially in men, should undergo cardiovascular risk screening to check for these disorders. These findings are limited, and they need to be confirmed by other observational studies.
Methods
Ethic statement
The institutional review board (IRB) of Severance Hospital at Yonsei University College of Medicine in Seoul, Korea approved this retrospective cohort study. The IRB waived the requirement to obtain informed consent, and this study was conducted in accordance with the tenets of the Declaration of Helsinki.
Data source
This retrospective cohort study used the NHIS-NSC 2002–2013 dataset, comprising a random sample of 1,025,340 subjects, which amounted to approximately 2.2% of the entire population in the Korean NHIS in 2002. Data were produced by the NHIS using a systematic sampling method for the purpose of research. Random sampling was used based on 1,476 strata. As all individuals in South Korea are enrolled in the NHIS, All available data in the NHIS are centralized and include complete information, including diagnostic codes, procedures, prescription drugs used, and personal information for inpatient and outpatient visits. Moreover, diagnostic codes based on the KCD have been used in South Korea, and they are comparable to the International Classification of Diseases (ICD).
Study population
The inclusion criteria were as follows: (1) patients in the study cohort who received medical care between 1st January 2003 and 31th December 2007 with a newly diagnosed RVO (KCD code H34.8, corresponding to ICD-9-CM codes 362.35, CRVO or 362.36, venous tributary [branch] occlusion); (2) subjects (comparison cohort) extracted using propensity-score matching (5 per each patient with RVO) from the database of 1 million subjects without RVO between January 2003 and December 2007; and (3) patients newly diagnosed as having AF (KCD code I48, corresponding to ICD-9-CM code 427.3, AF and flutter) from 2003–2013 to assess the outcome variable, AF occurrence. The exclusion criteria were as follows: (1) patients with chronic RVO before 2003; (2) patients with chronic AF before 2003; and (3) patients with AF occurrence before the occurrence of RVO, based on the patient’s visit date.
The variables for matching were as follows: (1) age (<50/50–59/60–69/70–79/≥80 years), (2) sex, (3) residential area, (4) household income (≤30%, 30–70%, and ≥70% of the median), and (5) year of enrollment (2003–2007). Comorbidities such as HF, hyperthyroidism, acute myocardial infarction, cerebrovascular disease, hypertension, DM, CKD, chronic lung disease, and liver disease were diagnosed based on the KCD, and they were defined as any diagnoses made from 2002–2007 (Supplementary Table 1).
Statistical analysis
Characteristics of the study population were presented with descriptive statistics. We matched the RVO and comparison groups based on propensity score matching; the propensity scores were estimated by logistic regression analysis to predict RVO occurrence, and we controlled for age, sex, residency, income, and year of enrollment. Matching was performed using the greedy 8→1 digit-matching macro with a propensity score. Once a match was made, the match was not considered again. From the preliminary analysis based on the formula by Schoenfeld40, we could achieve more than 80% power with a sample size of 10,000 (ratio of cases to controls, 1:5) and an AF incidence of 4% for detecting an HR of 1.32 or greater. Each patient was tracked for up to 11 years from the first visitation date with a diagnosis of RVO (RVO group) or a randomly selected visitation date in the matched year (control group) to the last follow-up date in 2013 or to the date of the first diagnosis of AF. The overall AF survival rate was described using a Kaplan-Meier survival curve from 2003–2013 (median, 7.7 years). An association between RVO and the prospective risk of AF development was evaluated using Cox proportional hazard model. HRs with 95% CIs were calculated using a multivariable model. For subgroup analyses, the groups were stratified according to age, sex, and the presence of comorbidities. As the proportions of heart failure (26.0%), hypertension (42.7%), DM (20.7%), and chronic lung disease (30.1%) were relatively high (>20%) in the entire cohort, these comorbidities were used for subgroup analyses; the groups were stratified into patients with heart failure, hypertension, DM, or chronic lung disease and those without these comorbidities. A significance level of α 5% was considered statistically significant. Stata/MP2, version 14.0 (StataCorp, College Station, TX, USA) and the SAS System for Windows, version 9.4 (SAS Institute Inc., Cary, NC, USA) were used to perform the analyses.
Additional Information
How to cite this article: Rim, T. H. et al. Evaluation of the Association Between Retinal Vein Occlusion and the Risk of Atrial Fibrillation Development: A 12-Year, Retrospective Nationwide Cohort Study. Sci. Rep. 6, 34708; doi: 10.1038/srep34708 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Rogers, S. L. et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology 117, 1094–1101, e1095 (2010).
Bertelsen, M. et al. Comorbidity in patients with branch retinal vein occlusion: case-control study. BMJ 345, e7885 (2012).
Rubinstein, K. & Jones, E. B. Retinal vein occlusion: long-term prospects: 10 years’ follow-up of 143 patients. Br J Ophthalmol 60, 148–150 (1976).
Hankey, G. J., Slattery, J. M. & Warlow, C. P. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ 302, 499–504 (1991).
Bruno, A. et al. Vascular outcome in men with asymptomatic retinal cholesterol emboli. A cohort study. Ann Intern Med 122, 249–253 (1995).
Elman, M. J., Bhatt, A. K., Quinlan, P. M. & Enger, C. The risk for systemic vascular diseases and mortality in patients with central retinal vein occlusion. Ophthalmology 97, 1543–1548 (1990).
Tsaloumas, M. D. et al. Nine year follow-up study of morbidity and mortality in retinal vein occlusion. Eye (Lond) 14, 821–827 (2000).
Klein, R., Klein, B. E., Moss, S. E. & Meuer, S. M. Retinal emboli and cardiovascular disease: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc 101, 173–180; discussion 180–172 (2003).
Cugati, S. et al. Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology 114, 520–524 (2007).
Hu, C. C., Ho, J. D. & Lin, H. C. Retinal vein occlusion and the risk of acute myocardial infarction (correction of infraction): a 3-year follow-up study. Br J Ophthalmol 93, 717–720 (2009).
Ho, J. D., Liou, S. W. & Lin, H. C. Retinal vein occlusion and the risk of stroke development: a five-year follow-up study. Am J Ophthalmol 147, 283–290, e282 (2009).
Rim, T. H., Kim, D. W., Han, J. S. & Chung, E. J. Retinal Vein Occlusion and the Risk of Stroke Development: A 9-Year Nationwide Population-Based Study. Ophthalmology, doi: 10.1016/j.ophtha.2015.01.020 (2015).
Rim, T. H., Oh, J., Kang, S. M. & Kim, S. S. Association between retinal vein occlusion and risk of heart failure: A 12-year nationwide cohort study. Int J Cardiol 217, 122–127 (2016).
Rim, T. H. et al. Retinal vein occlusion and the risk of acute myocardial infarction development: a 12-year nationwide cohort study. Sci Rep 6, 22351 (2016).
Andersson, T. et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case-control study. Eur Heart J 34, 1061–1067 (2013).
Chugh, S. S. et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129, 837–847 (2014).
Hayreh, S. S. et al. Ocular neovascularization with retinal vascular occlusion-III: incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology 90, 488–506 (1983).
Joffe, L., Goldberg, R. E., Magargal, L. E. & Annesley, W. H. Macular branch vein occlusion. Ophthalmology 87, 91–98 (1980).
Zegarra, H., Gutman, F. A. & Conforto, J. The natural course of central retinal vein occlusion. Ophthalmology 86, 1931–1939 (1979).
Kim, C. S. et al. Sectoral retinal nerve fiber layer thinning in branch retinal vein occlusion. Retina 34, 525–530 (2014).
Kernt, M. & Ulbig, M. W. Images in cardiovascular medicine. Wide-field scanning laser ophthalmoscope imaging and angiography of central retinal vein occlusion. Circulation 121, 1459–1460 (2010).
Wolf, P. A., Abbott, R. D. & Kannel, W. B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22, 983–988 (1991).
Koizumi, H., Ferrara, D. C., Brue, C. & Spaide, R. F. Central retinal vein occlusion case-control study. Am J Ophthalmol 144, 858–863 (2007).
Christiansen, C. B. et al. Retinal vein and artery occlusions: a risk factor for stroke in atrial fibrillation. J Thromb Haemost 11, 1485–1492 (2013).
Schnabel, R. B. et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet, doi: 10.1016/S0140-6736(14)61774-8 (2015).
Jeong, J. H. Prevalence of and risk factors for atrial fibrillation in Korean adults older than 40 years. J Korean Med Sci 20, 26–30 (2005).
Kerr, C. R. & Humphries, K. Gender-related differences in atrial fibrillation. J Am Coll Cardiol 46, 1307–1308 (2005).
Frost, L., Vestergaard, P. & Mosekilde, L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med 164, 1675–1678 (2004).
Humphries, K. H. et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation 103, 2365–2370 (2001).
Wang, T. J. et al. Temporal Relations of Atrial Fibrillation and Congestive Heart Failure and Their Joint Influence on Mortality The Framingham Heart Study. Circulation 107, 2920–2925 (2003).
Targher, G. et al. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One 8, e57183 (2013).
Benjamin, E. J. et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 271, 840–844 (1994).
Flammer, J. et al. The eye and the heart. Eur Heart J 34, 1270–1278 (2013).
Dean, J., Cruz, S. D., Mehta, P. K. & Merz, C. N. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol 12, 406–414 (2015).
Aoqui, C. et al. Microvascular dysfunction in the course of metabolic syndrome induced by high-fat diet. Cardiovasc Diabetol 13, 31 (2014).
Hajhosseiny, R., Matthews, G. K. & Lip, G. Y. The Metabolic Syndrome, Atrial Fibrillation and Stroke: Tackling An Emerging Epidemic. Heart Rhythm, doi: 10.1016/j.hrthm.2015.06.038 (2015).
Nystrom, P. K. et al. Obesity, metabolic syndrome and risk of atrial fibrillation: a Swedish, prospective cohort study. PLoS One 10, e0127111 (2015).
Tadic, M., Ivanovic, B. & Cuspidi, C. What do we currently know about metabolic syndrome and atrial fibrillation? Clin Cardiol 36, 654–662 (2013).
Nguyen, J. T. & Benditt, D. G. Atrial fibrillation susceptibility in metabolic syndrome: simply the sum of its parts? Circulation 117, 1249–1251 (2008).
Schoenfeld, D. A. Sample-size formula for the proportional-hazards regression model. Biometrics 39, 499–503 (1983).
Acknowledgements
This study used the National Health Insurance Service-National Sample Cohort from 2002–2013 database (NHIS-2016-2-113), which was released by the Korean National Health Insurance Service. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Contributions
T.H.R. wrote the initial draft of the manuscript. T.H.R., J.O., C.S.L., S.C.L., S.-M.K. and S.S.K. conceived the concept for this study. J.O., C.S.L., S.C.L. and S.-M.K. were involved in critical revision of the manuscript. T.H.R. and S.S.K. performed the statistical analysis. S.C.L., S.-M.K. and S.S.K. supervised the study. All authors reviewed the manuscript and approved the final version.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Rim, T., Oh, J., Lee, C. et al. Evaluation of the Association Between Retinal Vein Occlusion and the Risk of Atrial Fibrillation Development: A 12-Year, Retrospective Nationwide Cohort Study. Sci Rep 6, 34708 (2016). https://doi.org/10.1038/srep34708
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34708
This article is cited by
-
Risk of incident atrial fibrillation in patients presenting with retinal artery or vein occlusion: a nationwide cohort study
BMC Cardiovascular Disorders (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.