Abstract
Rats with normal renal function (Experiment 1, n = 12) and uninephrectomized (1/2Nx) rats (Experiment 2, n = 12) were fed diets with normal P (NP) and either normal (NF) or high fat (HF). Rats with intact renal function (Experiment 3, n = 12) were also fed NF or HF diets with high P (HP). Additionally, uremic (5/6Nx) rats (n = 16) were fed HP diets with NF or HF. Feeding the HF diets resulted in significant elevation of plasma FGF23 vs rats fed NF diets: Experiment 1, 593 ± 126 vs 157 ± 28 pg/ml (p < 0.01); Experiment 2, 538 ± 105 vs 250 ± 18 pg/ml (p < 0.05); Experiment 3, 971 ± 118 vs 534 ± 40 pg/ml (p < 0.01). Rats fed HF diets showed P retention and decreased renal klotho (ratio klotho/actin) vs rats fed NF diets: Experiment 1, 0.75 ± 0.06 vs 0.97 ± 0.02 (p < 0.01); Experiment 2, 0.69 ± 0.07 vs 1.12 ± 0.08 (p < 0.01); Experiment 3, 0.57 ± 0.19 vs 1.16 ± 0.15 (p < 0.05). Uremic rats fed HF diet showed more severe vascular calcification (VC) than rats fed NF diet (aortic Ca = 6.3 ± 1.4 vs 1.4 ± 0.1 mg/g tissue, p < 0.001). In conclusion, energy-rich diets increased plasma levels of FGF23, a known risk factor of cardiovascular morbidity and mortality. Even though FGF23 has major phosphaturic actions, feeding HF diets resulted in P retention, likely secondary to decreased renal klotho, and aggravated uremic VC.
Similar content being viewed by others
Introduction
Energy-dense foods (fast-foods) are implicated in the etiopathogenesis of dysmetabolic syndrome, a significant and growing health problem both in Western and developing countries1,2. In addition to their high caloric content, these foods are usually rich in phosphate (P), which is widely used as a food additive3,4. Moreover, the high fat content of fast-food is likely to increase P digestibility5, further aggravating P load. Phosphate overload may cause an increase in fibroblast growth factor 23 (FGF23), which is a known factor of cardiovascular risk6. Although there is an increasing awareness of the detrimental effects of the P content of fast-food, the influence of the caloric content of the diet on P balance has not been studied in detail.
Phosphate overload may be tolerated when renal function is intact; however, a decline in renal function, which is associated to high-fat intake and type II diabetes7, compromises P handling. In fact, in patients with chronic kidney disease (CKD), elevated serum phosphate plays a major role in the development of vascular calcification (VC)8. Phosphate promotes VC through a series of mechanisms, including increased serum CaxP product, which leads to precipitation of calcium salts, and phenotypic transdifferentiation of vascular smooth muscle cells (VSMCs) to osteogenic cells9,10.
We hypothesized that feeding a diet with high caloric content (high fat diet) would cause an increase in FGF23 in an attempt to control P balance, and would promote VC when renal function is impaired. Thus, the objectives of the present study were: (a) to evaluate changes in FGF23 and P balance induced by high fat diets in rats with normal and reduced renal function; and, (b) to investigate the influence of high fat diets on the development of VC in uremic rats.
Results
Studies on P balance
Phosphorus balance was studied in rats with intact and reduced (1/2Nx) renal function fed diets with normal P (NP) and either normal fat (NF) or high fat (HF); and in rats with normal renal function fed high P (HP) diets with NF or HF. In all experiments, rats fed HF diets reduced their food intake when compared when rats fed NF diets. Therefore rats fed HF diets ingested less P than rats fed NF diets. However, the ingested P was more readily absorbed in rats fed HF diets than in rats fed NF diets. This resulted in almost the same net absorption of P and thus in a nearly identical P load in NF vs HF groups: rats with intact renal function fed NP, 44.5 ± 3.2 mg/day vs 44.1 ± 2.4 mg/day; 1/2Nx rats fed NP, 42.1 ± 1.6 mg/day vs 47.3 ± 2.0 mg/day; rats with intact renal function fed HP, 94.5 ± 6.5 mg/day vs 95.9 ± 4.2 mg/day. Urinary P excretion was not different in rats fed HF or NF and NP. In rats fed HP, P excretion by urine was lower (p < 0.05) in rats fed HF diet, 85.0 ± 3.6 mg/day, than in rats fed NF diet, 101.3 ± 4.0 mg/day. In rats with intact renal function fed NP, a non-significant tendency to P retention was detected with HF diet, 14.8 ± 1.8 mg/day vs 12.9 ± 2.2 mg/day in rats fed NF diet. Phosphorus retention in rats on HF diets became significant (p < 0.05) in 1/2Nx rats fed NP, 19.3 ± 1.8 mg/day vs 10.0 ± 2.2 mg/day in rats on NF diet, and in rats fed HP, 10.9 ± 4.4 mg/day vs −6.8 ± 5.2 mg/day in rats on NF diet (Table 1).
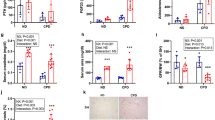
In the groups receiving NP, even though P load was similar, rats fed HF diets showed consistently higher plasma FGF23 concentrations than rats fed NF diets: rats with intact renal function fed NP, 593 ± 126 pg/ml vs 157 ± 28 pg/ml (p < 0.01), and 1/2Nx rats fed NP, 538 ± 105 pg/ml vs 250 ± 18 pg/ml (p < 0.05). Feeding a HP diet resulted in higher FGF23 levels than feeding NP diet and rats fed HP-HF diet also had significantly (p < 0.01) elevated FGF23, 995 ± 120 pg/ml, when compared with rats fed HP-NF diet, 547 ± 40 pg/ml (Fig. 1). HF diets elicited increased expression of FGF23 in bone. In a subset of rats fed NP, bone mRNA FGF23/GAPDH ratio increased 3 times in rats fed HF (n = 6) vs rats fed NF (n = 6) diets (3.6 ± 0.9 vs 1.1 ± 0.5, p < 0.05). It is interesting to note that even though plasma FGF23 concentrations were significantly higher in rats fed HF diets than in rats fed NF diets, urinary excretion of P was not increased in the animals fed HF diets (Table 1).
Influence of the fat content of the diet on fibroblast growth factor 23 (FGF23).
Plasma concentrations of FGF23 in rats fed normal fat (NF, white bars) or high fat (HF, black bars) diets in the studies on P balance. Experiment 1 (n = 12): rats with intact renal function fed 0.6% P, Experiment 2 (n = 12): 1/2Nx rats fed 0.6% P, Experiment 3 (n = 12): rats with intact renal function fed 1.2% P. ap < 0.05 vs NF in the same Experiment, bp < 0.05 vs NF in Experiment 1.
No significant differences between NF and HF groups were found in plasma concentrations of P, iCa, creatinine, total cholesterol, triglycerides and PTH. As expected, 1/2 Nx rats had higher plasma creatinine and urea. Similarly, rats fed HP tended to show higher PTH values. Plasma concentrations of calcidiol were similar in all groups and were not influenced by the increased caloric content in HF diets, except in rats fed HP-HF diet which had higher calcidiol. However, feeding HF diets resulted in significant reductions in plasma concentrations of calcitriol when compared with rats fed NF diets: rats with intact renal function fed NP, 11.3 ± 1.1 pg/ml vs 82.2 ± 11.7 pg/ml (p < 0.001); 1/2Nx rats fed NP, 20.8 ± 10.8 pg/ml vs 117.1 ± 23.7 pg/ml (p < 0.01); rats with intact renal function fed HP, 74.8 ± 25.9 pg/ml vs 143.0 ± 11.8 pg/ml (p < 0.05) (Table 2).
Figure 2 shows the levels of klotho and FGFR1 proteins in the kidney. Changes in renal klotho were well correlated with changes in plasma FGF23 concentrations. A significant decrease in renal klotho (ratio klotho/β-actin) was identified in all rats fed HF diets: rats with intact renal function fed NP, 0.75 ± 0.06 vs 0.97 ± 0.02 in rats fed NF diet (p < 0.01); 1/2Nx rats fed NP, 0.69 ± 0.07 vs 1.12 ± 0.08 in rats fed NF diet (p < 0.01); and rats with intact renal function fed HP, 0.57 ± 0.19 vs 1.16 ± 0.15 in rats fed NF diet (p < 0.05) (Fig. 2A). Feeding HF diets did not influence FGFR1 expression in the kidney (Fig. 2B).
Influence of the fat content of the diet on renal klotho and fibroblast growth factor receptor 1 (FGFR1).
(A) Western Blots of klotho and (B) FGFR1 obtained from renal tissue of rats fed normal fat (NF, white bars) or high fat (HF, black bars) diets in the studies on P balance. Experiment 1 (n = 12): rats with intact renal function fed 0.6% P, Experiment 2 (n = 12): 1/2Nx rats fed 0.6% P, Experiment 3 (n = 12): rats with intact renal funcion fed 1.2% P. ap < 0.05 vs NF in the same Experiment.
Studies on vascular calcification (VC)
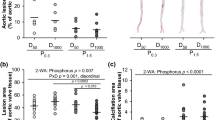
Uremic rats fed HP-NF diet only experienced very minor mineral deposition in vascular tissue: aortic calcium = 1.4 ± 0.1 mg/g tissue, aortic phosphorus = 0.1 ± 0.1 mg/g tissue. However, 5/6 Nx rats on HP-HF diet showed consistent increases in both aortic calcium (6.3 ± 1.4 mg/g tissue) and phosphorus (12.4 ± 7.2 mg/g tissue), p < 0.001 vs NF diet. Similar results were obtained in other soft tissues and extraskeletal calcification was also confirmed by von Kossa staining (Fig. 3). Feeding HF diets not only resulted in mineral deposition but also in a phenotypic transdifferentiation of the vessel wall that was evidenced by an increase in the expression of osteogenic genes. Thus, mRNA Runx-2/GAPDH ratio was increased by 2.8 fold, mRNA Osterix/GAPDH was increased by 2.4 fold and mRNA Sclerostin/GAPDH was increased by 3.8 fold in rats fed HF diet when compared with rats fed NF diet (Fig. 4).
Influence of the fat content of the diet on vascular calcification.
(A) Calcium (white bars) and phosphorus (black bars) content (mg/g of tissue) in the aortas of uremic (5/6 Nx) rats fed high 1.2% P and either normal fat (NF, n = 6) or high fat (HF, n = 10) diets in Experiment 4. (B) Von Kossa stained sections of the aortas of 5/6Nx rats fed high 1.2% P and either NF (left) or HF (right). Calcification foci are stained in brown. ap < 0.05 vs NF.
Influence of the fat content of the diet on the expression of osteogenic genes in aortic tissue.
Levels of mRNA Runx-2/GAPDH, mRNA Osterix/GAPDH and mRNA Sclerostin/GAPDH in the aortas of uremic (5/6 Nx) rats fed 1.2% P and either normal fat (NF, white bars) or high fat (HF, black bars) diets in Experiment 4 (n = 6 NF and 10 HF). ap < 0.05 vs NF.
No differences between uremic rats fed NF and HF diets were observed in plasma creatinine, urea, iCa, P and PTH. Plasma triglycerides, LDL cholesterol and HDL cholesterol tended to be elevated in rats fed HF diet but significant differences with rats fed NF diet were only found in total cholesterol (111.9 ± 6.4 vs 84.2 ± 3.5 mg/dl). Plasma FGF23 was significantly higher (11,366 ± 1,639 vs 4,799 ± 2,402 pg/ml, p < 0.05) in rats fed HF diet (Table 3) and was correlated with aortic calcium (r = 0.640, p = 0.001). The increase in FGF23 was accompanied by a marked but non-significant decrease in the expression of klotho (ratio klotho/β-actin) in the remnant kidney of 5/6 Nx rats, from 1.00 ± 0.39 in rats fed NF diet to 0.55 ± 0.16 in rats fed HF diet.
Discussion
This study was designed to investigate the effect of feeding energy-dense diets on P balance and VC in rats with normal and reduced renal function. Our results show that rats fed diets with high fat content decreased renal klotho expression, developed P retention due to impaired renal excretion of P, and increased plasma FGF23 concentrations. Moreover, uremic rats fed HF diets showed more severe VC than their normocaloric-fed counterparts.
The study experimental design was based on comparing the effect of diets with identical P concentration and different caloric content. For this purpose a high-fat diet, which is commercialized to induce obesity in rodents, was used. As previously reported11, during the experiments the rats fed HF diets reduced food consumption to maintain a constant caloric intake (around 50 kcal/day). Thus, after eating HF or NF diets for 30 days, no differences in body weight were observed between groups. This allowed to study the effect of the caloric content of the diet independent of obesity-mediated mechanisms (e.g. adipokines and other inflammatory mediators released by fat tissue). Although the reduction in food intake in rats fed HF diets resulted in a decreased P intake, in agreement with previous reports12 the intestinal absorption of P was increased in rats fed HF diets. The decrease in P intake was compensated by the increase in intestinal absorption of P in rats fed HF diets resulting in almost identical P load in HF vs NF groups.
Feeding HF diets was consistently associated to increases in plasma FGF23 concentrations in all experimental groups. Although FGF23 has been reported to be increased in obese people13 and a recent study identified energy intake as a potential predictor of plasma FGF23 concentrations14, to the best of our knowledge this is the first report demonstrating a direct relationship between ingestion of a high calories/high fat diet and increases in plasma concentrations of FGF23. Circulating levels of FGF23 are a prognostic factor for cardiovascular disease in patients with CKD15 and in the general population16. Thus, the demonstration of elevated FGF23 after feeding energy dense diets provides additional insight into the deleterious effect of these diets on cardiovascular health.
One of the most intriguing findings of the present work is that the increase in FGF23 elicited by HF diets was not accompanied by an increase in renal excretion of P. These data strongly suggest resistance to the phosphaturic action of FGF23. To promote phosphaturia in the kidney tubule, FGF23 needs to bind to the klotho-FGFR complex17. Therefore, the demonstration of reduced klotho protein levels in the kidneys of rats fed HF diet may explain the resistance to the phosphaturic action of FGF23 found in the present study. Renal klotho has been reported to be decreased in hyperlipidemic ApoE knockout mice and the authors suggested that hyperlipidemia-associated kidney injury might be responsible for the decreased renal expression of klotho18. While the renal toxicity of high fat diets is well documented19, additional mechanisms might be involved in the decrease of klotho induced by feeding HF diets. Klotho intervenes in energy regulation at different levels and klotho knockout mice display a lean phenotype20. Moreover, rats subjected to caloric restriction have been shown to increase renal klotho mRNA expression and klotho protein21. Thus, in addition to renal toxic effects, energy-dense diets may directly downregulate renal klotho. Klotho down-regulation by high caloric intake could have significant health consequences since, as shown in this study, it will lead to impaired renal excretion of P and P retention.
Feeding HF diets resulted in low plasma calcitriol concentrations and calcitriol was significantly lower in rats fed HF diets than in rats fed NF diets. The vitamin D content was identical in HF and NF diets. As explained above, the rats fed HF diets ingested less food and this might have resulted in decreased vitamin D intake; but, on the other hand, diets with high fat content have been reported to increase vitamin D absorption by the intestine22. In any case, plasma calcidiol concentrations were not decreased in rats fed HF diets. Since calcidiol is the vitamin D metabolite that best reflects nutritional intake23, the origin of the decreased plasma calcitriol in rats fed HF diets cannot be attributed to decreased dietary intake of vitamin D. The most likely explanation for the decreased calcitriol is a reduction in calcitriol synthesis secondary to the increase in FGF23, which is known to inhibit the 1-alpha-hydroxylase activity in the kidney24. The decrease in serum calcitriol after eating diets with high fat content may also have significant health implications because vitamin D deficiency is associated with cardiovascular disease, diabetes and metabolic syndrome25,26.
Extraskeletal calcification is a common feature in uremic patients and VC represents an important contributor to the high rate of cardiovascular mortality associated to CKD8. The inability to eliminate P plays a major role in the development of uremic VC10. Data from Experiments 1–3 indicated that HF diets resulted in P retention particularly when rats were fed HP and, to a lesser extent, with moderate (1/2 Nx) reduction in renal function. In Experiment 4 we evaluated the effect of feeding HP-HF diets to uremic (5/6 Nx) rats. In contrast to rats fed HP-NF diets, uremic rats fed HP-HF diets showed upregulation of osteogenic genes in vascular tissue and marked VC. In addition to P retention, feeding HF diets also resulted in moderate dislipidemia which may also have contributed to the development of VC. Our data demonstrate that although P retention associated to the ingestion of fast-food can be tolerated when renal function is normal, in individuals with a significant decrease in renal function ingestion of high-fat diets may lead to VC.
In conclusion, after being fed with high fat diets rats showed decreased renal klotho expression, increased circulating levels of FGF23 and decreased plasma concentrations of calcitriol. Reduced klotho resulted in resistance to the phosphaturic action of FGF23 and P retention. Moreover, uremic rats fed hypercaloric/high fat diets developed more severe extraosseous calcifications than their normocaloric-fed counterparts.
Methods
Ethics
All experimental protocols were reviewed and approved by the Ethics Committee for Animal Research of the University of Cordoba (Cordoba, Spain). All protocols were carried out in accordance with the approved guidelines. They followed the guidelines laid down by the Higher Council of Scientific Research of Spain following the normal procedures directing animal welfare (Real Decreto 223/88, BOE of 18 of March) and adhered to the recommendations included in the Guide for Care and Use of Laboratory Animals (US Department of Health and Human Services, NIH) and European laws and regulations on protection of animals, under the advice of specialized personnel.
Animals, surgical procedures and diets
Three months-old Wistar rats, provided by the Animal Housing Facilities of the University of Cordoba (Cordoba, Spain), were housed with a 12 h/12 h light/dark cycle. Appropriate measures were taken to ensure animal welfare and to address the basic behavioral and physiological needs of rats. Renal function was reduced by either 1/2 nephrectomy (Nx) or 5/6 Nx. The former was performed by unilateral Nx while the 5/6 Nx was accomplished in a two-step procedure that reduces the original renal mass by five-sixths leading to uremia. These procedures are described in detail elsewhere27. Briefly, animals were anesthetized using xylazine (5 mg/kg, ip) and ketamine (80 mg/kg, ip). For the first step of the 5/6 Nx, a 5- to 8-mm incision was made on the left mediolateral surface of the abdomen. The left kidney was exposed, and the two poles (2/3 of renal mass) were ablated. The kidney was inspected and returned to an anatomically neutral position within the peritoneal cavity. The abdominal wall and skin incisions were closed with sutures, and the rat was placed back into its home cage. After 1 week of recovery, in the second step, the animal was reanesthetized and a 5- to 8-mm incision was made on the right mediolateral surface of the abdomen. The right kidney was exposed and unencapsulated, the renal pedicle was clamped and ligated, and the kidney was removed. The ligated pedicle was returned to a neutral anatomical position and the abdomen and skin incisions closed with suture materials. The procedure for 1/2 Nx was identical to the second step of the 5/6 Nx. Phentanyl (0.2 mg/kg, ip) was used as analgesic agent.
Diets with two P concentrations were used in the experiments: normal P (0.6%) diet (NP) and high P (1.2%) diet (HP). Independent of their P content, diets had either a normal fat content (NF diet) with a 5% fat concentration that provided Metabolizable Energy = 3518 kcal/kg (Altromin C 1000, AltrominSpezialfutter GmbH, Germany) or a high fat content (HF diet) with a 35% fat concentration that provided Metabolizable Energy = 5241 kcal/kg (Altromin C 1090–60, AltrominSpezialfutter GmbH, Germany). All diets had 0.6% of Ca and 500 IU/g of vitamin D.
Experimental Design
Studies on P balance
These experiments were conducted with the rats housed in metabolic cages, allowing daily control of food and water intake and collection of urine and feces. During the first week the animals were adapted to the cages and received the control diet with 0.6% P and normal fat (NP-NF). Then, rats were switched to experimental diets in three Experiments:
Experiment 1- Rats with intact renal function were allotted to 2 groups (n = 6 each). Rats in group 1 were fed normal P and normal fat diet (NP-NF) and rats in group 2 were fed normal P and high fat diet (NP-HF) for 30 days. Water and food intake were recorded daily during the whole experiment. During the last week of the trial feces and urine were collected daily to assess P balance. At day 30, rats were sacrificed by exsanguination under general anesthesia (thiopental, 20 mg/kg, ip) to obtain blood and tissue samples.
Experiment 2- Rats with reduced (1/2 Nx) renal function (n = 12) were fed either normal P and normal fat (NP-NF, n = 6) or normal P and high fat (NP-HF, n = 6) diet for 30 days, following the same protocol as in Experiment 1.
Experiment 3- Rats with intact renal function were fed HP diet (1.2% P) and were allotted to 2 groups (n = 6 each). Rats in group 1 were fed normal fat diet (HP-NF) and rats in group 2 were fed high fat diet (HP-HF) for 30 days, following the same protocol as in Experiments 1 and 2.
Studies on vascular calcification (VC)
These studies (Experiment 4) were aimed at evaluating the effect of feeding HF diet on the development of VC in uremic rats. Sixteen rats were fed a NP diet with either normal fat content (NP-NF, n = 6) or high fat content (NP-HF, n = 10) for 45 days. At day 45 the first step of the 5/6 Nx was conducted and a week later the 5/6Nx was completed (day 52). After the second step of 5/6 Nx rats were switched to a HP (1.2%) diet. The fat content of the diet was maintained unchanged; thus rats received HP-NF (n = 6) or HP-HF (n = 10) diets. Rats were sacrificed at day 112 to obtain blood and tissue samples.
Blood chemistries
Blood for chemistry analyses was obtained at the time of sacrifice (intraperitoneal thiopental anesthesia plus exsanguination), from the abdominal aorta. Blood for measurements of ionized calcium levels was collected in heparinized syringes and immediately analyzed using a Ciba-Corning 634 ISE Ca2+/pH Analyzer (Ciba-Corning, Essex, England). Afterwards, plasma was separated by centrifugation and stored at −20 °C until assayed. Plasma creatinine, urea, tryglycerides, total cholesterol, HDL cholesterol, LDL cholesterol and phosphorus were measured by spectrophotometry (BioSystems SA, Barcelona, Spain). ELISA tests were used to quantify plasma FGF23 (Kainos Laboratories, Tokyo, Japan) and PTH (Immutopics, San Clemente, CA). Radioimmunoassay (Immunodiagnostic Systems Ltd, Boldon, UK) was used in plasma samples to determine 25-hydroxyvitamin D (calcidiol) and 1,25-dihydroxyvitamin D (calcitriol).
Urine and feces analysis
Fecal samples were dried, ashed and demineralized with 0.6 mmol/l HNO3 solution. Fecal P was measured by inductively coupled plasma mass spectrophotometry (ICP-MS, Perkin Elmer Elan DRC-e, Walthan, Massachusetts, USA). Urinary phosphorus was measured by spectrophotometry (BioSystems SA, Barcelona, Spain). Net intestinal absorption (equation (1)) and P balance (equation (2)) were calculated as follows:


Protein extraction and Western blot analysis
Proteins were isolated from renal tissue by using a lysis buffer containing HEPES (10 mmol/l), KCl (10 mmol/l), EDTA (0.1 mmol/l), EGTA (0.1 mmol/l), DTT (1 mmol/l), PMSF (0.5 mmol/l), protease inhibitor cocktail (70 μg/ml), and I-Gepal CA-630 (0.6%), pH 7.9 (Sigma Aldrich, St. Louis, MO). Protein concentration was determined by Bradford method. For Western Blot analysis, 50 μg of protein were electrophoresed on a 10% SDS-polyacrilamide gel (Invitrogen, Carlsbad, CA) and electrophoretically transferred (Transfer Systems, BioRad, Hercules, CA) from the gels onto nitrocellulose membranes (Invitrogen). The following steps were perfomed with gentle shaking. Membranes were incubated in TTBS-L solution [20 mMTris-HCl (pH 7.6), 0.2% Tween 20, 150 mMNaCl] (Sigma Aldrich), and 5% nonfat dry milk (Bio-Rad) at room temperature for 1 hour to avoid nonspecific binding. Membranes were then washed with TTBS buffer (the same composition as TTBS-L without nonfat dry milk) and incubated for 2 hours at room temperature with a mouse anti-FGFR1 antibody (GeneTex, Irvine, CA; 0.5 μg/ml) or rat anti-klotho antibody (Alpha Diagnostic Int, San Antonio, TX; 0.5 μg/ml). The membranes were then washed with TTBS buffer and inmunolabeled using a peroxidase-conjugated secondary antibody (1:5000 dilution; Santa Cruz Biotechnology Inc, Santa Cruz, CA). Finally, they were revealed on autoradiographic film using ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ). Beta-Actin was used as housekeeping protein to ensure equal loading of the gels. Protein levels were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Real-Time-Polymerase Chain Reaction (RT-PCR)
Study of bone FGF23 and aortic Runx-2, Osterix and Sclerostin mRNA was performed by Quantitative Real-Time PCR. Tibial bone or aorta were disrupted using liquid nitrogen and grinded thoroughly with a mortar. Aortic total RNA was extracted from the lysate using RNA extraction kit (RNeasy Fibrous Tissue Mini Kit, Qiagen, Germany). Bone RNA was extracted using the chloroform and isopropanol precipitation method and a treatment with DNAse I Amplification Grade (Sigma-Aldrich). Fifty ng of total RNA were used to analyze mRNA expression in the LightCycler thermal cycler system (Roche Diagnostics, Indianapolis, IN, USA). RT-PCR was performed in one step, using the QuantiTect SYBR Green RT-PCR kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s protocol. Primers for Runx-2, Osterix and GAPDH were designed with the free Oligo 7 software. The Sclerostin primer was purchased to Integrated DNA Technologies, Inc (San Diego, CA, USA). Primer sequences are listed below:
FGF23- F: 5′-TTGGATCGTATCACTTCAGC-3′,
R: 5′-TGCTTCGGTGACAGGTAG-3′;
Runx-2- F: 5′-CGG GAATGATGAGAACTACTC-3′,
R: 5′-GCG GTCAGAGAACAAACTAGGT-3′;
Osterix- F: 5′-GTACGGCAAGGCTTCGCATCTGA-3′,
R: 5′-TCAAGTGGTCGCTTCGGGTAAAG-3′;
GAPDH- F: 5′-AGGGCTGCCTTCTCTTGTGAC-3′,
R: 5′-TGGGTAGAATCATACTGGAACATGTAG-3′.
The expression of target genes was normalized to GAPDH as housekeeping and calculated according to the 2∆(∆CT) method.
Assessment of vascular calcification (VC)
Following sacrifice, the thoracic aorta, stomach and lungs were dissected and processed to study mineral content. Calcification was studied by measuring the tissue calcium and phosphorus content. The tissues were demineralized in 10% formic acid (aorta) and 150 mM HCl (stomach and lungs). The calcium and phosphorus content was measured in the supernatant according to a method previously described27. Fresh tissue was also fixed in 10% buffered formalin, embedded in paraffin, and cut into 3 μm sections. Paraffin-embedded sections of the aorta, lung and stomach were stained by the Von Kossa method to evaluate mineralization.
Statistics
Values are expressed as the mean ± standard error (SE). The difference between means for two different groups was determined by t-test; the difference between means for three or more groups was assessed by ANOVA. Fisher LSD test was used as a post-hoc procedure. A correlation study was carried out using the Pearson test. p < 0.05 was considered significant.
Additional Information
How to cite this article: Raya, A. I. et al. Energy-dense diets increase FGF23, lead to phosphorus retention and promote vascular calcifications in rats. Sci. Rep. 6, 36881; doi: 10.1038/srep36881 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wang, Y. C., McPherson, K., Marsh, T., Gortmaker, S. L. & Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 378, 815–825 (2011).
Popkin, B. M., Adair, L. S. & Ng, S. W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 70, 3–21 (2012).
Sarathy, S., Sullivan, C., Leon, J. B. & Sehgal, A. R. Fast food, phosphorus-containing additives, and the renal diet. J. Renal Nutr. 18, 466–470 (2008).
Uribarri, J. & Calvo, M. S. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin. Dial. 16, 186–188 (2003).
Frommelt, L. et al. Effects of low-carbohydrate, high-fat diets on apparent digestibility of minerals and trace elements in rats. Nutrition. 30, 869–875 (2014).
Panwar, B. et al. Fibroblast growth factor 23 and risk of incident stroke in community-living adults. Stroke. 46, 322–328 (2015).
Chen, J. et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann. Intern. Med. 140, 167–174 (2004).
Block, G. A. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 15, 2208–2218 (2004).
Villa-Bellosta, R., Millan, A. & Sorribas, V. Role of calcium-phosphate deposition in vascular smooth muscle cell calcification. Am. J. Physiol. Cell Physiol. 300, C210–C220 (2011).
Giachelli, C. M. The emerging role of phosphate in vascular calcification. Kidney Int. 75, 890–897 (2009).
Ainslie, D. A., Proietto, J., Fam, B. C. & Thorburn, A. W. Short-term, high-fat diets lower circulating leptin concentrations in rats. Am. J. Clin. Nutr. 71, 438–442 (2000).
Frommelt, L. et al. Effects of low-carbohydrate, high-fat diets on apparent digestibility of minerals and trace elements in rats. Nutrition. 30, 869–875 (2014).
Marsell, R. et al. Relation between fibroblast growth factor-23, body weight and bone mineral density in elderly men. Osteoporos. Int. 20, 1167–1173 (2009).
Di Giuseppe, R. et al. Potential predictors of plasma fibroblast growth factor 23 concentrations: cross-sectional analysis in the EPIC-Germany study. PLoS One. 10, e0133580 (2015).
Scialla, J. J. et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 25, 349–360 (2014).
Kestenbaum, B. et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ. Heart Fail. 7, 409–417 (2014).
Kuro-o, M. Overview of the FGF23-Klotho axis. Pediatr. Nephrol. 25, 583–590 (2010).
Sastre, C. et al. Hyperlipidemia-associated renal damage decreases Klotho expression in kidneys from ApoE knockout mice. PLoS One. 8, e83713 (2013).
Boini, K. M. et al. Activation of inflammasomes in podocyte injury of mice on the high fat diet: Effects of ASC gene deletion and silencing. Biochim. Biophys. Acta. 1843, 836–845 (2014).
Razzaque, M. S. The role of Klotho in energy metabolism. Nat. Rev. Endocrinol. 8, 579–587 (2012).
Miyazaki, T. et al. Klotho expression is induced by calorie restriction in adult male rats. Trace Nutrients Research. 27, 92–96 (2010).
Dawson-Hughes, B. et al. Dietary fat increases vitamin D-3 absorption. J. Acad. Nutr. Diet. 115, 225–230 (2015).
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
Dobnig, H. et al. Independent association of low serum 25- hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 168, 1340–1349 (2008).
Parker, J. et al. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 65, 225–236 (2010).
Lopez, I. et al. Calcimimetic R-568 decreases extraosseous calcifications in uremic rats treated with calcitriol. J. Am. Soc. Nephrol. 17, 795–804 (2006).
Acknowledgements
The work reported here was supported by a Spanish Government Grants from the Instituto de Salud Carlos III (PI14/00467-PI14/00638) with co-financing from European Funds and PI-0272-2014 from Fundacion Progreso y Salud, Junta de Andalucia.
Author information
Authors and Affiliations
Contributions
Study design: A.I.R., E.A.-T. and I.L. Study conduct: A.I.R., R.R., C.P. and E.D. Data collection: A.I.R., R.R., C.P., M.E.R.-O., E.D., J.R.M.-C. and Y.A. Data analysis: A.I.R., J.R.M.-C. and R.R. Data interpretation: A.I.R., R.R. and I.L. Drafting manuscript: E.A.-T. and I.L. Revising manuscript content: M.R., J.R.M.-C. and E.A.-T. E.A.-T. takes responsibility for the integrity of the data analysis. All authors reviewed and approved the final manuscript.
Ethics declarations
Competing interests
M.R. has received lecture fees from the following companies: Amgen, Abbott, Shire and Fresenius.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Raya, A., Rios, R., Pineda, C. et al. Energy-dense diets increase FGF23, lead to phosphorus retention and promote vascular calcifications in rats. Sci Rep 6, 36881 (2016). https://doi.org/10.1038/srep36881
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36881
This article is cited by
-
Direct regulation of fibroblast growth factor 23 by energy intake through mTOR
Scientific Reports (2020)
-
p38MAPK controls fibroblast growth factor 23 (FGF23) synthesis in UMR106-osteoblast-like cells and in IDG-SW3 osteocytes
Journal of Endocrinological Investigation (2019)
-
A high-fat diet stimulates fibroblast growth factor 23 formation in mice through TNFα upregulation
Nutrition & Diabetes (2018)
-
The production of fibroblast growth factor 23 is controlled by TGF-β2
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.