-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan E. Kaplan, Debra L. Hanson, David L. Cohn, John Karon, Susan Buskin, Melanie Thompson, Patricia Fleming, Mark S. Dworkin, Adult and Adolescent Spectrum of HIV Disease Project Investigators, When to Begin Highly Active Antiretroviral Therapy? Evidence Supporting Initiation of Therapy at CD4+ Lymphocyte Counts <350 cells/µL, Clinical Infectious Diseases, Volume 37, Issue 7, 1 October 2003, Pages 951–958, https://doi.org/10.1086/377606
Close - Share Icon Share
Abstract
We assessed the risk of acquired immunodeficiency syndrome (AIDS)-related opportunistic illness or death among persons first prescribed highly active antiretroviral therapy (HAART) in January 1996 or later in the Centers for Disease Control and Prevention's Adult and Adolescent HIV Spectrum of Disease Project. Patients were included if they were naive to antiretroviral drugs and had no history of AIDS-related opportunistic illness. Risk was assessed as a function of CD4+ lymphocyte count and human immunodeficiency virus load at the time of initiation of HAART in a Cox proportional hazards model. Hazard ratios for AIDS or death were 6.3, 3.5, and 1.7 for persons with baseline CD4+ cell counts of 0–49, 50–199, and 200–349 cells/µL, respectively, compared with the referent (CD4+ cell count ⩾500 cells/µL). HAART should not be deferred until the CD4+ cell count reaches <200 cells/µL. The increased hazard associated with CD4+ cell counts of 200–349 cells/µL was modest but supports initiation of HAART at CD4+ cell counts <350 cells/µL, particularly in patients with high virus loads.
The use of HAART has resulted in remarkable decreases in HIV morbidity and mortality in the industrialized world since its introduction in 1996 [1–3]. Initial guidelines were aggressive in their approach to the use of HAART and suggested that all HIV-infected persons with CD4+ lymphocyte counts <500 cells/µL, as well as those with high HIV plasma RNA levels (virus load [VL]), be offered therapy [4, 5]. However, the observations of acute and chronic toxicities associated with HAART, some of which may be fatal, as well as difficulty in adhering to complex regimens and concerns about development of antiretroviral drug resistance, quality of life, and cost, have tempered these recommendations [6–8]. In addition, several studies have shown that later initiation of therapy may be associated with effective responses to HAART [9–12]. Accordingly, current guidelines suggest deferring therapy until later in the course of infection—that is, until the CD4+ lymphocyte count decreases to <350 cells/µL [13] or <200 cells/µL [14]. However, the optimal stage of HIV infection at which to begin therapy has not been defined.
The ideal approach to answering the “when to start” question would be a randomized clinical trial, but the feasibility of such a trial may be limited by the willingness of patients and providers to participate and the sample size and length of time required to conduct a meaningful study. Therefore, observational data have assumed greater importance as a means for addressing this question. One of the most pertinent issues is the response to therapy when it is initiated at various CD4+ cell counts: do patients respond satisfactorily, regardless of the CD4+ cell count at which HAART is started? We assessed progression to AIDS-related opportunistic illness (OI) or death as a function of immunological and virological status at initiation of HAART by using data from the Centers for Disease Control and Prevention's Adult and Adolescent Spectrum of HIV Disease (ASD) Project, a medical record review surveillance project involving data from 11 US cities.
Patients And Methods
The details of the ASD Project have been reported elsewhere [15, 16]. Briefly, since 1990, HIV-infected persons ⩾13 years of age have been identified and enrolled in the ASD Project at initiation of care at selected facilities in Atlanta, Dallas, Denver, Detroit, Los Angeles, New York, San Antonio (TX), Seattle, Houston, New Orleans, and Bayamon (Puerto Rico), regardless of stage of HIV infection. Patients' medical records for the year before enrollment are reviewed for information about demographic characteristics, mode of HIV exposure, history of AIDS-defining illness, and other clinical and laboratory findings, including CD4+ cell counts, VL measurements, and prescription of drugs. During 6-month follow-up intervals, the records are reviewed for information about AIDS-defining conditions, other illnesses, laboratory tests, and prescription of medications (the exact dates of drug prescriptions during each interval have been collected only since 2000). From 1990 through December 2002, >50,000 persons were enrolled in the ASD Project. Since 1996, >30,000 of persons enrolled in the ASD Project have received care at a participating facility.
To study the time between initiation of HAART and either diagnosis of an AIDS-related OI or death (all causes), we searched the ASD database for persons who had no history of antiretroviral therapy and who were first prescribed antiretroviral drugs during a 6-month follow-up period. We then restricted the data set to persons who initiated antiretroviral therapy in January 1996 or later and had no history of an AIDS-defining illness. Finally, we restricted the data set to patients who initiated antiretroviral therapy with a HAART regimen and who had a CD4+ lymphocyte count measured within the 12 months before initiation of HAART (or within the 12 months before the midpoint of the 6-month interval during which HAART was initiated, if the exact date of initiation of therapy was unavailable). HAART was defined as a regimen including 2 nucleoside reverse-transcriptase inhibitors and at least 1 protease inhibitor, a nonnucleoside reverse-transcriptase inhibitor, or abacavir [13].
For persons for whom the exact date of initiation of HAART was known, the CD4+ cell count and VL at which HAART was initiated were defined as the most recent measurements within the 12-month period before HAART was initiated. For persons for whom the exact date of initiation of therapy was unavailable, baseline CD4+ cell counts and VL were defined as the most recent measurements in the 6-month interval before that in which HAART was initiated. We measured follow-up time from the date on which HAART was initiated or from the midpoint of the 6-month interval during which HAART was initiated (if the exact date was unknown). We excluded persons for whom the exact date of initiation was unknown who also received a diagnosis of an AIDS-related OI during the 6-month interval in which HAART was initiated, to ensure exclusion of end points that might have occurred before initiation of therapy. To assess the results with inclusion of all events, we performed an analysis that included data from the subset of patients for whom the date of HAART initiation was known. For all subjects, we included follow-up time through December 2002, the cutoff date for this analysis.
The Kaplan-Meier method was used to describe the estimated AIDS or death survival probabilities after initiation of HAART, according to the CD4+ lymphocyte count and VL at which HAART was initiated [17]. In the univariate analysis, differences in probabilities of progression to AIDS or death (i.e., differences in survival curves) were assessed by the generalized Wilcoxon statistic [18].
Temporal risk for AIDS or death associated with baseline CD4+ cell count and VL was also estimated by a Cox proportional hazards model [19]. Baseline CD4+ cell count was categorized as ⩾500, 350–499, 200–349, 50–199, or <50 cells/µL. Baseline VL was categorized as high (⩾100,000 copies/mL) or low (<100,000 copies/mL); this VL cutoff was selected on the basis of its ability to discriminate risk of disease progression in recent studies [10, 12, 20]. The multiple regression model controlled for potential confounding risk factors (i.e., age, sex, race, HIV exposure group, calendar year, and enrollment at a private vs. nonprivate health care facility). Because we were interested in the influence of baseline immunological and virological status, we did not include time-dependent covariates in the regression model; we used the intent-to-treat principle and did not consider changes in antiretroviral therapy or changes in CD4+ cell count or VL during the follow-up period. Validation of the proportionality assumption was assessed for each model covariate by plotting the estimated hazard versus time.
Baseline VL data were missing for 518 (19%) of the 2729 patients in the analysis. A multiple imputation procedure [21] was used to replace each missing value with a set of plausible values that represent the uncertainty about the correct value to impute. Ten imputed data sets were then analyzed, and the results were combined. Estimates are the average from the 10 complete-data estimates. Variance is the average of the 10 complete-data within-imputation variance plus the between-imputation variance. Although results were generated with and without multiple imputations, the tables and figures present estimates computed without the imputations (i.e., by using the VL measurements that were available).
Results
A total of 4976 persons began receiving antiretroviral therapy during a 6-month interval in January 1996 or later and had no history of AIDS-related OI. Of these, 2729 were included in the analysis; 2247 were excluded because they began receiving non-HAART regimens (n = 1825), had no CD4+ cell count recorded within the 12 months before initiation of HAART (n = 392), or developed an OI in the 6-month interval during which HAART was initiated (for persons whose exact date of initiation of HAART was unavailable; n = 30) (table 1).
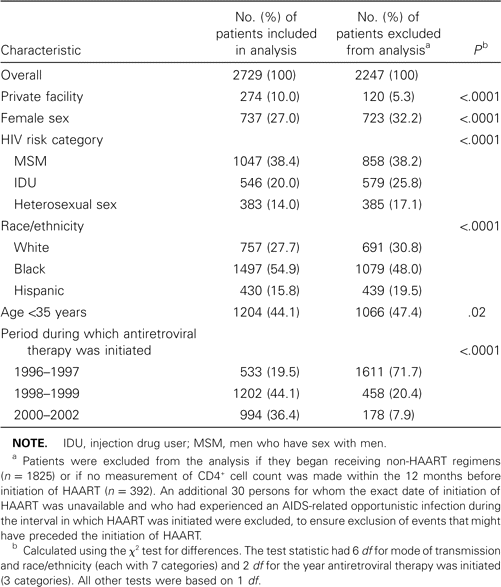
Characteristics of patients in the Adult and Adolescent Spectrum of HIV Disease Project who had no history of AIDS-related opportunistic illness and who began receiving antiretroviral therapy during 1996–2002.
Of the 2729 persons included in the analysis, 44% were <35 years old and 73% were men (table 1). Twenty-eight percent were white, 55% were black, and 16% were Hispanic. Thirty-eight percent were men who have sex with men, 20% were injection drug users (either men who have sex with men or heterosexual), and 14% were heterosexual non—injection drug users. Ten percent of patients were enrolled in private health care facilities. Patients excluded from the analysis were more likely to have started therapy during 1996 and 1997 than after 1997 and were less likely to have been enrolled in private facilities (table 1).
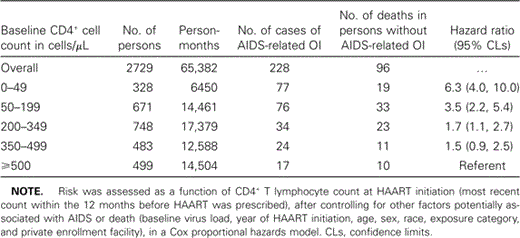
Of the 2729 persons in the analysis, the exact month in which HAART was initiated was known for 1467 (54%). Persons included in the study were followed up for a total of 65,382 person-months (median, 21 months). During this time, 324 persons progressed to AIDS or death, including 228 who had an AIDS-related OI and 96 who died without a history of such an illness (table 2).
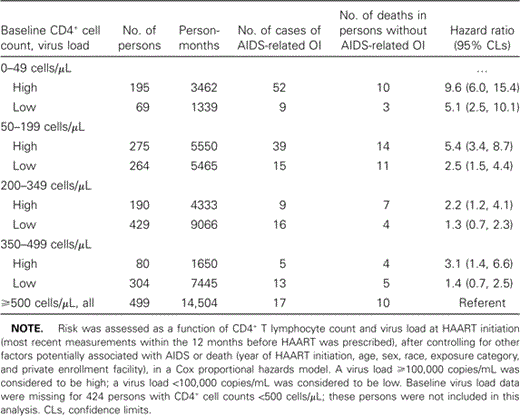
Outcomes and risk for AIDS-related opportunistic illness (OI) or death in persons who began receiving HAART with CD4+ counts at various levels.
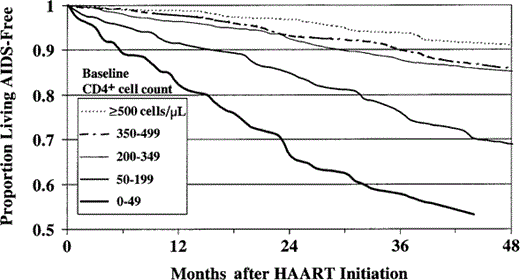
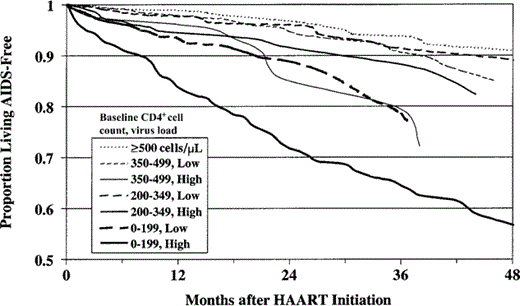
The estimated proportions of persons living without AIDS at times since initiation of HAART, by baseline CD4+ count, are shown in figure 1. The probabilities of progression to AIDS or death were highest among persons who had the lowest CD4+ cell counts (0–49 and 50–199 cells/µL) when HAART was initiated; these probabilities were significantly higher than those for persons who initiated therapy at CD4+ cell counts ⩾500 cells/µL (P < .0001). The probability of progression to AIDS or death was also higher among persons with baseline CD4+ cell counts of 200–349 cells/µL than among those with CD4+ cell counts ⩾500 cells/µL (P = .004). When subjects were stratified by both CD4+ cell count and VL, persons with CD4+ cell counts <200 cells/µL and either high or low VLs, persons with CD4+ cell counts of 200–349 cells/µL and high VLs, and those with CD4+ cell counts of 350–499 cells/µL and high VLs were at increased risk for progression to AIDS or death, compared with persons who initiated therapy at CD4+ cell counts ⩾500 cells/µL (those with high and with low VLs combined; P < .0001, P < .0001, P = .006, and P = .002, respectively; figure 2).
Proportion of persons in the Adult and Adolescent Spectrum of HIV Disease Project living without AIDS-related opportunistic illness after initiation of HAART, by CD4+ cell count at initiation of HAART. The probabilities of not progressing to AIDS or death among persons who began receiving HAART at CD4+ cell counts <50, 50–199, and 200–349 cells/µL were significantly lower than that among persons who began receiving HAART at CD4+ cell counts ⩾500 cells/µL, as tested by the generalized Wilcoxon statistic (P < .0001, P < .0001, and P = .004, respectively).
Proportion of persons in the Adult and Adolescent Spectrum of HIV Disease Project living without AIDS-related opportunistic illness after initiation of HAART, by CD4+ cell count and virus load (VL) at initiation of HAART. A VL ⩾100,000 copies/mL was considered to be high; a VL <100,000 copies/mL was considered to be low. The probability of not progressing to AIDS or death among persons who began receiving HAART at CD4+ cell counts <199 cells/µL (both high and low VL), at CD4+ cell counts of 200–349 cells/µL (high VL only), and at CD4+ cell counts of 350–499/µL (high VL only) were significantly lower than for those who began receiving HAART at CD4+ cell counts ⩾500 cells/µL (high and low VLs combined), as tested by the generalized Wilcoxon statistic (P < .0001, P < .0001, P = .006, and P = .002, respectively).
Results from the multiple regression models are shown in tables 2 and 3. Persons with the lowest CD4+ counts at initiation of HAART had the highest risk of progression to AIDS or death (hazard ratios [HRs] were 6.3 and 3.5 for persons with baseline CD4+ cell counts <50 and 50–199 cells/µL, respectively, compared with the referent CD4+ cell count of ⩾500 cells/µL; table 2). Risk was also significantly higher among those who began receiving HAART at CD4+ cell counts of 200–349 cells/µL (HR, 1.7; 95% confidence limits, 1.1, 2.7). Among persons with CD4+ cell counts of 200–349 cells/µL and those with CD4+ cell counts of 350–499 cells/µL, persons with high VLs were at greater risk of progression to AIDS or death than were those with CD4+ cell counts ⩾500 cells/µL (the referent group) (table 3).
Outcomes and risk for AIDS-related OI opportunistic illness (OI) or death in persons who began HAART with a variety of CD4+ cell counts and virus loads.
Because the exact date of initiation of HAART was unavailable for 1262 (46%) of study subjects, and because 30 of these persons were excluded from the analysis because they had experienced an AIDS-related OI in the interval during which HAART was initiated, we repeated the multiple regression analyses, limiting them to persons with known dates of initiation of HAART (n = 1467). In the analysis of risk as a function of baseline CD4+ cell count, the estimated HRs were similar (6.9, 3.8, and 1.5 for persons with baseline CD4+ cell counts of 0–49, 50–199, and 200–349 cells/µL, respectively), but the association was statistically significant only for those who began receiving HAART at CD4+ cell counts of 0–49 and 50–199 cells/µL (data not shown). In the analysis of risk as a function of baseline CD4+ cell count and VL, the point estimates of risk were 13.3, 5.8, 7.0, 2.6, 2.4, and 1.7 for persons with CD4+ cell counts of 0–49 cells/µL (high VL), 0–49 cells/µL (low VL), 50–199 cells/µL (high VL), 50–199 cells/µL (low VL), 200–349 cells/µL (high VL), and 350–499 cells/µL (high VL), respectively. Statistically significant risks were observed only for those with baseline CD4+ cell counts <200 cells/µL (both high and low VL; data not shown).
When the analyses were repeated with the inclusion of imputed VLs for persons for whom baseline VL data were missing, the estimated HRs and levels of statistical significance for each CD4+ cell count/VL group were similar, with 1 exception. The risk associated with initiation of HAART at a CD4+ cell count of 200–349 cells/µL and a low VL was statistically significant (HR, 1.7; 95% confidence limits, 1.1, 2.7; data not shown).
Discussion
This analysis offered an opportunity to address a component of the question of when to start HAART. We used data from a large observational database for an HIV-infected population that is diverse with regard to age, sex, race, HIV exposure group, geographic location, and type of health care setting. The study specifically addressed the hypothesis that patients will respond to HAART satisfactorily regardless of the CD4+ cell count at the time HAART is initiated. Our results disprove this hypothesis and indicate that the risk of progression to AIDS or death was highest among persons who had the lowest CD4+ counts when HAART was initiated. These data are consistent with, and therefore provide further support for, earlier observational cohort studies, which have shown that rates of disease progression and death are clearly increased among patients who begin receiving therapy when their CD4+ cell counts are <200 cells/µL [10–12, 20].
Our study also suggests that risk of progression may be higher, although to a lesser extent, among those who begin receiving HAART at CD4+ cell counts of 200–349 cells/µL, than among persons with baseline CD4+ cell counts ⩾500 cells/µL. These results were observed in both the Kaplan-Meier (univariate) and the Cox proportional hazards (multivariate) analyses and are consistent with the findings of another study, which suggested that risk is increased among patients who begin receiving therapy when their CD4+ cell counts are in this range [12]. Although these persons, as well as those with lower CD4+ cell counts, may respond well to HAART, our results suggest that, on average, persons who begin receiving therapy at CD4+ cell counts of 200–349 cells/µL are at greater risk.
In our analysis, a high baseline VL (⩾100,000 copies/mL) was associated with increased risk for progression to AIDS or death in all the CD4+ cell count strata analyzed, including CD4+ cell counts of 200–349 and 350–499 cells/µL, which suggests that VL may be useful in clinical decision making. In a study by Egger et al. [12] involving several European and North American cohorts, persons who began receiving HAART when their VLs were >100,000 copies/mL demonstrated higher rates of progression to AIDS or death or to death alone in virtually all CD4+ cell count strata that were analyzed. Recent studies have also demonstrated that persons who begin receiving therapy when their VLs are >100,000 copies/mL achieve viral suppression more slowly [9].
An important limitation of this study is the observational nature of the data; patients were not randomly assigned to groups with different schedules for initiation of HAART. There may be biases related to the stage of disease at which HAART was initiated that affect patient outcome. For example, patients who had the lowest CD4+ cell counts when they began receiving therapy may be less likely to engage in health-promoting behaviors, including adherence to HAART regimens. Wood et al. [22] recently reported that, among persons who began receiving HAART at CD4+ cell counts <200 cells/µL, outcome was dramatically improved once the analysis was adjusted for adherence. Peterson et al. [23] reported that when adherence was taken into consideration, rates of disease progression in persons who began receiving therapy at CD4+ cell counts of 200–350 cells/µL were no different from those among persons with baseline CD4+ cell counts >350 cells/µL at initiation of HAART. Hence, adherence may be an especially important determinant of outcome in the observational studies done to date [10–12, 20, 24, 25]. We included a variable for private versus nonprivate health care facility in the regression model, as a possible surrogate for health-seeking behavior; patients from private sites had a better prognosis, but the estimated risks associated with baseline CD4+ cell counts or VLs were independent of this result. An additional limitation is posed by the relatively brief period of follow-up (median, 21 months) in our study; it is possible that additional follow-up time would allow better discrimination between the risks associated with initiating HAART at various CD4+ cell count strata.
Although patients in our study began receiving HAART during a defined 6-month interval, the exact date of initiation of therapy was unavailable for nearly one-half (46%) of the subjects. Accordingly, we excluded 30 persons who developed an AIDS-related OI in the 6-month interval during which HAART was initiated, to ensure exclusion of end points that might have occurred before initiation of therapy. Therefore, we repeated our analyses, limiting them to the 1467 persons with known dates of initiation of therapy. It is noteworthy that the point estimates of risk in the multiple regression analyses were similar to those observed in the primary analyses. This supports the robustness of our findings.
The implications of our findings with regard to the guidelines for use of HAART should be approached cautiously. It is important to point out that these observations inform but do not directly address the question of when to initiate HAART. Rather, they address the very specific question of whether clinical progression differs according to the CD4+ cell count at which HAART is initiated. To better assess the question of when to initiate HAART, outcomes should be compared among persons at the same stage of HIV disease but who began receiving HAART at different times (i.e., immediate vs. deferred therapy). It is very possible, for example, that the lead time required for CD4+ cell counts to decrease from 500 to 350 cells/µL would compensate for a slightly decreased survival rate among persons who began receiving HAART at CD4+ cell counts of 200–349 cells/µL [26]. Recently, several investigators approached the “when to start” issue in this way [27–30]; however, the results were mixed, in part, perhaps, because of biases inherent in comparing persons who begin therapy with those who defer initiation of therapy in observational studies.
Despite these limitations, it appears clear that the patients who have the lowest CD4+ cell counts when they begin HAART are at the highest risk of progression to AIDS or death and do not experience the same clinical benefit as do patients who begin receiving therapy earlier. Thus, therapy should not be deferred until the CD4+ cell count decreases to <200 cells/µL. The increased risk seen among patients who begin receiving HAART at CD4+ cell counts of 200–349 cells/µL was modest, but the increase suggests that initiation of therapy at CD4+ cell counts of <350 cells/µL is prudent, particularly for persons with high VLs. This recommendation is in line with current guidelines, which reflect the results from similar observational studies [13]. This recommendation also allows initiation of therapy before the CD4+ cell count decreases to <200 cells/µL, at which point patients are at more-immediate risk for AIDS-related OIs, in addition to risk for suboptimal response to HAART.
A decision to initiate therapy when the patient's CD4+ cell count is 200–349 cells/µL should clearly be discussed with the patient, and the decision-maker should take into account current uncertainties related to disease progression, development of drug toxicities, emergence of antiretroviral drug resistance, and impact on quality of life. Additional research, including studies with longer periods of follow-up that compare patients who begin receiving therapy at CD4+ cell counts of 200–349 cells/µL with patients in other CD4+ cell count strata, is necessary to clarify current uncertainties.
Study Group Members
The Adult and Adolescent Spectrum of HIV Disease Investigators are as follows: M. Thompson and J. Gable (AIDS Research Consortium of Atlanta, Atlanta, GA); S. Odem and S. Melville (Texas Department of Health, Austin, TX); A. Davidson, D. L. Cohn, and C. Rietmeijer (Denver Department of Health and Hospitals, Denver, CO); L. L. Wotring and E. D. Mokotoff (Michigan Department of Community Health, Detroit); W. McNeely and K. Reynolds (Houston Department of Health and Human Services, Houston, TX); P. Keiser (University of Texas Southwestern, Dallas); J. Turner and D. Masters (Los Angeles County Department of Health Services, Los Angeles, CA); A. Morse and S. Broyles (Louisiana Office of Public Health, New Orleans); J. Sackoff (City of New York Department of Health, New York, NY); J. Otero, R. Hunter, and M. de los Angeles Gomez (University Central del Caribe, Bayamon, Puerto Rico); S. Miranda (Puerto Rico Department of Health, San Juan); and S. Buskin, S. G. Hopkins, and B. Sohlberg (Public Health—Seattle and King County, Seattle, WA).
Acknowledgments
We thank the Adult and Adolescent Spectrum of HIV Disease (ASD) Investigators; Pei-Chun Wan and Michael Adams, for data management in the ASD Project; and J. Curlew, R. Moseley, K. Distel, and C. Willis, for secretarial and graphics assistance.
References
Author notes
Present affiliations: Albuquerque, New Mexico (J.K.); New Jersey Department of Health and Senior Services, Trenton (P.F.); Illinois Department of Public Health, Chicago (M.S.D.).
Study group members are listed at the end of the text.





Comments