-
PDF
- Split View
-
Views
-
Cite
Cite
Michael J. Rybak, The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin, Clinical Infectious Diseases, Volume 42, Issue Supplement_1, January 2006, Pages S35–S39, https://doi.org/10.1086/491712
Close - Share Icon Share
Abstract
Vancomycin is one of only a few antibiotics available to treat patients infected with methicillin-resistant Staphylococcus aureus and methicillin-resistant, coagulase-negative Staphylococcus species. Therefore, understanding the clinical implications of the pharmacokinetic and pharmacodynamic properties of vancomycin is a necessity for clinicians. Vancomycin is a concentration-independent antibiotic (also referred to as a “time-dependent” antibiotic), and there are factors that affect its clinical activity, including variable tissue distribution, inoculum size, and emerging resistance. This article reviews the pharmacokinetic and pharmacodynamic data related to vancomycin and discusses such clinical issues as toxicities and serum concentration monitoring.
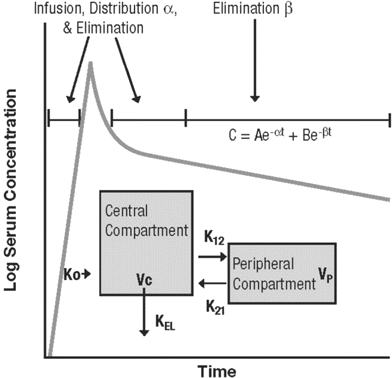
Vancomycin is a large glycopeptide compound with a molecular weight of ∼1450 Da [1]. It is not appreciably absorbed orally and is eliminated primarily via the renal route, with >80%–90% recovered unchanged in urine within 24 h after administration of a single dose [2]. The pharmacokinetic profile of vancomycin is complex and can be characterized by either a 2- or 3-compartment pharmacokinetic profile (figure 1) [3,4,5]. The drug is administered intravenously, with a standard infusion time of at least 1 h, to minimize infusion-related adverse effects. In patients with normal creatinine clearance, vancomycin has an α-distribution phase of ∼30 min to 1 h and a β-elimination half-life of 6–12 h. The volume of distribution is 0.4–1 L/kg [2, 4–7]. The binding of vancomycin to protein has been reported in the literature to range from 10% to 50% [8–11]. Factors that affect the overall activity of vancomycin include its tissue distribution, inoculum size, and protein-binding effects.
Schematic representation of a 2-compartment pharmacokinetic model, wherein C is the concentration, α and β are the respective elimination constants, e is the base of the natural logarithm, t is time, A and B are the respective zero time intercepts for α and β, Ko is the infusion rate constant, Vc is the volume of the central compartment, Vp is the volume of the peripheral compartment, K12 and K21 are intracompartmental rate constants, and KEL is the elimination rate constant from the central compartment.
Tissue Distribution
Vancomycin penetrates into most body spaces, although the concentrations obtained are variable and somewhat dependent on the degree of inflammation present. In studies examining the penetration of vancomycin into the CSF of patients with uninflamed meninges, fairly low concentrations have been demonstrated (range, 0–3.45 mg/L), with corresponding CSF-to-serum ratios of 0–0.18 [1, 12]. As expected, inflamed meninges improve penetration of vancomycin into the CNS, with reported concentrations of 6.4–11.1 mg/L and CSF-to-serum ratios of 0.36–0.48 [1, 12].
The penetration of vancomycin into the lung is highly variable. Cruciani et al. [13] investigated the penetration of vancomycin into the lung tissue of 36 patients undergoing a partial lobectomy. After intravenous administration of 1 g of vancomycin, concentrations ranged from 0 to 12.2 mg/L, with a mean concentration of 2.8 mg/L and a penetration of 41% [13]. In a recent study investigating the penetration of vancomycin into the epithelial lining fluid of healthy volunteers given 1 g of vancomycin every 12 h, the mean concentration at 12 h was 2.4 mg/L, which represented a 52% overall penetration rate [14]. However, in critically injured patients, penetration of vancomycin into epithelial lining fluid was more variable, ranging from 0 to 8.1 mg/L after several hours, with an overall blood-to—epithelial lining fluid penetration ratio of 6:1 [15]. Craig and Andes [16] recently compared the efficacy of vancomycin with that of oritavancin (a glycopeptide derivative similar to vancomycin) in a thigh and lung mouse infection model. Although the activity of oritavancin was comparable in both lung and thigh infection models, vancomycin activity was found to be 2–3-fold less potent in the lung infection model, compared with the thigh infection model.
Inoculum Size
The impact of inoculum on the activity of vancomycin has recently been examined in an in vitro pharmacodynamic model incorporating simulated endocardial vegetations against methicillin-susceptible and methicillin-resistant Staphylococcus aureus [17]. The activity of vancomycin, nafcillin, daptomycin, and linezolid was examined at a moderate inoculum of 106 log10 cfu/g and a high inoculum of 109 log10 cfu/g over a 72-h period. At the lower inoculum of 106 log10 cfu/g, vancomycin, nafcillin, and daptomycin demonstrated similar bactericidal activity; however, at the higher inoculum, nafcillin and vancomycin had little to no impact over the 72-h period, whereas the effectiveness of daptomycin was minimally affected. Although linezolid is bacteriostatic, it was not affected by the size of the inoculum.
Protein-Binding Effects
Although most studies have shown that the binding of vancomycin to protein is moderate (⩽50%), there are a number of in vitro assessments that have demonstrated a 1–8-fold increase in the MIC as a result of the presence of albumin, whereas the presence of serum has had a more variable effect [17–19].
Vancomycin Concentration And Killing Activity
A number of in vitro and animal studies have been performed to determine the relationship between vancomycin concentration and killing activity. Most in vitro killing curve experiments evaluating fixed vancomycin exposure concentrations as small increments of the MIC demonstrate that killing activity does not change as a function of increasing concentration [20]. Using an in vitro pharmacodynamic model that could mimic the elimination half-life of vancomycin, Larson et al. [21] evaluated the effects of concentration and bacterial activity against a strain of methicillin-resistant S. aureus. The administration of stepwise increasing clinical concentrations (range, 5–40 mg/L) resulted in no appreciable difference in killing. The postantibiotic effect of vancomycin is dependent on the concentration. As drug concentrations exceed the MIC by 2–4-fold, the postantibiotic effect was been reported to increase from 0.2 to 2 h for S. aureus and from 4.3 to 6.5 h for Staphylococcus epidermidis [22]. In vitro and neutropenic mouse thigh infection models have demonstrated that the area under the concentration curve (AUC) divided by the MIC (AUC/MIC) is the best predictor of the activity of vancomycin against methicillin-susceptible S. aureus (figure 2), methicillin-resistant S. aureus, and glycopeptide-intermediate S. aureus (GISA). However, in a Streptococcus pneumoniae nonneutropenic mouse peritonitis model, Knudsen et al. [24] demonstrated that the peak serum concentration divided by the MIC (peak/MIC) was the pharmacodynamic parameter with the most predictive value. Why peak/MIC has predictive value is unknown, but the reason may be one of several factors, including the species of organism, very high susceptibility to vancomycin, and the contribution of neutrophils to elimination of the pathogen.
Relationship between pharmacokinetic/pharmacodynamic indices for vancomycin and bacteriologic efficacy against methicillin-susceptible Staphylococcus aureus. This plot, which delineates the change in colony-forming units (cfu) in an experimental mouse infection model 3 different ways, suggests that the area under the curve divided by the MIC (AUC/MIC) is the most valuable pharmacokinetic/pharmacodynamic parameter for predicting the activity of vancomycin against methicillin-susceptible S. aureus. Peak/MIC, peak serum concentration divided by the MIC. Data are from Ebert [23].
Human Pharmacodynamic Studies
There are very few human studies evaluating the pharmacodynamics of vancomycin, and the findings of most of those studies have not been conclusive in determining which parameter has the most value in predicting patient outcome. The majority of studies have involved relatively small patient populations and patients with a variety of infection types. One prospective evaluation randomized 106 patients with S. aureus infections, including bacteremia and endocarditis, to achieve 3 different trough concentration targets of 5–10 mg/L, 10–15 mg/L, and 15–25 mg/L. No relationships were found between peak concentrations, trough concentrations, or pharmacodynamic parameters (e.g., peak/MIC, time above the MIC, or AUC/MIC) and organism eradication or overall patient outcome [25]. Moise-Broder et al. [26] examined the relationship between the vancomycin AUC/MIC and the outcomes of 108 patients with methicillin-resistant S. aureus pneumonia. An AUC/MIC value of ⩾400 was associated with a successful outcome, whereas an AUC/MIC value of <400 was associated with a lower eradication rate and a higher mortality rate (P = .005) [26]. A recent study examined the relationship between the AUC/MIC value and a successful outcome in 168 patients with S. aureus bacteremia. The MIC50 was 0.5 mg/L (range, 0.25–1.0 mg/L), and the median AUC/MIC value was 1072. Overall, in this study, no relationship was found between successful outcome and a specific AUC/MIC value [27].
The development of staphylococcal resistance to vancomycin has been associated with prolonged exposure to low serum concentrations of the drug. GISA infection and subsequent failure of vancomycin therapy have been reported since the middle of the 1990s. By definition, these strains have a vancomycin MIC of 8–16 mg/L. The majority of cases of GISA infection have occurred among patients receiving peritoneal dialysis or hemodialysis who had received suboptimal, prolonged, and repeated courses of vancomycin [28]. Most cases of GISA infection have involved serum concentrations of vancomycin that were consistently ⩽10 mg/L. Although the number of cases of GISA infection has remained low, there appears to be some evidence that this type of resistance has occurred in the past but may have been underreported because of our inability to detect these strains in the clinical laboratory [29]. The Centers for Disease Control and Prevention recommends the use of vancomycin screening plates of 6 mg/L, which may increase our ability to detect these strains [30]. S. aureus strains that display heteroresistance to glycopeptides (i.e., heteroresistant GISA strains) have also been reported to be associated with vancomycin therapy failure [30, 31]. These strains typically have an MIC of 1–4 mg/L but contain a subpopulation of cells that exhibit higher MIC values when plated onto agar plates containing vancomycin or when tested with a heavy inocula by use of the Etest (AB BIODISK) methods for antimicrobial susceptibility. Similar to GISA strains, these organisms are difficult to detect in the clinical laboratory, and their prevalence may be underreported [32]. Recent in vitro evaluations have demonstrated a relationship between exposure to low vancomycin serum concentrations and the development of heteroresistant GISA [33]. However, because of the difficulty in detecting these strains clinically, the overall prevalence and clinical significance of heteroresistant GISA have not been established [32, 34].
Toxicity
In recent years, there appears to be less controversy with regard to the relationship between serum vancomycin concentrations and toxicity. Historically, vancomycin toxicities were related to impurities in the manufacturing process [1]. Although most animal studies have not found that vancomycin causes nephrotoxicity, there have been a number of studies involving humans that have attempted to link elevated serum vancomycin serum concentrations with renal damage [35–39]. Most of these studies are retrospective, and definitions for nephrotoxicity are highly variable. In many cases, serum vancomycin concentrations were measured after an elevation in serum creatinine levels, making it uncertain which came first. Despite these drawbacks, the nephrotoxic potential of vancomycin is reported to be ⩽5% [37, 39]. In one of the largest investigations to date, Pestotnik et al. [40] reported that the incidence of nephrotoxicity among 1750 patients was 1.4%. Of interest, vancomycin appears to potentiate the nephrotoxicity of aminoglycosides, when administered in combination with this class of drugs. Both animal and human studies have concluded that the combination may increase the nephrotoxicity of aminoglycosides by, on average, 3–4-fold [37, 39, 41].
With respect to ototoxicity, the overall incidence appears to be low [42]. Despite clinical case reports of a relationship between vancomycin serum concentrations and ototoxicity, there are no animal models that have demonstrated this relationship. The majority of experts believe that this drug is not ototoxic [42–45].
The so-called therapeutic range most often quoted for vancomycin monitoring is peak and trough serum concentrations of 30–40 mg/L and 5–10 mg/L, respectively [46, 47]. As stated earlier, there is little evidence to support a relationship between specific serum concentrations and efficacy and toxicity. Because vancomycin is a concentration-independent, or time-dependent, antibiotic and because there are practical issues associated with determining a precise peak serum concentration with this multicompartment antibiotic, most clinicians have abandoned the routine practice of determining peak serum concentrations. Therefore, most clinicians rely solely on monitoring trough serum concentrations for this antibiotic.
The overall AUC/MIC value may be the pharmacodynamic parameter that best correlates with a successful outcome associated with the use of vancomycin; however, further studies seem to be warranted. The nephrotoxic and ototoxic effects of this drug are minimal and are not related to specific serum concentrations. Prolonged exposure to serum concentrations close to the MIC are associated with the emergence of resistance; therefore, it is important to maintain adequate serum concentrations in patients with fast or rapidly changing creatinine clearance. There are several body compartments in which penetration is poor, such as the lung and the CNS. It would also seem prudent to keep concentrations from being suboptimal in patients with pneumonia or meningitis, as well as in patients receiving vancomycin who are receiving dialysis for renal failure. The American Thoracic Society recently published guidelines for hospital-acquired, ventilator-associated, and health care—associated pneumonia. These guidelines recommend vancomycin trough concentrations of 15–20 mg/L for the treatment of methicillin-resistant S. aureus pneumonia. The recommendations are based on vancomycin susceptibility and pharmacokinetic/pharmacodynamic properties, as well as reported clinical experience, since no definitive clinical studies have evaluated the recommended serum concentrations with respect to patient outcome [48]. Although precise and aggressive pharmacokinetic adjustments via the successive measurement of serum concentrations may not be necessary for most infections, empirical adjustments made using standard pharmacokinetic equations or a validated nomogram that maintains trough serum concentrations at 4–5 times the MIC would seem to be reasonable. Higher concentrations may be needed for sequestered infections or in situations where vancomycin penetration has been documented to be poor. Further research examining the outcome of patients as it relates to vancomycin serum concentrations is warranted.
Acknowledgments
Potential conflicts of interest. M.J.R. receives grant and/or research support from Advancis, Bayer, Cubist, and Oscient, is a consultant for Advancis, Bayer, Cubist, and Oscient, and is on speakers' bureaus of Aventis, Bayer, and Cubist.




![Relationship between pharmacokinetic/pharmacodynamic indices for vancomycin and bacteriologic efficacy against methicillin-susceptible Staphylococcus aureus. This plot, which delineates the change in colony-forming units (cfu) in an experimental mouse infection model 3 different ways, suggests that the area under the curve divided by the MIC (AUC/MIC) is the most valuable pharmacokinetic/pharmacodynamic parameter for predicting the activity of vancomycin against methicillin-susceptible S. aureus. Peak/MIC, peak serum concentration divided by the MIC. Data are from Ebert [23].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/42/Supplement_1/10.1086_491712/1/m_42-Supplement_1-S35-fig002.gif?Expires=1716311715&Signature=DWx-PS1xSrdA3hmc5FAi68NkQNdsnztqDAM6bwvg0RZgKMoYI9G8MHD-Wb8w1uLYmPGW5ASmlEQ0cf8Y7P0q4f-v3IC03p5w77QAWow-Sey8As9GSGaQHi9g9SU0t6Il~zqSLwECHp6j34vTJmhI6VnbC5eWXpimRa2ACV-Tu35AESOFOFaPcZBYuxjKmGQPbhyz8Nay3AfAsna0c1hxccVyRA9fkbNVN-NCRnqyt9m~k70o8VERNWWkOd6KNXANT2Sq-KzlLARDQe28hMVuowKErByXETmSLH6a3KEovdrZjXVWCrJKI~geFlHJakiCPZe~cqQZiqEViDjMPpdyJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments