Abstract
Mast cell infiltration of tumour islets represents a survival advantage in non-small cell lung cancer (NSCLC). The phenotype and activation status of these mast cells is unknown. We investigated the mast cell phenotype in terms of protease content (tryptase-only [MCT], tryptase + chymase [MCTC]) and tumour necrosis factor-alpha (TNFα) expression, and extent of degranulation, in NSCLC tumour stroma and islets. Surgically resected tumours from 24 patients with extended survival (ES; mean survival 86.5 months) were compared with 25 patients with poor survival (PS; mean survival 8.0 months) by immunohistochemistry. Both MCT and MCTC in tumour islets were higher in ES (20.0 and 5.6 cells/mm2 respectively) compared to PS patients (0.0 cells/mm2) (p < 0.0001). Both phenotypes expressed TNFα in the islets and stroma. In ES 44% of MCT and 37% of MCTC expressed TNFα in the tumour islets. MCT in the ES stroma were more degranulated than in those with PS (median degranulation index = 2.24 versus 1.73 respectively) (p = 0.0022), and ES islet mast cells (2.24 compared to 1.71, p < 0.0001). Since both MCT and MCTC infiltrating tumour islets in ES NSCLC patients express TNFα, the cytotoxic activity of this cytokine may confer improved survival in these patients. Manipulating mast cell microlocalisation and functional responses in NSCLC may offer a novel approach to the treatment of this disease.
Similar content being viewed by others
Introduction
Lung cancer currently causes more deaths worldwide than any other malignancy and non-small cell lung cancer (NSCLC) accounts for the majority of these cases1. There is increasing evidence that the immune system plays a role in the regulation of cancer development2,3,4, and cells of the innate and adaptive immune responses have been implicated in both the progression and curtailment of tumour growth.
Mast cells are innate immune cells which arise in the bone marrow, circulate as progenitors, and differentiate following migration into tissue. They are found in all healthy tissues, where they contribute to tissue homeostasis and host defence, but are best known for their role in allergic diseases and asthma5. Their primary role is to respond rapidly to a tissue insult, initiating an appropriate program of tissue inflammation and repair. However, when exposed to a chronic insult, their ongoing activation may contribute to tissue damage, remodelling and fibrosis. Mast cells are an important component of immune cell infiltrates in tumours, but their role in tumour development and progression remains unclear6. In many situations they have been linked with tumour progression and metastasis7,8,9, and this is proposed to be mediated through their ability to promote angiogenesis via the release of autacoid mediators and pro-angiogenic chemokines and growth factors10,11. For example, the products of mast cells released during degranulation have been demonstrated in co-culture to enhance the migration of cervical cancer cells12. Increased histamine expression has also been shown to be associated with colorectal cancer and worsening tumour stage13, and heparin and bound cytokines/growth factors can promote neovascularisation14.
Taken together, these studies suggest that degranulating mast cells may be associated with tumour progression. However, Tataroglu has suggested that there is no correlation between intratumoural mast cells and angiogenesis in NSCLC15, and another study found no correlation between mast cells and survival in NSCLC16. However, the microlocalisation of mast cells within the tumour was not assessed. In contrast, we demonstrated that while mast cell numbers are similar in the tumour stroma of patients with surgically resected NSCLC irrespective of survival status, there is a marked survival advantage when mast cells are present within clusters of NSCLC tumour epithelial cells (islets)17.
Mast cells exhibit marked heterogeneity across species, within different organs within the same species, and even within the same organ5. Heterogeneity is evident with respect to ultrastructure, receptor expression, mediator content, immunological and non-immunological activation, and pharmacological responsiveness5. In humans, two common mast cell phenotypes are recognised based on their protease content: mast cells which contain tryptase only (MCT), and mast cells containing both tryptase and chymase (MCTC)18. MCTC predominate in the skin and connective tissue, and are also found in significant numbers in airway submucosal tissues18,19. MCT predominate in mucosal epithelia, and are also present in the lamina propria18,19. Their roles remain unclear, but their ability to release different proteases and cytokines18,19 suggests some roles which are mutually exclusive. Mast cell phenotype has been investigated in NSCLC before by Ibaraki20, who concluded that MCTC are associated with microvessel count, and thus, angiogenesis.
Tumour Necrosis Factor-alpha (TNFα) is an important cytokine produced by airway mast cells21. TNFα plays an important role in host defence and protects against cancer development as revealed by the increased incidence of cancer in patients receiving anti-TNFα therapy22,23. However, TNFα has been described by Szlosarek as having a paradoxical role in cancer, by inducing cell-mediated killing of certain tumours, as well as acting as a tumour promoter24. We have shown previously that increased expression of TNFα in the tumour islets of patients with NSCLC is independently associated with improved survival25. Tumour islet TNFα expression in extended survival patients was localised predominantly to macrophages of the M1 phenotype, and also mast cells identified by tryptase staining25,26. Whether the MCTC mast cell phenotype infiltrates the tumour islets, expresses TNFα, and confers a survival advantage has not been reported.
The microanatomical localisation of mast cells within NSCLC tissue appears critical to their role in disease progression. The primary aims of the present study were therefore to define the phenotype of mast cells within NSCLC stroma and islets in terms of their protease and TNFα content and their state of activation defined by the extent of degranulation.
Materials and Methods
Study Population
The study was approved by the Leicestershire Research Ethics Committee (approval reference number 6529). The methods in our study were carried out in accordance with our internal standardised protocols which are relevant guidelines to our study. The tissue specimens evaluated were from 49 patients with NSCLC who had undergone resection with curative intent at the University Hospitals of Leicester National Health Service Trust (Leicester, UK). These patients had resections during two periods: one dating from 1991–1994 and the second from January to December 1999. This cohort of patients has been described previously17. The patients from the 1991–1994 cohort had all died at the time of the study. 4 patients from the 1999 cohort were still alive and had provided written informed consent for the use of their tissue in research at the time of surgery.
Patients were selected for the study based on their survival, without knowledge of their previous tumour mast cell counts. 24 patients had extended survival (ES) (mean ± SEM 86.5 ± 8.8 months), and 25 patients had poor survival (PS) (8.0 ± 0.78 months). Patient characteristics are summarised in Table 1.
Immunohistology
The specimens studied were formalin-fixed and paraffin embedded. Only the advancing edge of the tumour was evaluated. Tissue sections of 4 μm thickness were cut onto glass slides and then de-waxed in xylene and rehydrated through graded alcohols. Antigen retrieval was carried out using Trilogy Antigen Retrieval solution (Cell Marque, Hot Springs, USA) in a pressure cooker (heated to 117.5 °C for 1 min and then cooled to 100 °C for 30 seconds). Antibodies for phenotypic analysis were all mouse antihuman mAb as follows: tryptase (clone AA1; Dakocytomation, Ely, Cambridgeshire, United Kingdom) as a specific marker for all mast cells, chymase (clone CCL1; Abcam, Cambridge, United Kingdom) as a specific marker for mast cells expressing chymase, and TNFα (clone P/T2; Abcam, Cambridge, United Kingdom). Immunostaining was performed using the Envision double-stain kit (Dakocytomation) according to the manufacturer’s instructions and as described previously17. Three slides were prepared for each patient: chymase versus tryptase, tryptase versus TNFα, and chymase versus TNFα. Peroxidase and 3,3′-diaminobenzidine tetrahydrochloride (brown reaction product), and alkaline phosphatase and fast red (red reaction product) were used to label cells expressing tryptase, chymase, and TNFα. Sections were then counterstained with haematoxylin and mounted in an aqueous mounting medium (BDH Chemicals Ltd, Poole, United Kingdom). Appropriate isotype controls were performed where the primary antibodies were replaced by irrelevant mouse mAb of the same isotype and at the same concentration as the specific primary mAb.
Analysis and Validation of Immunostaining
Analysis was performed blind with respect to the clinical outcome. The ten most representative high-power fields (x400) per slide were manually selected using an Olympus BX50 microscope (Olympus, Southall, United Kingdom). The respective areas of stroma and of tumour cell islets were then measured at x400 magnification using Scion image analysis software (Based on National Institutes of Health Image for Macintosh, modified for Windows [Scion Corp, Frederick, MD]). The number of nucleated cells with positive staining were then counted manually and expressed as cells/mm2 of stroma or tumour islets. Analysis was repeated for 10 patients to assess repeatability and validity. To identify mast cell phenotype, cells positive for chymase were counted as MCTC and all other mast cells were counted as MCT. To assess the number of MCT cells expressing TNFα, the number of MCTC cells expressing TNFα was subtracted from the total number of tryptase + cells (i.e. the total of MCT and MCTC) expressing TNFα.
A degranulation index score was established in order to assess the degree of degranulation by each individual mast cell as follows:
0 No degranulation
1 <33% degranulation
2 33–66% degranulation
3 >66% degranulation
Statistical Analysis
Statistical analyses were carried out using the GraphPad Prism software package (v. 6.02; GraphPad Prism Software Inc, San Diego, CA). For categoric analysis, the median value was used as a cut point to dichotomise the series. The χ2 test was used to test for relationships between categoric variables, and the Mann-Whitney nonparametric test was used to compare categoric with continuous variables. Spearman’s test was used to test correlation. Kaplan-Meier survival curves were used to look for correlations with survival and were compared with the use of the log-rank statistic. For the above comparisons, p < 0.05 was considered statistically significant.
Results
Patient Characteristics
Of the 49 patients studied, 45 had died at the time of analysis. 31 tumours were squamous, 7 adenocarcinoma, 6 large cell, and 5 other. 27 were stage I, 17 stage II, and 5 stage IIIa. 1 patient received additional chemotherapy and 4 received additional radiotherapy for later palliation (post surgery). The patient characteristics are summarised in Table 1.
Validation of Analysis
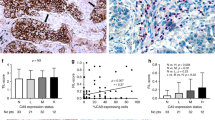
Clear and distinguishable staining was evident for tryptase, chymase and TNFα, and double-stained cells were readily identifiable (Fig. 1). Appropriate isotype controls were negative. Cells counts were repeated and the intraclass correlation coefficient was calculated as 0.797 (p < 0.01). This method of analysis has also been validated by our group previously17. The degranulation index was validated by two separate observers with an intraclass correlation of 0.668 (p < 0.05).
Examples of immunohistochemical double-staining for (A) chymase (brown) and tryptase (red) demonstrating the presence of MCT mast cells (red) and MCTC (reddish brown) (B) tryptase (brown) and TNFα (red) demonstrating the presence of TNFα in tryptase + mast cells, and (C) chymase (brown) and TNFα (red) demonstrating the expression of TNFα in MCTC mast cells. Arrowhead = double-stain cell. Black arrow = single-stain red cell. Grey arrow = single-stain brown cell.
Cellular Distribution
There were significantly more MCT in the tumour islets of the ES group (median, 20.0 cells/mm2 [IQR 5.7–27.9] compared to the PS group (median, 0.0 [IQR 0–1.2], p < 0.0001) (Fig. 2A). In contrast, in the stroma there was no significant difference between the MCT densities in the ES group (median, 36.0 cells/mm2 [IQR 17.9–57.06]) and the PS group (median, 34.1 cells/mm2 [IQR 4.6–80.1], p = 0.87) (Fig. 2B).
There were also significantly more MCTC in the tumour islets of the ES group (median, 5.6 cells/mm2 [IQR 2.6–16.6]) compared to the PS group (median, 0 [IQR 0–1.7], p = < 0.0001) (Fig. 2C). In contrast, in the stroma there was no significant difference between the MCTC densities in the ES group (median, 9.3 cells/mm2 [IQR 4.4–15.8]) and the PS group (median, 6.7 cells/mm2 [IQR 2.1–22.6], p = 0.61) (Fig. 2D). The percentage of mast cells which were MCT or MCTC in the tumour compartments are shown in Table 2.
The median density of MCT and MCTC expressing TNFα in the tumour islets of patients with ES was significantly greater (median 14.9 [IQR 5.6–33.76] and median 13.8 cells/mm2 [IQR 1.2–25.3] respectively) than in patients with PS (median 0.1 [IQR 0–7.9] and 0.0 cells/mm2 [IQR 0–1.2] respectively) (p = 0.0005 for MCT & p = 0.0018 for MCTC) (Fig. 3A and C). The median density of MCT and MCTC expressing TNFα in the stroma of patients with ES was 4.5 (IQR 0–28.5) and 11.7 (IQR 4.8–19.9) cells/mm2 respectively compared to 6.9 (range 0–23.1) and 4.4 (IQR 0–15.2) cells/mm2 respectively in with patients with PS (p = 0.932 for MCT and p = 0.053 for MCTC) (Fig. 3B and D). The median density of all cells expressing TNFα (mast cells and other cells) in tumour islets of patients with an above median survival was also noted to be significantly greater (71.1 cells/mm2) than in patients with a below median survival (12.7 cells/mm2) (p = 0.0035). The proportion of MCT and MCTC which expressed TNFα in the different tissue compartments are shown in Table 2.
Degranulation Index
In the stroma, MCT were degranulated to a greater degree in patients with ES than in those with PS (Fig. 4A, median [IQR] degranulation index 2.2 [2.0–2.5] compared to 1.83 [1.3–2.3], p = 0.021). There was no significant difference between ES and PS mast cell degranulation index for MCTC in stroma (Fig. 4B). MCT in the ES stroma were also more degranulated than MCT in the ES islets (degranulation index median 2.2 [IQR 2.0–2.5] compared to 1.7 [IQR 1.4–1.9], p < 0.0001) (Fig. 4C). There were insufficient cells for analysis of degranulation in the islets of PS patients.
Kaplan-Meier Survival Analysis
For further analysis, the data were divided into two groups above and below the median cell count values. Kaplan-Meier survival curves were plotted to investigate further the association of cell densities with survival. The log rank statistic was used to compare survival rates. In the tumour islets, patients with above median density of both MCT (Fig. 5A) and MCTC (Fig. 5B) had significantly greater predicted survival (p < 0.0001) but there was no correlation with stromal mast cell densities and survival (Fig. 5A and B). There was a positive association between survival and tumour islet density of mast cells (MCT and MCTC) expressing TNFα (p = 0.0004) (Fig. 6A and B). In contrast there were no survival differences evident using the same analysis on stromal cell counts (Fig. 6A and B).
Discussion
We have shown previously that mast cell infiltration of tumour islets in surgically resected NSCLC confers a marked survival advantage independently of tumour stage17. This study extends those findings by delineating the phenotype of mast cells within the tumour stroma and epithelial islets, and the extent of their degranulation. Of potential importance, both protease phenotypes are present within the tumour islets of patients with ES, they express TNFα, and the presence of both is associated with increased survival following surgical resection.
In healthy human airways mast cells are located predominantly in the lamina propria, and the density of mast cells there is similar to the density recorded here in the NSCLC tumour stroma21. Furthermore, the normal airway lamina propria ratio of MCT:MCTC is approximately 80:2019,27, which is again similar to that in the NSCLC stroma. This suggests that the homeostatic mechanisms regulating mast cell density and phenotype is similar in healthy bronchus and NSCLC stroma. Mast cells are rarely found in healthy airway epithelium, but those present have been reported to be almost exclusively of the MCT phenotype18. In steroid-naïve asthma, mast cells infiltrate the airway epithelium with an MCT:MCTC ratio of about 80:2019. This presumably occurs under the influence of epithelial-derived chemoattractants which are released in response to inhaled pro-inflammatory stimuli. It is interesting therefore that in NSCLC epithelial islets, mast cells are also present in some patients, that their presence correlates with ES, and that their protease distribution of 80:20 MCT:MCTC is similar to that seen in asthmatic airway epithelium. This suggests that in patients with ES, the tumour epithelium expresses a repertoire of chemoattractants, growth factors and cell-cell signals for the recruitment, differentiation and survival of both the MCT and MCTC phenotype, and that common mechanisms may exist for the recruitment of these cells in both asthma and NSCLC.
The correlation of improved survival with both MCT and MCTC mast cell phenotypes within the NSCLC tumour islets suggests that mast cells contribute to the anti-tumour immunological response, which also comprises infiltration by CD68+ macrophages17, natural killer and regulatory T cells28. There are many mast cell products which may contribute to the recruitment and activation of these other immune cells, but TNFα may be particularly important. This cytokine is stored pre-formed and secreted rapidly by mast cells in response to numerous diverse stimuli including IgE-dependent activation29,30, TLR-dependent activation31 and cell-cell contact with T cells32. It is therefore likely that the expression of TNFα by mast cells of both the MCT and MCTC phenotypes within the tumour islets has important biological consequences.
We have shown previously that the expression of TNFα in the tumour islets is also associated with improved survival25. These findings suggest that TNFα has a protective role when present in the NSCLC tumour islets, contributing to the limitation of tumour growth and dissemination. In mouse models mast cell-derived TNFα significantly increased T cell proliferation and cytokine production33 and IgE- and antigen-dependent mast cell enhancement of T cell activation required TNFα34. TNFα also induces cytotoxic activity in macrophages, and so mast cell-derived TNFα may play a pivotal role in the cytokine pathways influencing cytotoxic T cells and macrophages within the tumour islets, and thus have important consequences on cytotoxicity against tumour cells. This would be in keeping with other recently identified protective roles for mast cells in mammalian systems35.
We found no correlation between the density of MCTC and poor survival which conflicts with the results of Ibaraki and colleagues20 who reported a relationship between MCTC density and microvessel density in NSCLC. This difference may be due to the fact that in that study no assessment of the microlocalisation of mast cells within the tumour was made. In addition, we noted that patients with poor survival had low numbers of both phenotypes of mast cell within their tumour islets, in keeping with our previous findings17.
Our results also demonstrate that patients who have a better outcome have greater degranulation of their MCT subset in the NSCLC stroma. It is plausible that granular products such as heparin and proteases are able to disrupt the stroma after degranulation, thus inhibiting consequent tumour growth. Mast cell proteases are known to cause cell structural alterations and loss of the extracellular matrix integrity6. It is attractive to hypothesise that degranulation is the default and potentially protective mast cell anti-tumour response, but in poor prognosis patients there is inhibition of this with concomitant enhancement of detrimental factors such as pro-angiogenic cytokine production. In support of this, cyclooxygenase-2 and its product PGE2 are over-expressed in NSCLC36,37, and correlate with poor survival38. PGE2 is known to inhibit human lung mast cell degranulation39, but increase the synthesis and release of VEGF40,41. In addition, PGE2 is an inhibitor of human lung mast cell migration42, and so the increased production of PGE2 by poor prognosis tumours might also explain why these tumours fail to recruit mast cells to the tumour islets. Thus the expression of PGE2 in NSCLC and other tumours could be a key factor determining whether mast cells are protective or pro-tumorigenic, and explain the discrepancies in previous studies with regards to their role.
It is recognised that mast cells exhibit heterogeneity with regards to their cytokine content. In the lung, the MCT phenotype expresses predominantly IL-5 and IL-6, while the MCTC phenotype expresses IL-4 and IL-1319. This study demonstrates that in lung cancer at least, mast cells of both the MCT and MCTC phenotype express TNFα. The factors determining the expression of these cytokines in the MCT versus MCTC phenotype are not known and require further study.
In summary, we have shown that the density of the two mast cell phenotypes MCT and MCTC is increased in the tumour islets of patients with ES in NSCLC. The production of TNFα by both mast cell phenotypes within the tumour islets, and the degranulation of the MCT phenotype within the tumour stroma, may be particularly important for their interaction with other immune cells and the associated inhibition of tumour progression.
Manipulating mast cell microlocalisation and functional responses in NSCLC, for example through the inhibition of PGE2 production using cyclooxygenase-2 inhibitors, may offer a novel approach to the treatment of this disease.
Additional Information
How to cite this article: Shikotra, A. et al. Mast cell phenotype, TNFα expression and degranulation status in non-small cell lung cancer. Sci. Rep. 6, 38352; doi: 10.1038/srep38352 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J Cancer 136, E359–E386 (2015).
Galdiero, M. R., Varricchi, G. & Marone, G. The immune network in thyroid cancer. Oncoimmunology. 5, e1168556 (2016).
Orozco-Morales, M., Soca-Chafre, G., Barrios-Bernal, P., Hernandez-Pedro, N. & Arrieta, O. Interplay between Cellular and Molecular Inflammatory Mediators in Lung Cancer. Mediators. Inflamm. 2016, 3494608 (2016).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Bradding, P. & Arthur, G. Mast cells in asthma–state of the art. Clin Exp. Allergy 46, 194–263 (2016).
Marichal, T., Tsai, M. & Galli, S. J. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 1, 269–279 (2013).
Takanami, I., Takeuchi, K. & Naruke, M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer 88, 2686–2692 (2000).
Imada, A., Shijubo, N., Kojima, H. & Abe, S. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur. Respir. J. 15, 1087–1093 (2000).
Nagata, M. et al. Chymase-positive mast cells in small sized adenocarcinoma of the lung. Virchows Archiv 443, 565–573 (2003).
Galinsky, D. S. & Nechushtan, H. Mast cells and cancer–no longer just basic science. Crit Rev Oncol. Hematol. 68, 115–130 (2008).
Coussens, L. M. et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 13, 1382–1397 (1999).
Rudolph, M. I. et al. The influence of mast cell mediators on migration of SW756 cervical carcinoma cells. J Pharmacol Sci. 106, 208–218 (2008).
Cianchi, F. et al. The role of cyclooxygenase-2 in mediating the effects of histamine on cell proliferation and vascular endothelial growth factor production in colorectal cancer. Clin Cancer Res. 11, 6807–6815 (2005).
Bradding, P. & Holgate, S. T. Immunopathology and human mast cell cytokines. Crit. Rev. Oncol. Haematol. 31, 119–133 (1999).
Tataroglu, C., Kargi, A., Ozkal, S., Esrefoglu, N. & Akkoclu, A. Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC). Lung Cancer 43, 47–54 (2004).
Dundar, E. et al. The significance and relationship between mast cells and tumour angiogenesis in non-small cell lung carcinoma. J Int. Med Res. 36, 88–95 (2008).
Welsh, G. et al. Macrophage and mast cell invasion of tumour cell islets confers a marked survival advantage in non-small-cell lung cancer. J. Clin. Oncol 23, 8959–8967 (2005).
Irani, A. A. et al. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. USA 83, 4464–4468 (1986).
Bradding, P. et al. Heterogeneity of human mast cells based on cytokine content. J. Immunol. 155, 297–307 (1995).
Ibaraki, T. et al. The relationship of tryptase- and chymase-positive mast cells to angiogenesis in stage I non-small cell lung cancer. Eur. J Cardiothorac. Surg. 28, 617–621 (2005).
Bradding, P. et al. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol 10, 471–480 (1994).
Bongartz, T. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295, 2275–2285 (2006).
Stone, J. H. et al. Solid malignancies among patients in the Wegener’s Granulomatosis Etanercept Trial. Arthritis Rheum. 54, 1608–1618 (2006).
Szlosarek, P. W. & Balkwill, F. R. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 4, 565–573 (2003).
Ohri, C. M., Shikotra, A., Green, R. H., Waller, D. A. & Bradding, P. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer 10, 323 (2010).
Ohri, C. M., Shikotra, A., Green, R. H., Waller, D. A. & Bradding, P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33, 118–126 (2009).
Irani, A. M. & Schwartz, L. B. Mast cell heterogeneity. Clin. Exp. Allergy 19, 143–155 (1989).
Esendagli, G. et al. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer 59, 32–40 (2008).
Walsh, L. J. et al. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. USA 88, 4220–4224 (1991).
Ohkawara, Y. et al. Human lung mast cells and pulmonary macrophages produce tumor necrosis factor-alpha in sensitized lung tissue after IgE receptor triggering. Am. J. Respir. Cell Mol. Biol. 7, 385–392 (1992).
Varadaradjalou, S. et al. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol 33, 899–906 (2003).
Bhattacharyya, S. P. et al. Activated T lymphocytes induce degranulation and cytokine production by human mast cells following cell-to-cell contact. J Leukoc. Biol 63, 337–341 (1998).
Nakae, S. et al. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc. Natl. Acad. Sci. USA 102, 6467–6472 (2005).
Nakae, S. et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol 176, 2238–2248 (2006).
Voehringer, D. Protective and pathological roles of mast cells and basophils. Nat. Rev Immunol 13, 362–375 (2013).
Wolff, H. et al. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 58, 4997–5001 (1998).
Huang, M., Sharma, S., Mao, J. T. & Dubinett, S. M. Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol 157, 5512–5520 (1996).
Khuri, F. R. et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 7, 861–867 (2001).
Kay, L. J., Yeo, W. W. & Peachell, P. T. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol 147, 707–713 (2006).
Abdel-Majid, R. M. & Marshall, J. S. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol 172, 1227–1236 (2004).
Detoraki, A. et al. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol 123, 1142–9, 1149 (2009).
Duffy, S. M., Cruse, G., Cockerill, S. L., Brightling, C. E. & Bradding, P. Engagement of the EP2 prostanoid receptor closes the K+ channel KCa3.1 in human lung mast cells and attenuates their migration. Eur J Immunol 38, 2548–2556 (2008).
Acknowledgements
Funding for this study was provided by the James Maxwell Grant Prophit Fellowship awarded to Dr Chandra Ohri by the Royal College of Physicians, London, UK. The work was also supported by the National Institute for Health Research Leicester Respiratory Biomedical Research Unit. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Contributions
C.O. carried out the immunohistochemical staining, slide analysis, statistical analysis and prepared the manuscript. A.S. assisted with microtomy and carried out the immunohistochemical staining, slide analysis and statistical analysis. D.W. provided the samples for analysis and prepared the manuscript. P.B. conceived the study, participated in its design and coordination, and prepared the manuscript. All authors have read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shikotra, A., Ohri, C., Green, R. et al. Mast cell phenotype, TNFα expression and degranulation status in non-small cell lung cancer. Sci Rep 6, 38352 (2016). https://doi.org/10.1038/srep38352
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38352
This article is cited by
-
Mast cells inhibit colorectal cancer development by inducing ER stress through secreting Cystatin C
Oncogene (2023)
-
Lung cancer-derived extracellular vesicles: a possible mediator of mast cell activation in the tumor microenvironment
Cancer Immunology, Immunotherapy (2020)
-
The Role of Mast Cells in IgE-Independent Lung Diseases
Clinical Reviews in Allergy & Immunology (2020)
-
Role of Mast Cells in Shaping the Tumor Microenvironment
Clinical Reviews in Allergy & Immunology (2020)
-
High mast cell density indicates a longer overall survival in oral squamous cell carcinoma
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.