Abstract
Major depressive disorder (MDD) and schizophrenia (SZ) are considered two distinct psychiatric disorders. Yet, they have considerable overlap in symptomatology and clinical features, particularly in the initial phases of illness. The amygdala and prefrontal cortex (PFC) appear to have critical roles in these disorders; however, abnormalities appear to manifest differently. In our study forty-nine drug-naïve, first-episode MDD, 45 drug-naïve, first-episode SZ, and 50 healthy control (HC) participants from 13 to 30 years old underwent resting-state functional magnetic resonance imaging. Functional connectivity (FC) between the amygdala and PFC was compared among the three groups. Significant differences in FC were observed between the amygdala and ventral PFC (VPFC), dorsolateral PFC (DLPFC), and dorsal anterior cingulated cortex (dACC) among the three groups. Further analyses demonstrated that MDD showed decreased amygdala-VPFC FC and SZ had reductions in amygdala-dACC FC. Both the diagnostic groups had significantly decreased amygdala-DLPFC FC. These indicate abnormalities in amygdala-PFC FC and further support the importance of the interaction between the amygdala and PFC in adolescents and young adults with these disorders. Additionally, the alterations in amygdala-PFC FC may underlie the initial similarities observed between MDD and SZ and suggest potential markers of differentiation between the disorders at first onset.
Similar content being viewed by others
Introduction
Psychiatric diagnosis is challenging, particularly during the initial phases of illness. As a field, we still rely heavily on longitudinal observation for definitive diagnosis, often resulting in considerable delay in appropriate diagnosis and treatment. With converging evidence of significant neural abnormalities by the time of illness onset, early identification and intervention is increasingly critical in efforts to substantially alter the trajectory of psychiatric illnesses such as schizophrenia (SZ) and major depressive disorder (MDD). MDD and SZ are severely disabling psychiatric disorders that have long been conceptualized as two distinct illnesses. However, MDD and SZ have considerable overlap in behavioral manifestations and impairments in emotional processing and cognitive functioning1,2,3, particularly during their initial phases4,5,6,7,8. Frequently, prodromal SZ resembles a major depressive episode and psychotic symptoms are present in first episode MDD, complicating differentiation between the two disorders at initial onset5. In first episode psychosis, significant negative symptoms, which traditionally has been viewed as hallmarks of SZ, have been observed in both SZ and MDD, as well as depressive symptoms in first episode SZ4,5. These overlaps in presentation at initial onset challenge effective treatment interventions during the early course of these disorders and ultimately the potential for marked improvement in clinical outcomes. Treatment approaches toward MDD and SZ differ in targets, pharmacologic agents, use of somatic therapy, and duration of treatment, and hence accurate diagnosis is important in determining appropriate treatment for individuals with these disorders. Neural biomarkers could vastly improve differentiation between psychiatric disorders such as MDD and SZ, especially at first onset. However studies comparing first-episode MDD and SZ are very limited.
Recent reviews of the literature indicate similar and different expression of structural and functional brain abnormalities in MDD and SZ9,10,11,12,13,14. In particular, two key brain regions involved in emotional and cognitive processing, the amygdala and prefrontal cortex (PFC), have been strongly implicated in both MDD and SZ. [In this article, the PFC is defined as the dorsal lateral PFC (DLPFC), critically engaged in cognitive regulation of emotion15 and working memory task16; the dorsal medial PFC including the dorsal anterior cingulated cortex (dACC) and its anterior section of frontal cortices– closely linked with conflict monitoring and reward processing and cognitive-motor functions17,18,19; and the ventral PFC (VPFC) including the orbitofrontal cortex (OFC), the inferior and rostral frontal cortices, ventral and rostral components of the ACC, mainly associated with emotional20 and hedonic processing21]. Postmortem histological studies indicated volume/cell munbers, levels of neurotransmitter receptors and gene expression in both the amygdala and PFC in MDD and SZ22,23,24,25,26,27,28,29,30. Structural and functional magnetic resonance imaging (MRI) studies demonstrate amygdala abnormalities in MDD and SZ with respect to size, density and activation. Previous structural MRI studies provided support MDD enlarged amygdala volume31,32 or reduced amygdala volume33,34 and decreased amygdala gray matter (GM) concentration35 and SZ reported decreased amygdala volume and GM density36,37,38. Functional MRI MDD demonstrated increased activation in the amygdala39,40,41,42 and SZ altered functional activation in amygdala43,44. Most interestingly, in the PFC, MDD and SZ appear to have some differential involvement of PFC subregions based on other MRI studies. Morphological and functional alterations have been shown primarily in the DLPFC39,45,46,47 and VPFC46,48 in MDD, though dACC deficits have also been observed49, whereas the majority of the MRI studies report abnormalities in the DLPFC46,50,51 and dACC17 in SZ, as well as the dorsomedial PFC (DMPFC)52. Connectivity studies have found abnormalities in PFC-amygdala functional connectivity (FC) in both MDD and SZ39,53,54,55,56, including altered FC between the DLPFC and amygdala39,53 and the VPFC and amygdala54,55 in MDD, and abnormalities in DLPFC-amygdala FC in SZ56.
We are not aware of any study directly comparing the resting-state FC (rsFC) between the amygdala and PFC in drug-naïve, first-episode MDD and SZ. Studies of drug naïve, first episode populations minimize confounding factors of medication effects and illness chronicity in understanding the development of psychiatric disorders. In this study, we performed a seed-based analysis of rsFC between the amygdala and PFC in first-episode, drug-naïve MDD and SZ, and healthy control (HC) participants aged 13 to 30 years. We selected this specific age range due to the critical brain changes that occurs during this period in the development of MDD and SZ57,58,59. Consistent with previous MRI studies in MDD or SZ, we hypothesized that (1) amygdala-PFC rsFC would be altered in the MDD and SZ groups, compared to the HC group, (2) there would be similarities in amygdala-DLPFC rsFC abnormalities between the MDD and SZ group, (3) there would be differences in amygdala to other regions of PFC rsFC abnormalities between the MDD and SZ group, for example, amygdala-VPFC rsFC abnormalities in MDD and amygdala-dACC rsFC alterations in SZ.
Methods
Subjects
This study totally recruited 94 drug naïve, first episode MDD and SZ aged from 13 to 30 years. Participants included 49 drug-naïve, first episode MDD (mean age 19.35 ± 6.03 years, 32 females), 45 drug-naïve, first episode SZ (mean age 18.42 ± 3.84 years, 26 females), and 50 HC individuals (mean age 18.18 ± 3.92 years, 33 females). MDD and SZ participants were recruited from the outpatient clinics at the Department of Psychiatry, First Affiliated Hospital of China Medical University, Shengyang China. HC participants were recruited from Shengyang, China using community advertisement. The presence or absence of Axis I diagnoses were independently determined by two trained psychiatrists using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) in participants ≥18 years old and the Schedule for Affective Disorders and Schizophrenia for School-Age Children-present and Lifetime Version (K-SADS-PL) in participants younger than 18 years old. MDD and SZ participants respectively met the DSM-IV criteria for MDD or SZ without any other current or lifetime Axis I disorders, including substance abuse and dependence. Efforts were made to follow-up on subjects and confirm their diagnosis, particularly those in the early course of illness. Of the 45 subjects with SZ, 37 were within the first year of initial symptom onset and DSM-IV criteria for SZ. At one year follow-up, these subjects continued to meet DSM-IV criteria for SZ. Of the total 49 MDD subjects in this study, 44 remain as MDD at two year follow-up, and 5 remain as MDD at one year follow-up. HC participants did not have any first-degree relatives with Axis I disorders. For all three groups, individuals were excluded if any of the following were present: (1) any MRI contraindications; (2) history of head trauma with loss of consciousness 5 or more minutes or any neurological disorder; and (3) any concomitant major medical disorder. All participants were right handed and scanned within 24 hours of initial contact with the research team. All participants provided written informed consent after detailed description of the study. If participants were younger than 18 years old, participants gave written informed assent, and their parent/legal guardian provided written informed consent after receiving a detailed description of the study. The study was approved by the Institutional Review Board of China Medical University.
Symptom measures using the Hamilton Depression Rating Scale (HAMD), the Brief Psychiatric Rating Scale (BPRS) and Hamilton Anxiety Rating Scale (HAMA) and neuropsychological function using the Wisconsin Card Sorting Test (WCST) were obtained in all three groups.
MRI data acquisition
MRI data was acquired using a GE MR Signa HDX 3.0 T MRI scanner at the First Affiliated Hospital, China Medical University, Shenyang, China. Head motion was minimized with restraining foam pads. A standard head coil was used for radio frequency transmission and reception of the nuclear magnetic resonance signal. The participants were asked to keep their eyes closed but remain awake during the scan. FMRI images were acquired using a spin echo planar imaging (EPI) sequence, parallel to the anterior–posterior commissure (AC–PC) plane with the following scan parameters: repetition time (TR) = 2000 ms; echo time (TE) = 40 ms; image matrix = 64 × 64; field of view (FOV) = 24 × 24 cm2; 35 contiguous slices of 3 mm and without gap; scan time 6 min 40 s.
Data processing
The resting-state fMRI data preprocessing was carried out by using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8) and the Resting-State fMRI Data Analysis Toolkit (REST) (www.restfmri.net). The first 10 images were deleted, and then the data underwent further preprocessing, including slice timing correction, head motion correction, spatial normalization and smoothing. Head motion parameters were computed by estimating translation in each direction and the angular rotation about each axis for each volume. Datasets were excluded if head motion was >3 mm maximum displacement in any of the x, y or z directions or 3° of any angular motion throughout the course of the scan. To access head motion confounder, we compared the mean framewise displacement60 among three groups, the result showed no significant differences in head motion when comparing the three groups (p = 0.81). Spatial normalization was performed using a standard EPI template from the Montreal Neurological Institute (MNI). The voxel size was resampled to 3 × 3 × 3 mm3. Spatial smoothing was performed with an 8-mm full-width at half maximum (FWHM) Gaussian filter. Linear detrending and temporal bandpass (0.01–0.08 Hz) filtering were performed to remove low-frequency drifts and physiological high-frequency noise. Linear regression of head motion parameters, global mean signal, white matter signal and cerebrospinal fluid signal were performed to remove the effects of the nuisance covariates.
Functional connectivity analysis
Functional connectivity analysis was performed using correlation analysis between the seed amygdala ROI and PFC mask in a voxel-wise manner using REST. The correlation coefficients were then transformed to Z-values using the Fisher r-to-z transformation. The amygdala was selected as the region-of-interest (ROI). The bilateral amygdala ROI was defined according to the automated anatomical labeling (AAL) template61 contained in REST, which has been resampled to 3 × 3 × 3 mm3. The BOLD time series of the voxels within the ROI were averaged to generate the reference time series for the ROI. A PFC mask was created using the normalized T1-weighted high-resolution images of all participants, which were skull-stripped using BrainSuite2 (http://brainsuite.usc.edu). The PFC mask included Brodmann areas (BA) 9–12, 24, 25, 32, and 44–47. Only voxels within this mask were further analyzed.
Statistical analysis
Demographic and clinical characteristics were analyzed using IBM SPSS Statistics for Windows, Version 21.0 (Armonk, NY, USA). A one-way ANOVA was used to compare rsFC among the three groups. The contrast map threshold was set at p < 0.05 for each voxel with a cluster size of at least 40 voxels (1080 mm3), corrected for multiple comparison as determined by AlphaSim (see program AlphaSim by B.D. Ward in AFNI software. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). Z-values were extracted from the PFC regions showing significant differences among the three groups. Post hoc two-sample t-test of the Z-values between each pair group (HC vs. MDD, HC vs. SZ, MDD vs. SZ) were performed using SPSS. Statistical significance was determined by p < 0.05.
Results
Demographics, clinical characteristics and cognitive function
Demographics, clinical characteristics and cognitive function of participants are shown in Table 1. There were no significant differences in age or gender (p > 0.6), or education (p = 0.05) among the three groups. And there was also no significant difference in duration of illness between MDD and SZ groups (p = 0.88). Significant differences in HAMD, HAMA, and BPRS scores (p < 0.01) were observed among the three groups. Post-hoc analyses showed higher HAMD and HAMA scores in the MDD group compared to the SZ and HC group and in the SZ group compared to the HC group. BPRS scores were significantly higher in the SZ group when compared to the MDD and HC groups. Significant differences were also observed in WCST-correct responses, WCST-categories completed and WCST-total errors scores among the three groups (p < 0.05). Post-hoc analyses showed lower WCST-correct responses, WCST-categories completed and WCST-total errors scores in the SZ group compared to the MDD and HC group.
Amygdala-PFC Connectivity
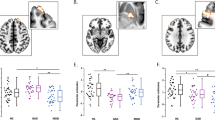
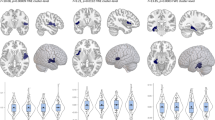
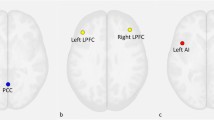
There were significant differences in amygdala- VPFC, amygdala-DLPFC and amygdala- dorsal ACC (dACC) rsFC in the three group comparison (p < 0.05, corrected) (Table 2; Fig. 1).
Post hoc pairwise comparisons found significantly decreased rsFC between the amygdala and VPFC in the MDD group, when compared with the HC and SZ groups (p < 0.05, corrected). No significant difference in amygdala-VPFC rsFC was observed between the HC and SZ groups. Amygdala-DLPFC rsFC was significantly decreased in the MDD and SZ groups, compared to the HC group (p < 0.05, corrected). Significantly decreased rsFC between the amygdala and dACC was found in the SZ group, compared to the HC group. No significant differences were shown in amygdala-dACC rsFC in the MDD group when compared to the HC and SZ groups (p < 0.05, corrected) (Fig. 2).
Clinical variables
Exploratory analyses did not reveal any significant correlations between rsFC in regions showing significant group differences and HAMD, BPRS, HAMA scores, and WCST in the MDD and SZ groups.
Discussion
As far as we are aware, this is the first study to investigate rsFC abnormalities between the amygdala and PFC in drug-naïve, first-episode adolescents and young adults with MDD and SZ. Our findings support similarities and differences in amygdala-PFC rsFC abnormalities between drug-naïve, first episode adolescents and young adults with MDD and SZ. Consistent with our previous hypotheses in this study, significant differences were found in rsFC between the amygdala and the VPFC, DLPFC, and dACC in the three group comparison (MDD, SZ, and HC). Both the MDD and SZ groups demonstrated significant decreases in amygdala-DLPFC rsFC when compared to the HC group. Differences between the MDD and SZ group were observed in amygdala-VPFC and amygdala-dACC rs FC. The MDD group had significantly decreased rsFC between the amygdala and VPFC, compared to the HC and SZ groups. Significant decrease in amygdala-dACC rsFC was found only in the SZ group when compared to the HC group. Results also suggest different effects of MDD and SZ on amygdala-dACC rsFC with the MDD group showing intermediate effects, although no significant difference in amygdala-dACC rsFC was observed between the MDD and SZ groups or the MDD and HC groups. The observed similarities and differences in amygdala-PFC rsFC may reflect neural mechanisms that underlie the clinical observations of symptom overlap during initial illness and divergence in longitudinal course in MDD and SZ. However, we did not observe any significant correlation between amygdala-PFC rsFC and symptom measures using the HAMD, BPRS, or HAMA.
Interestingly, our findings seem to reflect general conceptualization and differentiation of MDD and SZ: MDD is a disorder of primarily emotional processing and regulation whereas cognitive deficits predominate in SZ. Previous studies implicate the VPFC and amygdala as core components of the cortico-limbic circuitry and interactions in emotional processing20,62, especially in an affective disorder54,55,56,63. For example, our previous study by Liu et al. reported decreased rsFC between the amygdala and the VPFC in BD56, and our another resting-state fMRI study identified a negative correlation in VPFC-amygdala activity in BD63. In this study, decreased amygdala-VPFC rsFC in MDD was shown, consistent with our previous findings by Tang et al. and by Kong et al. of decreased functional connectivity amygdala-left VPFC and amygdala-left rostral PFC in treatment-naïve MDD54,55. We did not observe similar findings in the SZ group.
The dACC has been associated with various functions including conflict monitoring, reward processing and cognitive-motor functions and is also thought to be a critical component in the salience network in SZ64. These functions may be relevant to the development of psychotic symptoms such as delusions and hallucinations65. The ventral ACC is mainly activated within emotional process, whereas the dorsal ACC mostly engages in cognitive tasks66. For example, greater improvement in depressed bipolar adolescents was associated with baseline higher activity in ventral ACC to mild happy faces during emotion processing by fMRI67. The patients with schizophrenia had abnormalities in activation of the dorsal ACC in cognitive functioning by fMRI68. We found significantly altered amygdala-dACC rsFC in the SZ group when compared to the HC group; this was not shown in the MDD group. Our study results also suggest intermediate effects on amygdala-dACC rsFC in MDD, which would be consistent with the presence of more subtle cognitive impairments in MDD compared to SZ.
Decreased rsFC between the amygdala and DLPFC were found in both the MDD and SZ groups in this study, consistent with previous studies in MDD and SZ39,53,56. The DLPFC is thought to be involved in cognitive regulation of emotion, working memory, planning and cognitive flexibility69. It may be necessary to translate information about value into goal representations and to maintain such information so that it can be implemented as action plans to achieve the desired outcome70. The evidence indicated the involvement of the DLPFC in emotional processing and regulation and cognition abnormalities in the DLPFC in both MDD and SZ. For example, depressed subjects displayed decreased DLPFC activity in response to cognitive tasks39, and patients with SZ reduced engagement of the DLPFC within the working memory network71. In addition, the meta-analytic results showed lower response in the dorsolateral prefrontal cortex in individuals with major depressive disorder than in healthy subjects72 and the review supported physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia73. Although the mechanism of interaction between DLPFC and amygdala remains unknown, our findings confirmed that the interaction of DLPFC-amygala could be closely associated with the pathophysiology of MDD and SZ.
Exploratory analyses did not reveal any significant relationship between rsFC in regions showing significant group differences and symptom measures and WCST scores in MDD and SZ. The reasons for this are unclear. The measures used likely may not sufficiently capture the impairments in emotional processing and cognitive function related to the rsFC alterations observed herein as previously discussed. They may be too broad and overinclusive, and more targeted and refined approaches are needed to further examine the relationship between rsFC alterations and clinical symptoms and neuropsychological function. However, our findings implicate greater severity of impairments in SZ with given greater magnitude of rsFC alteration in SZ than MDD. This is consistent with the predicted clinical course and outcome for these disorders. Future studies should include more comprehensive and refined symptom and neuropsychological measurements to further understand the relationship between brain function and clinical and cognitive manifestations.
There are limitations in this study. Educational level was marginally different among the three groups (p = 0.05), and it may have contributed to our findings. Educational level has been previously shown to impact brain function74. Further, as the study included subjects early in the course of illness, the results herein should be interpreted with caution. Follow-up studies have found that 20 to 40% of adolescents with MDD develop BD within a period of 5 years after the onset of depression75. The longitudinal diagnostic stability for these subjects remains to be seen with ongoing efforts for follow-up and diagnostic confirmation.
In summary, the findings of this study support our a prior hypotheses that there are abnormalities in amygdala-PFC rsFC in drug-naïve, first episode adolescent and young adult with MDD and SZ and that there are similarities and differences in these abnormalities between the two disorders. Both MDD and SZ appear to have similar alterations in amygdala-DLPFC rsFC. Abnormalties amygdala-VPFC rsFC may be specific to MDD whereas those in amygdala-dACC rsFC may be more related to and severe in SZ. These findings may reflect the neural mechanisms underlying the initial similarities and then divergence in clinical course in adolescent and young adult with MDD and SZ. Moreover, they may indicate potential differentiating markers at first-onset that may improve early diagnosis, intervention and treatment in adolescent and young adult with MDD and SZ. And the clinicians could treat patients in the 2 disorders using physical therapy related brain regions.
Additional Information
How to cite this article: Wei, S. et al. Similarities and differences of functional connectivity in drug-naïve, first-episode adolescent and young adult with major depressive disorder and schizophrenia. Sci. Rep. 7, 44316; doi: 10.1038/srep44316 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Li, Y. et al. Vol. 14 24 (2015).
Olsen, E. K., Bjorkquist, O. A., Bodapati, A. S., Shankman, S. A. & Herbener, E. S. Associations between trait anhedonia and emotional memory deficits in females with schizophrenia versus major depression. Psychiatry research 230, 323–330 (2015).
Jaffe, F., Markov, D. & Doghramji, K. Sleep-disordered breathing: in depression and schizophrenia. Psychiatry (Edgmont) 3, 62–68 (2006).
Lyne, J. et al. Prevalence of item level negative symptoms in first episode psychosis diagnoses. Schizophrenia research 135, 128–133 (2012).
Rosen, C. et al. Phenomenology of first-episode psychosis in schizophrenia, bipolar disorder, and unipolar depression: a comparative analysis. Clinical schizophrenia & related psychoses 6, 145–151 (2012).
Aston, J. et al. First self-perceived signs and symptoms in emerging psychosis compared with depression. Early intervention in psychiatry 6, 455–459 (2012).
Romm, K. L. et al. Depression and depressive symptoms in first episode psychosis. The Journal of nervous and mental disease 198, 67–71 (2010).
Hafner, H. et al. Schizophrenia and depression: challenging the paradigm of two separate diseases–a controlled study of schizophrenia, depression and healthy controls. Schizophrenia research 77, 11–24 (2005).
Gong, Q. & He, Y. Depression, neuroimaging and connectomics: a selective overview. Biological psychiatry 77, 223–235 (2015).
Wheeler, A. L. & Voineskos, A. N. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Frontiers in human neuroscience 8, 653 (2014).
Cox, S. R. et al. A systematic review of brain frontal lobe parcellation techniques in magnetic resonance imaging. Brain structure & function 219, 1–22 (2014).
Kerestes, R., Davey, C. G., Stephanou, K., Whittle, S. & Harrison, B. J. Functional brain imaging studies of youth depression: a systematic review. NeuroImage 4, 209–231 (2014).
Lener, M. S. & Iosifescu, D. V. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Annals of the New York Academy of Sciences 1344, 50–65 (2015).
Zhou, Y., Fan, L., Qiu, C. & Jiang, T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neuroscience bulletin 31, 207–219 (2015).
Davidson, R. J., Pizzagalli, D., Nitschke, J. B. & Putnam, K. Depression: perspectives from affective neuroscience. Annual review of psychology 53, 545–574 (2002).
Barch, D. M., Sheline, Y. I., Csernansky, J. G. & Snyder, A. Z. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biological psychiatry 53, 376–384 (2003).
Gilleen, J., Shergill, S. S. & Kapur, S. Impaired subjective well-being in schizophrenia is associated with reduced anterior cingulate activity during reward processing. Psychological medicine 45, 589–600 (2015).
Kerns, J. G. et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. The American journal of psychiatry 162, 1833–1839 (2005).
Heckers, S. et al. Anterior cingulate cortex activation during cognitive interference in schizophrenia. The American journal of psychiatry 161, 707–715 (2004).
Frodl, T. et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biological psychiatry 67, 161–167 (2010).
Kringelbach, M. L. The human orbitofrontal cortex: linking reward to hedonic experience. Nature reviews 6, 691–702 (2005).
Rubinow, M. J. et al. Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain structure & function(2014).
Feyissa, A. M., Chandran, A., Stockmeier, C. A. & Karolewicz, B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Progress in neuro-psychopharmacology & biological psychiatry 33, 70–75 (2009).
Weidenhofer, J., Bowden, N. A., Scott, R. J. & Tooney, P. A. Altered gene expression in the amygdala in schizophrenia: up-regulation of genes located in the cytomatrix active zone. Molecular and cellular neurosciences 31, 243–250 (2006).
Sathyasaikumar, K. V. et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophrenia bulletin 37, 1147–1156 (2011).
Altshuler, L. L. et al. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar disorders 12, 541–549 (2010).
Rajkowska, G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological psychiatry 48, 766–777 (2000).
Benes, F. M. & Berretta, S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25, 1–27 (2001).
Benes, F. M. Searching for unique endophenotypes for schizophrenia and bipolar disorder within neural circuits and their molecular regulatory mechanisms. Schizophrenia bulletin 33, 932–936 (2007).
Mellios, N. et al. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biological psychiatry 65, 1006–1014 (2009).
van Eijndhoven, P. et al. Amygdala volume marks the acute state in the early course of depression. Biological psychiatry 65, 812–818 (2009).
Weniger, G., Lange, C. & Irle, E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. Journal of affective disorders 94, 219–229 (2006).
Kronenberg, G. et al. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. Journal of psychiatric research 43, 1112–1117 (2009).
Tang, Y. et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry research 156, 83–86 (2007).
Lee, H. Y. et al. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: an optimized voxel-based morphometry study. Journal of affective disorders 133, 128–136 (2011).
Bois, C. et al. Hippocampal, amygdala and nucleus accumbens volume in first-episode schizophrenia patients and individuals at high familial risk: A cross-sectional comparison. Schizophrenia research 165, 45–51 (2015).
Meisenzahl, E. M. et al. Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophrenia research 104, 44–60 (2008).
Exner, C., Boucsein, K., Degner, D., Irle, E. & Weniger, G. Impaired emotional learning and reduced amygdala size in schizophrenia: a 3-month follow-up. Schizophrenia research 71, 493–503 (2004).
Siegle, G. J., Thompson, W., Carter, C. S., Steinhauer, S. R. & Thase, M. E. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological psychiatry 61, 198–209 (2007).
Anand, A. et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological psychiatry 57, 1079–1088 (2005).
Arnone, D. et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. The American journal of psychiatry 169, 841–850 (2012).
Pulcu, E. et al. Increased amygdala response to shame in remitted major depressive disorder. PloS one 9, e86900 (2014).
Pankow, A. et al. Altered amygdala activation in schizophrenia patients during emotion processing. Schizophrenia research 150, 101–106 (2013).
Mier, D. et al. Evidence for altered amygdala activation in schizophrenia in an adaptive emotion recognition task. Psychiatry research 221, 195–203 (2014).
Amico, F. et al. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J Psychiatry Neurosci 36, 15–22 (2011).
Kinou, M. et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophrenia research 150, 459–467 (2013).
Shen, T. et al. Increased cognition connectivity network in major depression disorder: a FMRI study. Psychiatry investigation 12, 227–234 (2015).
Chen, F. et al. The effect of body-mind relaxation meditation induction on major depressive disorder: A resting-state fMRI study. Journal of affective disorders 183, 75–82 (2015).
Bertocci, M. A. et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychological medicine 42, 1417–1428 (2012).
Nenadic, I. et al. Brain structure in schizophrenia vs. psychotic bipolar I disorder: A VBM study. Schizophrenia research 165, 212–219 (2015).
Slifstein, M. et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA psychiatry 72, 316–324 (2015).
Raij, T. T., Korkeila, J., Joutsenniemi, K., Saarni, S. I. & Riekki, T. J. Association of stigma resistance with emotion regulation - functional magnetic resonance imaging and neuropsychological findings. Comprehensive psychiatry 55, 727–735 (2014).
Lu, Q. et al. Impaired prefrontal-amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neuroscience letters 523, 125–130 (2012).
Tang, Y. et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychological medicine 43, 1921–1927 (2013).
Kong, L. et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci 38, 417–422 (2013).
Liu, H. et al. Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophrenia bulletin 40, 469–477 (2014).
Jernigan, T. L., Trauner, D. A., Hesselink, J. R. & Tallal, P. A. Maturation of human cerebrum observed in vivo during adolescence. Brain 114(Pt 5), 2037–2049 (1991).
Giedd, J. N. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences 1021, 77–85 (2004).
Kochunov, P. et al. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biological psychiatry 73, 482–491 (2013).
Yan, C. G. et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 76, 183–201 (2013).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289 (2002).
Ghashghaei, H. T., Hilgetag, C. C. & Barbas, H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuro Image 34, 905–923 (2007).
Chepenik, L. G. et al. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry research 182, 207–210 (2010).
White, T. P., Joseph, V., Francis, S. T. & Liddle, P. F. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophrenia research 123, 105–115 (2010).
Cleghorn, J. M. et al. Regional brain metabolism during auditory hallucinations in chronic schizophrenia. Br J Psychiatry 157, 562–570 (1990).
Bush, G., Luu, P. & Posner, M. I. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences 4, 215–222 (2000).
Diler, R. S. et al. Neural correlates of treatment response in depressed bipolar adolescents during emotion processing. Brain imaging and behavior 7, 227–235 (2013).
Cordes, J. S. et al. Cognitive and neural strategies during control of the anterior cingulate cortex by fMRI neurofeedback in patients with schizophrenia. Frontiers in behavioral neuroscience 9, 169 (2015).
Sligte, I. G., Wokke, M. E., Tesselaar, J. P., Scholte, H. S. & Lamme, V. A. Magnetic stimulation of the dorsolateral prefrontal cortex dissociates fragile visual short-term memory from visual working memory. Neuropsychologia 49, 1578–1588 (2011).
Barch, D. M., Pagliaccio, D. & Luking, K. Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Current topics in behavioral neurosciences(2015).
Kyriakopoulos, M. et al. Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry 51, 911–920 e912 (2012).
Hamilton, J. P. et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. The American journal of psychiatry 169, 693–703 (2012).
Callicott, J. H. et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10, 1078–1092 (2000).
Ardila, A. & Moreno, S. Neuropsychological test performance in Aruaco Indians: an exploratory study. Journal of the International Neuropsychological Society: JINS 7, 510–515 (2001).
Birmaher, B. et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar disorders 11, 52–62 (2009).
Acknowledgements
The authors were supported by research grants from the National Natural Science Foundation of China (81571331, Wang, 81271499, Yanqing Tang and 81101012, Feng Wu), Liaoning Education Foundation (Pandeng Scholar, Fei Wang), the Liaoning Science and Technology Foundation (2008225010, Yanqing Tang and 2011225018, Fei Wang), the Liaoning Doctor Scientific Foundation (20111099, Feng Wu), National Institute of Health (K01MH086621, Fei Wang), the National Alliance for Research on Schizophrenia and Depression (Fei Wang) and the Klingenstein Foundation (Fei Wang). National Keyresearch and Development Program (2016YFC090430, Fei Wang), National High Tech Development Plan (863) (2015AA020513, Fei Wang), National Keyresearch and Development Program (2016YFC1306900, Yanqing Tang), and the Liaoning Education Foundation (L2015591, Shengnan Wei).
Author information
Authors and Affiliations
Contributions
F.W. and Y.T. designed the experiment. H.G. and M.C. acquired the data. S.W., H.G., M.C., Q.Z., Y.Z. and X.J. analyzed the data. S.W. and F.W. wrote the manuscript. All the authors discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wei, S., Womer, F., Geng, H. et al. Similarities and differences of functional connectivity in drug-naïve, first-episode adolescent and young adult with major depressive disorder and schizophrenia. Sci Rep 7, 44316 (2017). https://doi.org/10.1038/srep44316
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44316
This article is cited by
-
Cortical thickness distinguishes between major depression and schizophrenia in adolescents
BMC Psychiatry (2021)
-
Understanding complex functional wiring patterns in major depressive disorder through brain functional connectome
Translational Psychiatry (2021)
-
Altered amygdala-based functional connectivity in individuals with attenuated psychosis syndrome and first-episode schizophrenia
Scientific Reports (2020)
-
Resting-state functional hypoconnectivity of amygdala in clinical high risk state and first-episode schizophrenia
Brain Imaging and Behavior (2020)
-
Age-specific effects of structural and functional connectivity in prefrontal-amygdala circuitry in women with bipolar disorder
BMC Psychiatry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.