-
PDF
- Split View
-
Views
-
Cite
Cite
Paul K. S. Chan, Outbreak of Avian Influenza A(H5N1) Virus Infection in Hong Kong in 1997, Clinical Infectious Diseases, Volume 34, Issue Supplement_2, May 2002, Pages S58–S64, https://doi.org/10.1086/338820
Close - Share Icon Share
Abstract
The first outbreak of avian influenza A(H5N1) virus in humans occurred in Hong Kong in 1997. Infection was confirmed in 18 individuals, 6 of whom died. Infections were acquired by humans directly from chickens, without the involvement of an intermediate host. The outbreak was halted by a territory-wide slaughter of more than 1.5 million chickens at the end of December 1997. The clinical spectrum of H5N1 infection ranges from asymptomatic infection to fatal pneumonitis and multiple organ failure. Reactive hemophagocytic syndrome was the most characteristic pathologic finding and might have contributed to the lymphopenia, liver dysfunction, and abnormal clotting profiles that were observed among patients with severe infection. Rapid diagnosis with the use of reverse-transcription polymerase chain reaction and monoclonal antibody-based immunofluorescent assay were of great clinical value in the management of the outbreak. The experience of the H5N1 outbreak in Hong Kong underscores the importance of continuous surveillance of influenza virus strains in humans and in other animal species.
The propensity for drastic changes in the surface glycoproteins (hemagglutinin and neuraminidase) of influenza A viruses has resulted in several catastrophes in human history [1]. The most severe recorded pandemic was the emergence of a swinelike H1N1 subtype, which resulted in >20 million deaths in 1918–1919. H1N1 continued to circulate until 1957, at which time it was supplanted by the H2N2 subtype; in 1968, H2N2 was replaced by another new human strain, H3N2. However, when H1N1 reappeared in 1977, it cocirculated with H3N2 in the human population, rather than replacing the previously circulating epidemic strain. In 1997, influenza A virus subtype H5N1, which previously had been confined to avian species, was isolated for the first time from a human. The epidemic of influenza A(H5N1) virus in the Hong Kong Special Administrative Region of the Peoples' Republic of China (HKSAR) has received worldwide attention and may be the first example of successful containment of a potentially global pandemic of influenza.
Epidemiology
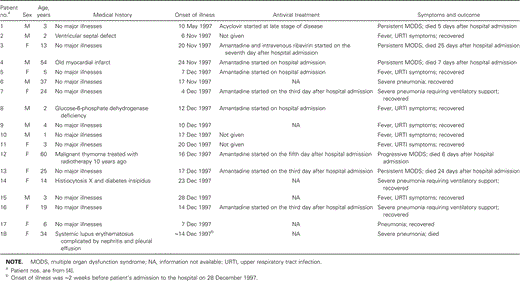
Human and poultry outbreaks. The first case of H5N1 infection identified in a human occurred in May 1997. No other cases were reported for 6 months; then, in November and December 1997, the second wave of the epidemic occurred, and an additional 17 cases were detected. These 18 cases were scattered in different residential districts without apparent geographic clustering. Of the 18 individuals with confirmed cases of influenza A(H5N1) virus infection, 8 were male, and 10 were female. Ages ranged from 1 to 60 years, and one-half of the individuals were <12 years of age. Eight cases occurred in children attending preschool, 1 in a primary-school student, 3 in high-school students, 2 in domestic helpers, 2 in housewives, 1 in an office worker, and 1 in a retiree. Six of these individuals died, and 12 made a full recovery (table 1) [2–7].
Characteristics of 18 patients who acquired influenza A(H5N1) virus infection during the 1997 epidemic in Hong Kong.
Epidemiologic and molecular evidence suggests that poultry was the source of the 1997 H5N1 outbreak in humans [8]. Investigations revealed that large-scale outbreaks of fatal avian influenza had occurred in chicken farms in the northwestern part of Hong Kong (Lau Fau Shan and Yuen Long) in March and April 1997, just before the first human case was discovered. Subsequently, similar outbreaks of fatal avian influenza were detected in October, November, and December, just before and at approximately the same time as the second wave of the epidemic in humans. The virus strains isolated from these avian outbreaks were confirmed to be influenza A virus subtype H5N1. Sequence comparisons of all 8 RNA segments from the human and avian isolates showed >99% sequence homology [8–11]. The sequencing data and the temporal relationship between avian and human outbreaks strongly suggest that a direct chicken-to-human cross-species transmission of the virus occurred, without the involvement of an intermediate host (e.g., swine) as a “mixing vessel” [11].
When the direct source of human influenza A(H5N1) virus infection was determined to have been poultry, and once it was known that almost 20% of the chickens in the markets were infected with this virus, the HKSAR government made a decision, on 28 December 1997, to slaughter all the poultry in farms and markets in Hong Kong. The operation started on 29 December 1997 and resulted in the removal of 1.5 million chickens from the territory before it ended on 31 December. At the same time, import of live poultry from mainland China, the only source of live poultry for Hong Kong, was temporarily suspended to allow a complete cleansing of the local environment. The depopulation of poultry has successfully stopped further spread of the epidemic.
Human-to-human transmission. Although chicken-to-human transmission had been identified as the initial source of human infection, whether H5N1 could be efficiently transmitted from human to human was a source of concern, because this would have indicated that another influenza pandemic was likely to emerge. A case-control study of 15 patients hospitalized for influenza A(H5N1) virus disease was conducted, using control subjects matched by age, sex, and neighborhood of residence [11]. Exposure to live poultry in the week before the onset of illness was found to be significantly associated with H5N1 disease, whereas traveling, eating or preparing poultry products, and recent exposure to persons with respiratory illness, including persons with known H5N1 infection, showed no significant association. A few cohort studies were conducted among individuals known to be infected. These studies demonstrated that 6 of 51 household contacts, 1 of 26 tour-group members, and 0 of 47 coworkers exposed to H5N1-infected persons tested positive for H5 antibody [13]. The results suggest that human-to-human transmission might have occurred through close physical contact with H5N1-infected patients, whereas social exposure to an infected individual was not associated with H5N1 infection. In another cohort study, which focused on health care workers recruited from 3 different hospitals at which H5N1-infected patients had been admitted [14], a significantly higher rate of seropositivity for H5N1 was found among exposed health care workers (8 [3.7%] of 217 persons) than among nonexposed health care workers (2 [0.7%] of 309 persons), which provides epidemiologic evidence of transmission from infected patients to health care workers. In summary, epidemiologic studies suggest that human-to-human transmission of H5N1 infection occurred but that the efficiency was low.
Origin of H5N1. Several molecular epidemiologic studies have been carried out to trace the source of the lethal H5N1 strain. The hemagglutinin gene of influenza A/goose/Guangdong/1/96(H5N1), which was isolated from a sick goose during an outbreak in Guangdong province, China, in 1996, was found to be similar to that of H5N1 viruses isolated in Hong Kong in 1997 [15]. However, the other genes were different, in particular the neuraminidase gene, which did not have the 19-amino acid deletion in the stalk region that was found in the Hong Kong H5N1 viruses [9]. Because farms in Guangdong are the major source of live poultry for Hong Kong, it is postulated that the hemagglutinin genes of the H5N1 viruses isolated during the Hong Kong outbreak might have derived from a virus similar to the A/goose/Guangdong/1/96 virus or shared a common progenitor with this goose pathogen, whereas the other 7 gene segments of the Hong Kong H5N1 viruses might have derived from ⩾1 other progenitors [9].
The possibility that genetic reassortment between H9N2 and H5N1 occurred has been raised, because these 2 subtypes cocirculated in poultry in Hong Kong during the 1997 epidemic. Sequence analysis showed that the internal genes PB1 and PB2 of a quail H9N2 virus (A/quail/Hong Kong/G1/97) were closely related to those of the H5N1 viruses [15]. These findings suggest that the H5N1 influenza viruses isolated in Hong Kong in 1997 were reassortants that might have obtained internal gene segments from A/quail/Hong Kong/G1/97 or vice versa; the available evidence supports the former.
Another possible gene donor is the teal. An H6N1 virus isolated from a teal in 1997 in Hong Kong (A/teal/Hong Kong/W312/97) showed >98% nucleotide homology with the human influenza A/Hong Kong/156/97(H5N1) in the 6 internal genes. In addition, the neuraminidase gene sequence showed 97% nucleotide homology with that of the human H5N1 virus; in particular, the neuraminidase protein of both viruses had the same 19-amino acid deletion in the stalk region [17]. Thus, the H6N1 virus isolated from the teal in Hong Kong in 1997 showed a high degree of homology with the Hong Kong H5N1 isolates in 7 of 8 gene segments. These data suggest that the H6N1 virus circulating in teals in Hong Kong may have been a donor of the neuraminidase gene, as well as all 6 internal genes, for the H5N1 virus.
In summary, the present evidence indicates that the H5N1 strains that caused the Hong Kong 1997 outbreak were reassortants from multiple cocirculating avian influenza virus strains [18–21]. This stresses the importance of continuous surveillance and characterization of influenza virus strains circulating in aquatic birds and poultry, as well as in humans [22].
Clinical Manifestations
The clinical manifestations associated with H5N1 infection in humans range from asymptomatic infection to mild upper respiratory illness, severe pneumonia, and multiple organ failure. The ratio of symptomatic cases to asymptomatic cases is not known, because it is not possible to precisely define the number of asymptomatic cases. The recorded case-fatality rate was alarmingly high, at 33.3% (6 of 18 cases), during the 1997 epidemic in Hong Kong. Early presentations included fever, headache, malaise, myalgia, sore throat, cough, and runny nose. Conjunctivitis and gastrointestinal symptoms were seen in some patients. These influenza-like illnesses are similar to those associated with the prevailing human influenza virus subtypes, H1N1 and H3N2. At the early stage of the illness, it is difficult to predict which patients will progress to severe disease. In the 1997 epidemic, 7 of 18 patients recovered after a period of mild upper respiratory illness. Of the 11 patients who developed pneumonia, 6 eventually died as a result of acute respiratory distress syndrome or multiple organ dysfunction syndrome [3, 3].
The clinical course of progression of severe H5N1 infection seems to be distinct from that of severe diseases observed during influenza pandemics that occurred before the 1997 epidemic [3]. None of the patients with severe cases had evidence of secondary bacterial pneumonia, which was the prevailing cause of death in influenza pandemics. Although Acinetobacter baumanii, Stenotrophomonas maltophilia, and Pseudomonas aeruginosa were isolated from a few patients after the patients had begun receiving ventilatory support, these bacterial pathogens could not be regarded as the primary cause of respiratory deterioration. In addition, cases of H5N1 infection were characterized by rapid clinical progression, with detection of signs of lower respiratory tract involvement on or soon after hospital admission (most patients were admitted within 1 week of the onset of illness) and progression to a disease stage at which the patient required ventilatory support within a few days after admission. One striking feature that was observed in all patients with severe cases, but not in patients with mild cases, was the early onset of lymphopenia. Pancytopenia occurred in 2 patients with severe infections. Elevated serum transaminase levels also were detected before respiratory deterioration in most of the patients with severe cases. Prolonged clotting times were also documented in some patients with severe cases soon after admission. Impaired renal function was a common finding in patients with severe infection, but it usually was seen at a later stage.
In summary, patients with severe H5N1 infection developed severe primary viral pneumonia, lymphopenia, impaired liver function, prolonged clotting times, and renal impairment, in some instances, at ∼1–2 weeks after the onset of symptoms. Experience from the documented cases indicates that chest radiography is a mandatory part of initial assessment, and measurement of the lymphocyte count is the most valuable parameter for identification of patients who are at the verge of progression to severe infection.
The determinant of the severity of human H5N1 infection remains a mystery. Mild cases of infection were found mainly in young children [3, 4], but this observation may well be biased by confounding factors. For example, mild cases of infection in young children are more likely to be reported than cases in older individuals, because the threshold for seeking medical attention for children is usually lower. In the 1957 and 1986 influenza A pandemics, most patients with primary influenza pneumonia had active cardiovascular, pulmonary, or renal diseases or alcoholism or were pregnant. In contrast, underlying medical illness seems not to be a necessary risk factor for severe H5N1 infection. Among the patients with fatal cases, only 1 patient, who had systemic lupus erythematosus complicated by nephritis, could be regarded as having had an active underlying disease. It has been shown that the hemagglutinin gene sequences of H5N1 contain multiple basic amino acids adjacent to the cleavage site, a motif associated with highly pathogenic avian influenza A viruses [9]. Thus, not only lack of previous exposure to the virus but the intrinsically high virulence of avian influenza A(H5N1) virus is a key determinant of the high fatality rate among infected humans. In fact, the fatality rate was also high during the outbreaks observed in chickens.
The efficacy of antiviral therapy for H5N1 disease cannot not be assessed, because experience is limited. Intravenous ribavirin was tried, in addition to amantadine, for 1 of the patients who eventually died. Amantadine was used for most of the patients with severe infections. However, the majority of those patients were already at a critical stage of the disease when the drug was administered, and amantadine is known to be most effective when it is given at an early stage of disease (within 2 days of onset).
Pathology
A full postmortem examination was carried out for 2 of the 6 patients with fatal cases of infection (a 13-year-old Chinese girl and a 25-year-old Filipino woman) in the 1997 epidemic [23]. Both patients had received amantadine treatment, and the 13-year-old girl had also received intravenous ribavirin. These 2 patients died of acute respiratory distress syndrome and multiple organ failure 1 month after the onset of illness. Both patients showed prominent features of reactive hemophagocytic syndrome. Histiocytes exhibiting active hemophagocytosis were found in bone marrow, lymph nodes from various sites, spleen, lungs, and in some liver sections from both patients. Hemophagocytic histiocytes were also found over the meninges of the 13-year-old girl. Other findings in these patients were also similar, including extensive central lobular necrosis, fatty changes, and extramedullary hematopoiesis in the liver and prominent acute tubular necrosis, congestion, and swelling in the kidneys.
Paramortem bone marrow aspirate and liver and renal biopsy samples were available from a 3-year-old boy, the first case patient to be identified, who died 10 days after the onset of illness without receiving any anti-influenza virus agent [7]. The bone marrow showed reactive changes, including active granulopoiesis with left shift, active erythropoiesis, and reactive histiocytes with occasional hemophagocytic activity. The liver biopsy sample showed microvesicular fatty changes and multiple Councilman bodies with scanty inflammatory cells. However, no viral inclusions were seen. The renal biopsy sample revealed vacuolation and vesicular changes of proximal tubules, but no abnormal inflammatory cell infiltration or viral inclusions were seen.
Postmortem lung and renal biopsy specimens were obtained from a 54-year-old man who died 11 days after the onset of illness. Amantadine was administered on admission to the hospital (6 days after the onset of illness). The lung sections revealed areas of hemorrhage, and fibrinous exudates were found in air spaces. The pneumocytes appeared to be reactive, but no viral inclusions were seen, and only sparse interstitial lymphocytic infiltration was found. The renal biopsy specimen showed extensive acute tubular necrosis. However, interstitial inflammatory cell infiltration was not observed.
Because multiple organ failure had early onset in patients with severe infection, attempts were made to detect H5N1 in multiple extrapulmonary tissues in which lytic viral infection might have contributed to organ dysfunction. H5-specific reverse-transcription PCR (RT-PCR) was performed on fresh postmortem tissue samples, and H5-specific immunostaining was performed on paraffin-embedded tissues. None of the extrapulmonary specimens examined, which included bone marrow, lymph nodes, and spleen, liver, and renal tissues, yielded results that were positive for H5N1. The only specimen that tested positive for the H5 antigen was the postmortem lung biopsy specimen taken from the 54-year-old patient.
Overall, the most prominent pathologic feature observed was a reactive hemophagocytic syndrome. Because it has been postulated that reactive hemophagocytic syndrome is a cytokine-driven condition triggered by various causes, levels of cytokines were measured retrospectively in the available serum samples. Elevation of soluble IL-2 receptor, IL-6, and IFN-γ levels was demonstrated in the serum samples obtained within the first 10 days after the onset of illness from both patients for whom a full postmortem examination was done.
In summary, the available clinicopathologic findings lead us to postulate that, in patients who have severe influenza A(H5N1) virus infection, the initial replication of viruses in the respiratory tract might trigger a stage of hypercytokinemia and result in reactive hemophagocytic syndrome; this accounted for at least some of the observed lymphopenia and various degrees of pancytopenia, impaired liver function, abnormal clotting profiles, and, eventually, multiple organ failure. Although a wide tissue tropism has been demonstrated in mouse models of H5N1 infection [24–27], evidence for extrapulmonary infection with H5N1 in humans is still lacking. However, one must realize that the human materials available for viral study were limited. Further study is needed to examine the tissue tropism of H5N1 in humans.
Laboratory Diagnosis
Influenza A(H5N1) virus can be cultured using cell lines (Madin-Darby canine kidney cells and LLCMK2 cells) that are routinely used for the detection of influenza viruses. In contrast to the usual human influenza virus strains, H1-H3, most H5N1 isolates produce an easily detectable cytopathic effect after 4–5 days of incubation. The cytopathic effect appears as diffuse patchy detachment of the cell monolayer. A preliminary identification of H5N1 from cell culture can be obtained by H5-specific RT-PCR or by immunostaining with use of H5-specific monoclonal antibodies. A few options exist for rapid detection of H5N1 viruses from clinical samples: (1) direct detection, by IFA with use of a pool of H5-specific monoclonal antibodies, from nasopharyngeal or endotracheal aspirates and bronchoalveolar lavage samples; (2) direct detection from throat swabs, nasopharyngeal or endotracheal aspirates, or bronchoalveolar lavage samples by H5-specific RT-PCR; and (3) centrifugation-enhanced 2-day shell vial culture from respiratory tract samples, followed by immunostaining with use of H5-specific monoclonal antibodies. During the second wave of the outbreak, a combination of these rapid tests was applied to diagnosis in hospitalized patients, and the results were promising. Our experience showed that RT-PCR and shell vial culture consistently provide a high degree of sensitivity and specificity, whereas the performance of direct detection with H5-specific IFA depends largely on the quality of the samples used. In general, the presence of at least 10 influenza virus-positive cells in clinical samples (as determined by the presence of influenza A virus-specific monoclonal antibodies) is required for the result from the H5-specific IFA to be reliable. Rapid diagnosis of H5N1 in hospitalized patients proved to be of high clinical value in the management of the 1997 outbreak. It created the opportunity for early administration of antiviral therapy, for establishment of appropriate isolation precautions, and for effective investigation of contacts. Because influenza-like illness is a common presentation for many diseases with causes other than influenza virus infection, rapid diagnosis allowed earlier relief of unnecessary anxiety related to the emergence of this new virus.
Ongoing Infection-Control Measures
It has been 4 years since the 1997 H5N1 outbreak in HKSAR. Since the alarming outbreak, the HKSAR government has put new policies in place that are aimed at prevention and early detection of reemergence of an epidemic of infection with H5N1 or a similar cross-species influenza virus [28]. Before poultry may be imported into Hong Kong, chickens must be segregated in designated farms in mainland China for 5 days and tested for H5 infection. Implementation of a policy to segregate chickens from waterfowl at all levels of the industry, from import to retail, has been recommended. Poultry surveillance for H5N1 in farms has been introduced, as have new licensing conditions covering hygiene and management practices. All flocks must be serologically tested during the growing period. With these surveillance procedures and policies, it is hoped that reassortment of influenza virus genes between avian species can be minimized and that cross-species transmission of new influenza virus strains from birds to humans can be prevented.
In March 1999, the surveillance system detected antibodies to H5N1 in a shipment of geese from Guangdong province. Although the birds already had been marketed when antibody results became available, H5N1 viruses were isolated from the cages that housed the birds. Genetic evaluation of these isolates (A/environment/Hong Kong/437/99) showed that the hemagglutinin gene sequences are similar to those in the H5N1 viruses that caused the 1997 epidemic in Hong Kong, and all 8 segments are closely related to A/goose/Guangdong/1/96 [29]. In February 2001, H5N1 virus was detected in surveillance fecal samples taken from a retail market in the Western District. In neither instance was the detection of H5N1 associated with poultry deaths. However, in May 2001, clusters of chicken deaths were found in retail markets throughout Hong Kong. H5N1 viruses were again isolated and shown to be genetically different from the 1997 H5N1 isolates. The HKSAR government decided to carry out a second territory-wide slaughter of poultry, and the import of live birds was temporarily suspended.
A tight monitoring system for human influenza virus infection is still being maintained. In March 1999, another avian influenza virus subtype, H9N2, was isolated from 2 young children with upper respiratory symptoms who made full recoveries [29]. However, up to the time of writing (January 2002), no case of H5N1 infection has been detected in a human since the first poultry slaughter, in 1997.
Although the H5N1 outbreak has been a disaster for human health and for the poultry industry in Hong Kong, it provided evidence that scientific investigations can play a major role in combating emerging infections. The success in containing the spread of H5N1 is due to joint efforts of the local and international public health authorities, research scientists, hospital administrators, and front-line health care workers.
References
- polymerase chain reaction
- reactive hemophagocytic syndrome
- blood coagulation
- chickens
- disease outbreaks
- avian influenza
- hong kong
- lymphopenia
- orthomyxoviridae
- pneumonia
- virus diseases
- infections
- antibodies
- viruses
- multiple organ dysfunction syndrome
- surveillance, medical
- coagulation process
- liver dysfunction
- rapid diagnosis
- host (organism)





Comments