-
PDF
- Split View
-
Views
-
Cite
Cite
David L. Paterson, The Role of Antimicrobial Management Programs in Optimizing Antibiotic Prescribing within Hospitals, Clinical Infectious Diseases, Volume 42, Issue Supplement_2, January 2006, Pages S90–S95, https://doi.org/10.1086/499407
Close - Share Icon Share
Abstract
Managing serious infections is a balance between providing timely and appropriate broad-spectrum empirical therapy for individual patients, which has been consistently shown to improve outcomes, and reducing unnecessary use of antimicrobial agents, which may contribute to the development of antimicrobial resistance. To control the spread of antimicrobial resistance, hospitals commonly implement programs designed to optimize antimicrobial use, supported by infection-control measures. Hospital-based antimicrobial management programs—also called “antimicrobial stewardship programs”—are primarily based on education coupled with a “front-end” approach (i.e., restricting the availability of selected antimicrobial agents) or a “back-end” approach (i.e., reviewing broad-spectrum empirical therapy and then streamlining or discontinuing therapy, as indicated, on the basis of culture and susceptibility testing results and clinical response). Institutional efforts to optimize antimicrobial use should concentrate on patient outcomes, should have multidisciplinary support, and should use a combination of interventions customized to the needs, resources, and information technology infrastructure of the health care institution.
Clinicians and policy makers who formulate decisions about the treatment of nosocomial infections face a challenge. They must strike a balance between the benefits of aggressive empirical antimicrobial therapy and the emergence and spread of antimicrobial-resistant pathogens associated with excessive use of antimicrobial agents. Early administration of appropriate antimicrobial agents that have microbiological activity against the organisms most likely to be the cause of an individual's infection has been consistently shown to improve outcomes for patients. More than 2 decades ago, in a study by Kreger et al. [1], appropriate empirical antimicrobial therapy reduced by 50% the frequency of shock among 612 patients with sepsis due to gram-negative bacteria [2]. Since then, numerous investigators have documented the effect of antimicrobial therapy on mortality [3–8]. For instance, in a prospective study of 655 infected patients who required admission to an intensive care unit (ICU), Kollef et al. [3] found that the rate of infection-associated mortality was significantly lower among patients who received appropriate antimicrobial therapy (i.e., the organism cultured was susceptible to the antimicrobials, as determined by in vitro testing), compared with patients who received inappropriate therapy (mortality rate, 18% vs. 42%; relative risk, 2.37; P < .001).
On the basis of these data, many clinicians “in the trenches” select and use broad-spectrum antimicrobial agents for treatment for as long as is possible. This approach, although possibly useful for the treatment of individual patients, has far-reaching ramifications with regard to the emergence and spread of antimicrobial resistance [9], when followed on a widespread basis. Infections increasingly are caused by multidrug-resistant staphylococci (methicillin- and vancomycin-resistant Staphylococcus aureus) [10–13] and gram-negative bacilli [14, 15], which may drastically diminish the efficacy of antimicrobial therapy. Furthermore, in the face of a limited number of new antimicrobial agents currently in development that have microbiological activity against multidrug-resistant gram-negative bacteria (the only examples of which are doripenem and tigecycline), the emergence of multidrug resistance is looming. Linden et al. [16] identified infection due to multidrug-resistant Pseudomonas aeruginosa in 23 liver transplant recipients treated between January 1996 and February 2003 at the University of Pittsburgh Medical Center (UPMC; Pittsburgh, PA) and for whom colistin was the only treatment option. At approximately the same time, an outbreak due to multidrug-resistant P. aeruginosa occurred in an ICU in Belgium [17]. Resistance characterization of the epidemic clone showed overexpression of the chromosomal cephalosporinase AmpC, decreased expression of the porin OprD, and up-regulation of the MexXY efflux pump system, which lead to resistance to all antimicrobial agents, with the exception of colistin. Numerous other examples of multidrug resistance in P. aeruginosa and Acinetobacter baumannii exist [18].
Development Of Resistant Strains In Hospitals
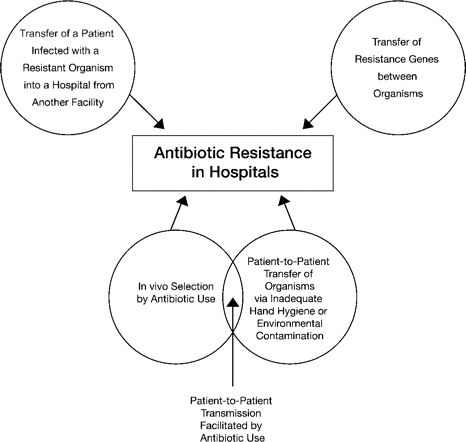
An understanding of how antimicrobial-resistant organisms enter hospitals will better prepare health care professionals to prevent their occurrence. Emergence of resistant strains in hospitals occurs when a patient infected with a resistant pathogen is transferred to the hospital from another facility, by horizontal transfer between patients, through selection based on antimicrobial use, and by transfer of resistance genes (figure 1).
Transfer of antimicrobial-resistant pathogens from health care facilities to hospitals has been well documented. After a terrorist bombing in Bali, victims initially treated at field hospitals developed infections due to multidrug-resistant Acinetobacter organisms, which were isolated when the patients were later transferred to and treated in Australia [19]. Similarly, infection with multidrug-resistant A. baumannii has been identified in patients at military hospitals where service members injured in Afghanistan, Iraq, or Kuwait were treated [20]. Quale et al. [18] described the clonal emergence of carbapenem-resistant A. baumannii in 15 hospitals in the New York metropolitan area, a finding that clearly indicates the potential for hospital-to-hospital transfer of resistant organisms.
Patient-to-patient transfer of multidrug-resistant pathogens within a hospital is also well documented. For example, in the outbreak described by Deplano et al. [17], 16 of the 18 affected patients had P. aeruginosa isolates with an identical serotype and PFGE pattern. Contamination of both the ICU environment and the hands of a nurse were detected, and contact precautions controlled transmission of the epidemic clone.
Antimicrobial Management Programs
Various interventions have been used in hospitals to control the spread of antimicrobial resistance. Infection-control measures, such as isolating patient contacts, the cohorting of patients, performing environmental cultures, and removing contaminated devices (e.g., bronchoscopes and faucet aerators), are often instituted to decrease horizontal transfer of antimicrobial resistance. In fact, effective infection-control practices and implementation of hygiene barriers are the foundation of any effort to control the spread of antimicrobial resistance [21]. A complementary approach to decreasing the emergence of antimicrobial resistance relies on institutional programs that optimize antimicrobial use. In addition to ensuring that antimicrobial resistance is not promoted, these antimicrobial management programs for infection control typically have 2 other objectives: to ensure that the empirical antimicrobials used are appropriate and adequate and to ensure that antimicrobials are not wasted, thus controlling acquisition costs. Some antimicrobial management programs (also called “antimicrobial stewardship programs”) in hospitals are based entirely on education, whereas others follow a “front-end approach,” a “back-end approach,” or a combination of practices [22].
Education as a Means to Decrease Antimicrobial Resistance
An education program, which is designed to increase physicians' understanding about the scope and consequences of antimicrobial resistance and appropriate use of antimicrobial agents, is relatively inexpensive and easy to implement in hospitals [23]. The influence of such programs is limited by (1) lack of institutional support; (2) only short-lived, if any, changes in prescribing practices; and (3) inadequately documented correlation between the educational initiative and the decrease in antimicrobial resistance. Therefore, many institutions supplement education with a formal antimicrobial stewardship program.
Use of a Front-End Approach as a Means to Decrease Antimicrobial Resistance
With the front-end approach, restrictions are placed on the availability of certain antimicrobial agents. Examples of such restrictions include the need for preapproval before the administration of restricted agents, use of special antimicrobial request forms, and antimicrobial cycling.
Need for preapproval. At the UPMC, use of a very broad array of antimicrobial agents (e.g., third-generation cephalosporins, fluoroquinolones, carbapenems, or β-lactam/β-lactamase inhibitor combinations) for non-ICU patients requires preapproval. The process in practice at the UPMC involves (1) dedicating a mobile telephone to receive calls regarding the approval of antimicrobial agents, (2) having the prescriber call the number of the dedicated mobile telephone before writing an order for a restricted antimicrobial agent, (3) discussion of the appropriateness of the choice of antimicrobial for treatment, (4) assignment of an approval number before the antimicrobial agent is dispensed, and (5) prescribing an alternative antimicrobial treatment if the initial request is denied. A large outbreak of nosocomial Clostridium difficile—associated disease (2.7 cases/1000 patient discharges from the hospital in 1999 vs. 6.8 cases/1000 patient discharges from the hospital in 2000/2001) with unexpectedly high rates of associated morbidity and mortality prompted the medical staff to embrace a front-end restriction policy. Results of a case-control study showed that the use of ceftriaxone (OR, 5.4), clindamycin (OR, 4.8), or levofloxacin (OR, 2.0) was an independent risk factor for C. difficile infection [24]. Other investigators have also associated such “collateral damage”—namely, the selection of C. difficile infection—with cephalosporin or fluoroquinolone use [25].
Since October 2002 and the onset of the antimicrobial restriction program at the UPMC, there has been a >50% reduction in the overall use of antimicrobials associated with the development of C. difficile infection (i.e., levofloxacin, clindamycin, and ceftriaxone). Ciprofloxacin, another restricted agent, was also used less frequently. In fact, the total number of defined daily doses (i.e., number of defined daily doses/100 patient-days) of antimicrobial agents used at our institution has decreased by ∼10%. Since implementation of the antimicrobial restriction program, there has been a reduction in the number of nosocomial C. difficile infections, although the rate has not returned to its baseline value. In addition, enhanced infection-control precautions were introduced.
There are a number of practical issues that a hospital must address when establishing an antimicrobial restriction program. For instance, prescribing physicians must have confidence in the person determining the appropriateness of antimicrobial requests. There is a need for salary relief for the “antimicrobial police force,” because attending infectious diseases (ID) physicians will probably be unwilling to participate without receiving adequate compensation, and because ID fellows may not be ideal. In one study conducted at the Hospital of the University of Pennsylvania (Philadelphia), a dedicated antimicrobial management team (comprised of a clinical pharmacist and an attending ID physician who provided backup) was more effective than an ID fellow, on the basis of the rate of appropriateness of recommendations (87% vs. 47%, respectively; P < .001), the clinical cure rate (64% vs. 42%, respectively; P = .007), and the treatment failure rate (15% vs. 28%, respectively; P = .03) [26].
Success of antimicrobial restriction in hospitals varies on the basis of the genesis of the antimicrobial resistance program. Clinicians caring for critically ill patients are unlikely to endorse and willingly follow an antimicrobial restriction program instigated by administrators to save money, whereas they may be more sympathetic to a program supported by their ID colleagues to address specific issues in the ICU (e.g., antimicrobial resistance, use of antimicrobial agents for colonization vs. infection, and excessively prolonged duration of treatment). The keys to implementation of a program using a front-end approach are discussion with and endorsements from the clinical departments that will be affected (e.g., the ICU, surgical department, emergency department, and outpatient clinics, as well as other affiliated hospitals).
Antimicrobial management programs are appropriate in ICUs [27], although they are more challenging in ICUs than in non-ICU settings. At the UPMC, antipseudomonal agents (e.g., piperacillin/tazobactam and cefepime) were available without restrictions in critical care units, whereas fluoroquinolones, carbapenems, and linezolid required preapproval for use. With this policy in place, a review of inappropriate empirical therapy for Pseudomonas infection revealed 2 major causes: repeated use of an antimicrobial agent that the patient had received previously and use of an antimicrobial agent to which a previously cultured organism was resistant (e.g., prior recovery of a cefepime-resistant Enterobacter isolate predicted resistance to cefepime in a more recently recovered Pseudomonas isolate) [28]. To encourage the selection of appropriate therapy, we created a ventilator-associated pneumonia (VAP) order set, which established principles for management very much like those now included in the guidelines for the management of nosocomial pneumonia recently published by the American Thoracic Society and the Infectious Diseases Society of America [29]. In our VAP order set, we encourage: (1) use of quantitative cultures of bronchoalveolar lavage (BAL) specimens rather than sputum cultures; (2) use of an antipseudomonal carbapenem if there was previous use of or resistance to piperacillin/tazobactam or cefepime; (3) use of empirical combination regimens instead of monotherapy; (4) reassessment of the need for antimicrobial agents on day 3 of therapy; and (5) discontinuation of therapy on day 8 of therapy.
Implementation of an antimicrobial management system in the critical care setting is not without barriers, especially in locations without computer-based order entry. Paper-based order sets require reprinting and distribution to all relevant caregivers when guidelines are updated, with out-of-date forms destroyed. Oftentimes, physicians do not have immediate access to the protocols and order sheets when they want to initiate antimicrobial agents for their critically ill patients. Among the more sophisticated approaches was a computerized decision-support system linked to computer-based medical records that was developed by Burke and colleagues at the LDS Hospital in Salt Lake City, Utah, to assist physicians in the use of antimicrobial agents [30]. During a 1-year period during which the computerized anti-infective management program was used in an ICU, the investigators documented statistically significant reductions in the numbers of adverse events, excess drug dosages, and antimicrobial susceptibility mismatches, as well as reductions in the length of hospital stay and costs, compared with the findings noted during the 2-year preintervention period. Of note, the same group of investigators also showed that computer support stabilized hospital-wide antimicrobial resistance patterns over multiple years [31].
Antimicrobial cycling. Antimicrobial cycling, or the scheduled rotation of antimicrobial agents, has been instituted as a means to control the development of antimicrobial resistance. This approach is based on the notion that withdrawing the use of an antimicrobial agent for a defined period limits the pressure that stimulates antimicrobial resistance. The purported benefits of antimicrobial cycling have included reduction in antimicrobial resistance among gram-negative organisms, improved antimicrobial prescribing, and reductions in the incidence of nosocomial infection (primarily VAP) and associated mortality rates [32]. There is a need for critical evaluation of published studies, because the methods used and the uncontrolled confounding variables may have led to unjustified enthusiasm for this approach.
Use of a Back-End Approach as a Means to Decrease Antimicrobial Resistance
A back-end approach to antimicrobial management permits empirical use of broad-spectrum antimicrobial agents, followed by postprescription review and, then, by streamlining (de-escalation) or discontinuing antimicrobial therapy on day 2 or 3, if this decision is supported by culture and susceptibility testing results and by the patient's clinical response. When an antimicrobial management program that uses a back-end approach is developed at an institution, one must be mindful that clinicians may have various reservations about streamlining or discontinuing therapy, such as wanting to “stick with a good thing” or considering their patient to be too sick for a change in treatment.
A prospective, multicenter study was recently conducted at 5 tertiary care centers to evaluate acceptance of a standardized postprescription antimicrobial review [33]. Patients receiving a broad-spectrum antimicrobial agent (i.e., a fluoroquinolone, a β-lactam/β-lactamase inhibitor combination, vancomycin, a third- or fourth-generation cephalosporin, or carbapenem) for 48 h were identified, justification of their therapy was assessed, and a recommendation was made to the health care provider by an ID physician if antimicrobial therapy was deemed to be unjustified on the basis of standard criteria. A total of 399 (29%) of 1379 reviewed courses of broad-spectrum therapy were unjustified. The Centers for Disease Control and Prevention's 12-step classification to prevent antimicrobial resistance among hospitalized patients captured the major types of antimicrobial misuse [34]. It found that, for 41% of cases, the spectrum was too broad on the basis of the results of microbiological testing (step 3); for 33% of cases, antimicrobial therapy was unnecessary (step 10); for 16%, vancomycin was unnecessary (step 9); and, for 1%, colonization/contamination was being treated (steps 7/8). Health care providers commonly accepted recommendations to modify or stop broad-spectrum antimicrobial agents. At one of the participating centers, an automated postprescription review system facilitated nearly 6-fold more interventions than did a prior-approval process [35].
Variants of the back-end approach have been used. At some hospitals, a list of antimicrobial agents that require assessment is generated on a daily basis, and the medical charts of patients receiving the targeted antimicrobial agents are reviewed. If therapy is considered to be inappropriate, an automatic stop order is executed, and either the attending physician is contacted by telephone or a note is written in the patient's medical chart. At other medical centers, administration of anti-infective agents with good oral bioavailability (e.g., fluoroquinolones, fluconazole, and linezolid) is switched from parenteral to oral administration for clinically stable patients who are taking other medications orally. This strategy was evaluated in a randomized, controlled study by Paladino et al. [36], who showed that the clinical and bacteriological outcomes and safety profiles for patients whose treatment is switched from intravenously to orally administered ciprofloxacin after 3 days were comparable to those for patients who continued receiving parenteral therapy. Also, switching from intravenously to orally administered therapy resulted in a savings of $293/patient (in 1991 US dollars). At the UPMC, switching from intravenously to orally administered antimicrobial therapy is automated and is widely accepted by physicians. At other institutions, culture results and antimicrobial susceptibility testing data from the microbiology laboratory are compared with data on antimicrobial use obtained from the pharmacy, to identify patients for whom empirical therapy can be streamlined.
An innovative approach to streamlining antimicrobial therapy was studied by Singh et al. [37] from the VA Medical Center in Pittsburgh, Pennsylvania. Patients with suspected VAP and a high (i.e., >6) Clinical Pulmonary Infection Score (CPIS; a composite score based on temperature, blood leukocyte counts, presence of tracheal secretions, PaO2/FIO2, chest radiograph findings, pulmonary infiltrate progression, and tracheal aspirate culture results) were treated with antimicrobial agents at the discretion of their physician. Patients with suspected VAP and a CPIS of ⩽6 at baseline were randomized to receive either a single antimicrobial agent, which was discontinued on day 3 of treatment if the CPIS remained ⩽6, or standard therapy (with dose and duration determined by the treating physician). Patients who had therapy discontinued on day 3 developed antimicrobial resistance and/or superinfection significantly less often than did patients who were treated with a more prolonged course of therapy (rate of development of resistance, 15% vs. 35%, respectively; P = .017) and had a lower 30-day mortality rate (13% vs. 31%, respectively; P = .06).
Effect On Antimicrobial Resistance?
Given the multitude of institutional approaches to antimicrobial stewardship, the important question is: do any of these approaches have a meaningful effect on antimicrobial resistance? The answer to this question depends on whether the aim of the antimicrobial management program is to arrest an outbreak of multidrug-resistant pathogens or to have a sustained effect on endemic resistance. There are many examples of situations for which changes in antimicrobial agent prescribing practices had a significant effect on outbreaks of resistant pathogens. As described earlier, antimicrobial restriction decreased nosocomial C. difficile infections at our institution. It must be noted that enhanced infection-control measures, including environmental decontamination, were also introduced in response to the outbreak of C. difficile infection. Rahal et al. [38] reported a 44% hospital-wide decrease in extended-spectrum β-lactamase (ESBL)—producing Klebsiella isolates after restriction of cephalosporins (an 80% decrease in use) at 1 university-affiliated hospital. Quale et al. [39] documented a significant decrease in the point prevalence of fecal colonization with vancomycin-resistant enterococci (from 47% to 15%; P < .001) and a gradual decrease in the number of patients with culture-positive clinical specimens after restriction of the use of third-generation cephalosporins and vancomycin. White et al. [40] showed that antimicrobial stewardship had an effect on antimicrobial susceptibility in Acinetobacter species.
There are much fewer data on the effect of antimicrobial restriction on endemic resistance. Antimicrobial restriction at the UPMC has resulted in sustained improvement in hospital-wide susceptibility of Pseudomonas organisms to quinolones, cefepime, and piperacillin/tazobactam. These data suggest that endemic resistance can be improved with an antimicrobial management program. We hypothesize that, by improving antimicrobial susceptibilities, patients are more likely to receive appropriate empirical antimicrobial therapy, but this outcome remains to be measured.
In summary, management of serious infections is a balance between optimizing empirical therapy for individual patients, which consistently has been shown to improve outcomes, and reducing unnecessary antimicrobial use, which may contribute to antimicrobial resistance. Institutional efforts to optimize antimicrobial use should concentrate on patient outcomes, receive multidisciplinary support, and use a combination of interventions customized to the needs, resources, and information technology infrastructure of the institution.
Acknowledgments
Potential conflicts of interest. D.L.P. has received research grant support from Elan, Merck, Pfizer, and AstraZeneca and serves on the speakers' bureaus of Elan, Merck, Pfizer, Roche, and Cubist.





Comments