-
PDF
- Split View
-
Views
-
Cite
Cite
Elinore F. McCance-Katz, David E. Moody, Patrick F. Smith, Gene D. Morse, Gerald Friedland, Patricia Pade, Jennifer Baker, Anika Alvanzo, Peter Jatlow, Petrie M. Rainey, Interactions between Buprenorphine and Antiretrovirals. II. The Protease Inhibitors Nelfinavir, Lopinavir/Ritonavir, and Ritonavir, Clinical Infectious Diseases, Volume 43, Issue Supplement_4, December 2006, Pages S235–S246, https://doi.org/10.1086/508188
Close - Share Icon Share
Abstract
We examined drug interactions between buprenorphine, an opioid partial agonist available by prescription for treatment of opioid dependence, and the protease inhibitors (PIs) nelfinavir (NFV), ritonavir (RTV), and lopinavir/ritonavir (LPV/R). Opioid-dependent, buprenorphine/naloxone-maintained, human immunodeficiency virus (HIV)-negative volunteers (n = 10 per PI) participated in 24-h pharmacokinetic studies, before and after administration of each PI. Symptoms of opiate withdrawal and excess were determined before and after PI administration. PI pharmacokinetics were determined and compared between opiate-dependent participants and healthy control participants (n = 15 per PI). Administration of RTV, but not of NFV or LPV/R, resulted in a significant increase in the buprenorphine area under the concentration-time curve (AUC). Symptoms of opiate excess, however, were not observed. Buprenorphine had no significant effects on PI AUC. Adjustments of doses of either buprenorphine or NFV, LPV/R, or RTV are not likely to be necessary when these drugs are administered for the treatment of opioid dependence and HIV disease.
Adherence to medical regimens among injection drug users is often poor [1, 2]. Despite the large number of drug abusers with HIV disease, HAART is frequently underutilized in this population, because of the difficulties experienced in obtaining adherence adequate to maintain viral suppression [3–5]. It has been possible, however, to engage many drug abusers successfully in HIV clinical care within drug treatment programs and in flexible outpatient settings [6–8]. The provision of drug abuse treatment facilitates adherence to treatment of HIV disease in this population. The course of HIV disease among drug users appears to be similar to that in other populations [9], and abundant evidence indicates that the rate of HIV disease progression can be slowed among injection drug users by use of medical interventions [9, 10]. It is also clear that HIV and substance abuse treatment in injection drug users is often complicated by exposure to multiple daily doses of illicit drugs, such as heroin and cocaine administered by self-injection, in addition to other drugs of abuse, as well as by the existence of high rates of comorbid diseases, particularly hepatitis C [11].
Optimal clinical care of this population requires treatment of both substance dependence and HIV disease. The opioid pharmacotherapy of choice for such individuals has traditionally been methadone; however, the concomitant administration of methadone with any of several antiretroviral agents has been shown to be associated with clinically significant adverse events [12]. Our research group has reported on the interactions between methadone and nelfinavir (NFV), ritonavir (RTV), and lopinavir/ritonavir (LPV/R). NFV has been shown to significantly decrease methadone exposure in those who are coadministered standard clinical doses of these medications, although most individuals receiving concomitant therapy do not show evidence of opiate withdrawal [13]. LPV/R has been associated with opiate withdrawal symptoms in methadone-maintained patients with trough plasma methadone concentrations in the lower end of the therapeutic plasma concentration range [14].
Buprenorphine has recently been shown to be equivalent to methadone in the treatment of opioid-dependent patients [15]. In the United States, buprenorphine is marketed as a sublingual formulation of buprenorphine/naloxone (4:1) [16]. Naloxone has been added to buprenorphine in a combination tablet to prevent diversion to injection use by opioid-addicted injection drug users. Naloxone is poorly absorbed by the sublingual route and does not alter buprenorphine opioid agonist effects. Given the adverse drug interactions observed between methadone and several protease inhibitors (PIs) and the availability of buprenorphine/naloxone for the treatment of opioid dependence by qualified physicians outside of specialized opiate treatment programs (e.g., methadone maintenance treatment programs), it is of interest to know whether the drug interactions identified between methadone and antiretroviral medications extend to buprenorphine. If buprenorphine were associated with fewer interactions with HIV drugs, compared with those associated with methadone—particularly those leading to opiate withdrawal—its use in the treatment of opioid-dependent patients with HIV disease could be associated with greater adherence to clinical regimens and improved clinical outcomes.
The goals of the present study included the following: (1) to determine whether the pharmacokinetics of buprenorphine, administered sublingually as buprenorphine/naloxone, are affected by coadministration of the PI medications NFV, RTV, and LPV/R; (2) to determine whether the pharmacokinetics of these PIs are affected by coadministration of buprenorphine; and (3) to determine whether clinically significant pharmacodynamic effects or toxicities occur when buprenorphine/naloxone is administered simultaneously with these PIs.
Methods
Procedures
Each of the 3 PI studies included 10 buprenorphine/naloxone-maintained (16/4 mg daily, sublingually administered) individuals and 15 (LPV/R or RTV) or 16 (NFV) control participants without opioid dependence. The study was open label and composed of both (1) a within-subject component, which examined the effect of PI administration (1250 mg of NFV twice daily for 5 days, 100 mg of RTV twice daily for 7 days, or 400/100 mg of LPV/R twice daily for 7 days) on buprenorphine disposition; and (2) a between-subject component, which examined the effect of buprenorphine on the disposition of the PIs. The study design has been described elsewhere [13, 14]. Eligible individuals provided written, voluntary, informed consent in accordance with university institutional review board-approved protocols. Opioid-dependent participants received buprenorphine/naloxone treatment for their opioid addiction at no charge and were offered monetary compensation for their participation in the pharmacokinetic studies. Control participants were offered monetary compensation for their time and effort in the study protocol.
Study procedures included standardized and validated measures of opiate withdrawal by clinician rating (Objective Opiate Withdrawal Scale; scale range, 0–12; scores ⩾3 indicate moderate withdrawal symptoms) [17] and of cognitive impairment by use of the Mini-Mental State Examination [18] for opioid-dependent participants (scale range, 0–30; scores <27 indicate cognitive impairment). Adverse symptoms were recorded for all participants, by use of an Adverse Symptoms Checklist (ASC) that queried for a wide range of adverse experiences, including changes in energy, gastrointestinal symptoms, CNS effects, genitourinary symptoms, and other somatic complaints, scored for severity on an ordinal scale (0, not present; 1, mild; 2, moderate; 3, severe [maximum possible total score, 87]). These ratings were evaluated at baseline, after stabilization with buprenorphine (before antiretroviral administration), and at completion of the PI dosing period and were evaluated for control subjects before and at completion of PI administration.
Biochemical assays
Buprenorphine and metabolite concentrations were determined using liquid chromatography-electrospray ionization-tandem mass spectrometry, as has been described elsewhere [19]. PI concentrations were quantified using a previously published simultaneous high-performance liquid chromatography assay [20].
Pharmacokinetic analysis
The pharmacokinetic parameters of buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, norbuprenorphine-3-glucuronide, NFV, M8 (the active metabolite of NFV), LPV, and RTV were determined as appropriate for each participant. Buprenorphine pharmacokinetics were determined after sublingual administration of the opioid only and again after continued buprenorphine/naloxone treatment and PI administration for the duration described above. In the control group, PI pharmacokinetic parameters were determined after the PIs had been administered as described for buprenorphine/naloxone-maintained individuals. The 24-h area under the concentration-time curve (AUC0–24,), minimum plasma concentration (Cmin), maximum plasma concentration (Cmax), time of Cmax (Tmax), and sublingual (buprenorphine) or oral (NFV, LPV, and RTV) clearance (Cl/F) were determined using the noncompartmental analysis module of WinNonLin Professional software, version 3.2 (Pharsight). For the metabolites, Cl/F was calculated on the basis of the administered dose of parent compound. The F term thereby represents the fraction of parent drug that is ultimately converted to the metabolite. For purposes of noncompartmental analysis, drug concentrations that were lower than the limit of quantitation were expressed as one-half of the limit. All pharmacokinetic parameters were summarized and displayed by treatment period. Urine results were calculated as a percentage of the daily dose, with a molar conversion for metabolites: % of daily dose = (amount) (molecular weight of buprenorphine) (molecular wight of metabolite)−1 (daily dose)−1. Renal clearance was calculated as follows: (amount in urine) (AUC0–24)−1.
Statistical analysis
The sample size calculations and statistical analysis for the present study have been described elsewhere [21]. Briefly, Student's paired t test was used to test the significance of the differences in pharmacokinetic parameters for buprenorphine (within-subject analyses). The Wilcoxon test was used for the within-subject comparison of the values of Tmax. Differences in pharmacokinetic parameters for PIs in the control groups versus the buprenorphine/naloxone-treated groups (between-group comparisons) were obtained by use of the Kruskal-Wallis test or, for Tmax, the Mann-Whitney test. A difference was considered to be statistically significant if P ⩽ .05 (2-tailed). Comparisons of subject characteristics were made by single-factor analysis of variance.
Results
Study Participants
A total of 25 individuals participated in each of the 3 PI protocols undertaken in the present study. For each PI studied, 10 opioid-dependent participants who were receiving a stable, daily, sublingual, dose of buprenorphine/naloxone (16/4 mg) and who were otherwise physically healthy and without current mental disorders other than substance use disorders completed the study. For each PI, 15 control participants who were matched by age, sex, and weight to opioid-dependent participants completed pharmacokinetic studies. The demographic characteristics of the study participants are listed in table 1. There were no significant differences in age, race, weight, or sex. Opioid-dependent participants received a stable dose of buprenorphine/naloxone for at least 2 weeks before study entry. On the basis of clinical assessment, opioid-dependent participants were stabilized (with stabilization defined as a lack of opiate withdrawal symptoms, cessation of opiate craving, and cessation of opiate use as determined by urine toxicology screen) with a 16/4-mg daily dose of buprenorphine/naloxone administered sublingually. Concomitant medication use in this sample was rare and included only hydrochlorothiazide for 1 participant in the buprenorphine-RTV group (hydrochlorothiazide use for otherwise well-controlled hypertension was permitted because it is renally excreted and would not alter the pharmacokinetics of the study drugs). The abuse of substances other than opioids was a common occurrence, in both the buprenorphine/naloxone and control groups, with cocaine abuse most prevalent, followed by cannabis abuse (in opioid-dependent participants) and alcohol abuse (no participants met diagnostic criteria for alcohol dependence) (table 1). Moderate cigarette smoking was common among both buprenorphine/naloxone-maintained participants and control participants, but daily use reported by all participants was <1 pack/day (range, 0.2–0.6 pack/day). Injection drug use was common among the opioid-dependent participants (range, 20%–50%), with nasal insufflation the preferred route of administration among the remaining opioid-dependent participants. Serological testing showed that 30% of the opioid-dependent participants in the buprenorphine-NFV and buprenorphine-RTV study had hepatitis C, although no participants in the buprenorphine-LPV/R study and no control participants had evidence of acute hepatitis C (table 1).
Characteristics of study participants, by study group.
| Nelfinavir | Lopinavir/ritonavir | Ritonavir | ||||
| Characteristic | With buprenorphine/naloxonea (n = 10) | Control (n = 16) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) |
| Age, years | 34 ± 2.5 | 41 ± 2.1 | 34 ± 2.7 | 38 ± 2.3 | 37 ± 2.4 | 40 ± 2.3 |
| Weight, kg | 77.2 ± 4.6 | 84.2 ± 4.5 | 84.1 ± 5.7 | 80.5 ± 3.9 | 83.1 ± 5.2 | 77.4 ± 4.0 |
| Buprenorphine/naloxone dose, mg/day | 16/4 | NA | 16/4 | NA | 16/4 | NA |
| Female sex, no. | 5 | 6 | 4 | 7 | 5 | 6 |
| Race, no. | ||||||
| African American | 6 | 6 | 8 | 6 | 7 | 6 |
| White | 3 | 6 | 1 | 4 | 2 | 6 |
| Hispanic | 0 | 3 | 0 | 5 | 0 | 3 |
| Mixed | 1 | 1 | 1 | 0 | 1 | 0 |
| Substance use disorder, no. | ||||||
| Opioid dependence | 10 | 0 | 10 | 0 | 10 | 0 |
| Cocaine abuse | 5 | 5 | 3 | 3 | 5 | 2 |
| Cannabis abuse | 5 | 0 | 1 | 2 | 2 | 1 |
| Alcohol abuse | 1 | 4 | 1 | 0 | 1 | 0 |
| Injection drug use, no. (%) | 5 (50) | NA | 2 (20) | NA | 4 (40) | NA |
| Nicotine use | ||||||
| Packs/day | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 |
| Total years smoking | 11.8 ± 3.1 | 10.8 ± 2.8 | 10.9 ± 3.5 | 7.3 ± 2.8 | 12.6 ± 3.9 | 8.3 ± 3.2 |
| HCV positive, no. (%) | 3 (30) | 0 | 0 | 0 | 3 (30) | 0 |
| AST concentration,b U/L | ||||||
| Before administration | 30.8 ± 5.5 | 23.6 ± 1.6 | 29.3 ± 2.6 | 22.5 ± 1.9 | 31.0 ± 3.7 | 20.9 ± 2.1 |
| After administration | 26.1 ± 3.1 | 28.0 ± 6.2 | 23.3 ± 1.8 | 18.3 ± 1.3c | 28.7 ± 4.6 | 21.2 ± 4.5 |
| ALT concentration,b U/L | ||||||
| Before administration | 34.6 ± 5.6 | 24.4 ± 3.4 | 32.6 ± 6.1 | 28.3 ± 4.3 | 29.2 ± 2.7 | 20.5 ± 3.8 |
| After administration | 31.6 ± 4.7 | 25.7 ± 5.9 | 23.3 ± 3.0 | 17.5 ± 1.9d | 25.0 ± 2.9 | 15.9 ± 2.4 |
| Adverse symptoms, ASC score | ||||||
| Before administration | 7.5 ± 1.7 | 4.3 ± 0.9 | 7.6 ± 2.4 | 2.1 ± 1.4 | 8.1 ± 2.7 | 1.6 ± 0.61 |
| After administration | 10.3 ± 3.1 | 5.2 ± 1.0 | 4.9 ± 2.0 | 5.7 ± 1.2 | 7.6 ± 2.2 | 1.3 ± 0.56 |
| Nelfinavir | Lopinavir/ritonavir | Ritonavir | ||||
| Characteristic | With buprenorphine/naloxonea (n = 10) | Control (n = 16) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) |
| Age, years | 34 ± 2.5 | 41 ± 2.1 | 34 ± 2.7 | 38 ± 2.3 | 37 ± 2.4 | 40 ± 2.3 |
| Weight, kg | 77.2 ± 4.6 | 84.2 ± 4.5 | 84.1 ± 5.7 | 80.5 ± 3.9 | 83.1 ± 5.2 | 77.4 ± 4.0 |
| Buprenorphine/naloxone dose, mg/day | 16/4 | NA | 16/4 | NA | 16/4 | NA |
| Female sex, no. | 5 | 6 | 4 | 7 | 5 | 6 |
| Race, no. | ||||||
| African American | 6 | 6 | 8 | 6 | 7 | 6 |
| White | 3 | 6 | 1 | 4 | 2 | 6 |
| Hispanic | 0 | 3 | 0 | 5 | 0 | 3 |
| Mixed | 1 | 1 | 1 | 0 | 1 | 0 |
| Substance use disorder, no. | ||||||
| Opioid dependence | 10 | 0 | 10 | 0 | 10 | 0 |
| Cocaine abuse | 5 | 5 | 3 | 3 | 5 | 2 |
| Cannabis abuse | 5 | 0 | 1 | 2 | 2 | 1 |
| Alcohol abuse | 1 | 4 | 1 | 0 | 1 | 0 |
| Injection drug use, no. (%) | 5 (50) | NA | 2 (20) | NA | 4 (40) | NA |
| Nicotine use | ||||||
| Packs/day | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 |
| Total years smoking | 11.8 ± 3.1 | 10.8 ± 2.8 | 10.9 ± 3.5 | 7.3 ± 2.8 | 12.6 ± 3.9 | 8.3 ± 3.2 |
| HCV positive, no. (%) | 3 (30) | 0 | 0 | 0 | 3 (30) | 0 |
| AST concentration,b U/L | ||||||
| Before administration | 30.8 ± 5.5 | 23.6 ± 1.6 | 29.3 ± 2.6 | 22.5 ± 1.9 | 31.0 ± 3.7 | 20.9 ± 2.1 |
| After administration | 26.1 ± 3.1 | 28.0 ± 6.2 | 23.3 ± 1.8 | 18.3 ± 1.3c | 28.7 ± 4.6 | 21.2 ± 4.5 |
| ALT concentration,b U/L | ||||||
| Before administration | 34.6 ± 5.6 | 24.4 ± 3.4 | 32.6 ± 6.1 | 28.3 ± 4.3 | 29.2 ± 2.7 | 20.5 ± 3.8 |
| After administration | 31.6 ± 4.7 | 25.7 ± 5.9 | 23.3 ± 3.0 | 17.5 ± 1.9d | 25.0 ± 2.9 | 15.9 ± 2.4 |
| Adverse symptoms, ASC score | ||||||
| Before administration | 7.5 ± 1.7 | 4.3 ± 0.9 | 7.6 ± 2.4 | 2.1 ± 1.4 | 8.1 ± 2.7 | 1.6 ± 0.61 |
| After administration | 10.3 ± 3.1 | 5.2 ± 1.0 | 4.9 ± 2.0 | 5.7 ± 1.2 | 7.6 ± 2.2 | 1.3 ± 0.56 |
NOTE. Data are mean ± SE, unless otherwise indicated. ALT, alanine aminotransferase; ASC, Adverse Symptoms Checklist; AST, aspart ateaminotransferase; HCV, hepatitis Cvirus; NA, not applicable.
Buprenorphine/naloxone maintenance treatment.
Normal range, 0–35U/L.
P = .04.
P =.01.
Characteristics of study participants, by study group.
| Nelfinavir | Lopinavir/ritonavir | Ritonavir | ||||
| Characteristic | With buprenorphine/naloxonea (n = 10) | Control (n = 16) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) |
| Age, years | 34 ± 2.5 | 41 ± 2.1 | 34 ± 2.7 | 38 ± 2.3 | 37 ± 2.4 | 40 ± 2.3 |
| Weight, kg | 77.2 ± 4.6 | 84.2 ± 4.5 | 84.1 ± 5.7 | 80.5 ± 3.9 | 83.1 ± 5.2 | 77.4 ± 4.0 |
| Buprenorphine/naloxone dose, mg/day | 16/4 | NA | 16/4 | NA | 16/4 | NA |
| Female sex, no. | 5 | 6 | 4 | 7 | 5 | 6 |
| Race, no. | ||||||
| African American | 6 | 6 | 8 | 6 | 7 | 6 |
| White | 3 | 6 | 1 | 4 | 2 | 6 |
| Hispanic | 0 | 3 | 0 | 5 | 0 | 3 |
| Mixed | 1 | 1 | 1 | 0 | 1 | 0 |
| Substance use disorder, no. | ||||||
| Opioid dependence | 10 | 0 | 10 | 0 | 10 | 0 |
| Cocaine abuse | 5 | 5 | 3 | 3 | 5 | 2 |
| Cannabis abuse | 5 | 0 | 1 | 2 | 2 | 1 |
| Alcohol abuse | 1 | 4 | 1 | 0 | 1 | 0 |
| Injection drug use, no. (%) | 5 (50) | NA | 2 (20) | NA | 4 (40) | NA |
| Nicotine use | ||||||
| Packs/day | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 |
| Total years smoking | 11.8 ± 3.1 | 10.8 ± 2.8 | 10.9 ± 3.5 | 7.3 ± 2.8 | 12.6 ± 3.9 | 8.3 ± 3.2 |
| HCV positive, no. (%) | 3 (30) | 0 | 0 | 0 | 3 (30) | 0 |
| AST concentration,b U/L | ||||||
| Before administration | 30.8 ± 5.5 | 23.6 ± 1.6 | 29.3 ± 2.6 | 22.5 ± 1.9 | 31.0 ± 3.7 | 20.9 ± 2.1 |
| After administration | 26.1 ± 3.1 | 28.0 ± 6.2 | 23.3 ± 1.8 | 18.3 ± 1.3c | 28.7 ± 4.6 | 21.2 ± 4.5 |
| ALT concentration,b U/L | ||||||
| Before administration | 34.6 ± 5.6 | 24.4 ± 3.4 | 32.6 ± 6.1 | 28.3 ± 4.3 | 29.2 ± 2.7 | 20.5 ± 3.8 |
| After administration | 31.6 ± 4.7 | 25.7 ± 5.9 | 23.3 ± 3.0 | 17.5 ± 1.9d | 25.0 ± 2.9 | 15.9 ± 2.4 |
| Adverse symptoms, ASC score | ||||||
| Before administration | 7.5 ± 1.7 | 4.3 ± 0.9 | 7.6 ± 2.4 | 2.1 ± 1.4 | 8.1 ± 2.7 | 1.6 ± 0.61 |
| After administration | 10.3 ± 3.1 | 5.2 ± 1.0 | 4.9 ± 2.0 | 5.7 ± 1.2 | 7.6 ± 2.2 | 1.3 ± 0.56 |
| Nelfinavir | Lopinavir/ritonavir | Ritonavir | ||||
| Characteristic | With buprenorphine/naloxonea (n = 10) | Control (n = 16) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) | With buprenorphine/naloxonea (n = 10) | Control (n = 15) |
| Age, years | 34 ± 2.5 | 41 ± 2.1 | 34 ± 2.7 | 38 ± 2.3 | 37 ± 2.4 | 40 ± 2.3 |
| Weight, kg | 77.2 ± 4.6 | 84.2 ± 4.5 | 84.1 ± 5.7 | 80.5 ± 3.9 | 83.1 ± 5.2 | 77.4 ± 4.0 |
| Buprenorphine/naloxone dose, mg/day | 16/4 | NA | 16/4 | NA | 16/4 | NA |
| Female sex, no. | 5 | 6 | 4 | 7 | 5 | 6 |
| Race, no. | ||||||
| African American | 6 | 6 | 8 | 6 | 7 | 6 |
| White | 3 | 6 | 1 | 4 | 2 | 6 |
| Hispanic | 0 | 3 | 0 | 5 | 0 | 3 |
| Mixed | 1 | 1 | 1 | 0 | 1 | 0 |
| Substance use disorder, no. | ||||||
| Opioid dependence | 10 | 0 | 10 | 0 | 10 | 0 |
| Cocaine abuse | 5 | 5 | 3 | 3 | 5 | 2 |
| Cannabis abuse | 5 | 0 | 1 | 2 | 2 | 1 |
| Alcohol abuse | 1 | 4 | 1 | 0 | 1 | 0 |
| Injection drug use, no. (%) | 5 (50) | NA | 2 (20) | NA | 4 (40) | NA |
| Nicotine use | ||||||
| Packs/day | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 |
| Total years smoking | 11.8 ± 3.1 | 10.8 ± 2.8 | 10.9 ± 3.5 | 7.3 ± 2.8 | 12.6 ± 3.9 | 8.3 ± 3.2 |
| HCV positive, no. (%) | 3 (30) | 0 | 0 | 0 | 3 (30) | 0 |
| AST concentration,b U/L | ||||||
| Before administration | 30.8 ± 5.5 | 23.6 ± 1.6 | 29.3 ± 2.6 | 22.5 ± 1.9 | 31.0 ± 3.7 | 20.9 ± 2.1 |
| After administration | 26.1 ± 3.1 | 28.0 ± 6.2 | 23.3 ± 1.8 | 18.3 ± 1.3c | 28.7 ± 4.6 | 21.2 ± 4.5 |
| ALT concentration,b U/L | ||||||
| Before administration | 34.6 ± 5.6 | 24.4 ± 3.4 | 32.6 ± 6.1 | 28.3 ± 4.3 | 29.2 ± 2.7 | 20.5 ± 3.8 |
| After administration | 31.6 ± 4.7 | 25.7 ± 5.9 | 23.3 ± 3.0 | 17.5 ± 1.9d | 25.0 ± 2.9 | 15.9 ± 2.4 |
| Adverse symptoms, ASC score | ||||||
| Before administration | 7.5 ± 1.7 | 4.3 ± 0.9 | 7.6 ± 2.4 | 2.1 ± 1.4 | 8.1 ± 2.7 | 1.6 ± 0.61 |
| After administration | 10.3 ± 3.1 | 5.2 ± 1.0 | 4.9 ± 2.0 | 5.7 ± 1.2 | 7.6 ± 2.2 | 1.3 ± 0.56 |
NOTE. Data are mean ± SE, unless otherwise indicated. ALT, alanine aminotransferase; ASC, Adverse Symptoms Checklist; AST, aspart ateaminotransferase; HCV, hepatitis Cvirus; NA, not applicable.
Buprenorphine/naloxone maintenance treatment.
Normal range, 0–35U/L.
P = .04.
P =.01.
Interaction between Buprenorphine and PIs
Effect of NFV on buprenorphine
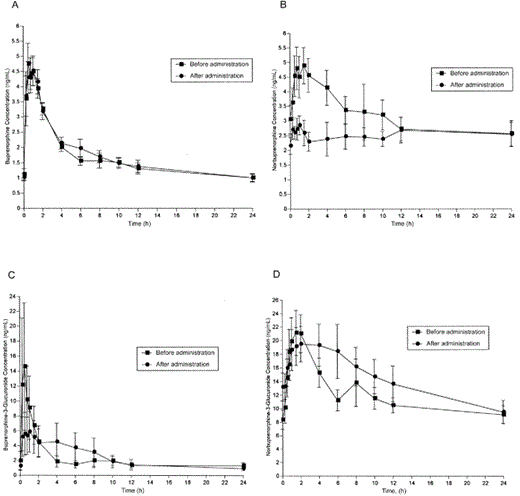
The pharmacokinetic parameters of buprenorphine before and after NFV administration are shown in table 2. Figure 1 graphically represents concentrations of buprenorphine; the active metabolite, norbuprenorphine; and the conjugated metabolites buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, over a 24-h dosing interval. Coadministration of NFV with buprenorphine for 5 days had few effects on the pharmacokinetics of buprenorphine and its metabolites. The AUC0–24 and Cmax values for norbuprenorphine were modestly decreased, but only the decrease in Cmax was statistically significant. Increases in Tmax for norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide were statistically significant but clinically inconsequential. Consistent with the minimal effects of NFV on buprenorphine disposition, there were no significant changes in the amounts of buprenorphine and its metabolites recovered in the urine over the dosing interval, nor were there significant changes in the respective renal clearance values (data not shown).
Effect of nelfinavir (NFV) on buprenorphine and buprenorphine metabolites.
| Substance, pharmacokinetic parameter | Value before NFV administration | Value after NFV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.5 ± 3.3 | 41.2 ± 4.3 | NS |
| Cl/F, L/h | 428.8 ± 33.0 | 441.8 ± 61.7 | NS |
| Cmax, ng/mL | 5.9 ± 0.74 | 5.1 ± 0.47 | NS |
| Tmax, h | 0.875 (0.25–2.0) | 0.875 (0.25–1.5) | NS |
| Cmin, ng/mL | 0.84 ± 0.09 | 0.93 ± 0.13 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 75.4 ± 10.5 | 60.8 ± 8.9 | NS |
| Cl/F, L/h | 255.1 ± 36.4 | 305.5 ± 36.3 | NS |
| Cmax, ng/mL | 5.8 ± 0.81 | 3.5 ± 0.49 | .04 |
| Tmax, h | 1.25 (0.25–8.0) | 2.5 (0.25–24.0) | .05 |
| Cmin, ng/mL | 2.1 ± 0.32 | 1.8 ± 0.24 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 53.5 ± 21.1 | 55.4 ± 27.2 | NS |
| Cl/F, L/h | 540.3 ± 89.3 | 740.1 ± 190.6 | NS |
| Cmax, ng/mL | 15.7 ± 27.0 | 6.4 ± 2.8 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 288.1 ± 37.1 | 344.5 ± 56.0 | NS |
| Cl/F, L/h | 63.1 ± 6.8 | 59.4 ± 10.1 | NS |
| Cmax, ng/mL | 23.3 ± 3.5 | 23.1 ± 3.5 | NS |
| Tmax, h | 2.3 (1.0–8.0) | 3.4 (1.0–8.0) | .05 |
| Substance, pharmacokinetic parameter | Value before NFV administration | Value after NFV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.5 ± 3.3 | 41.2 ± 4.3 | NS |
| Cl/F, L/h | 428.8 ± 33.0 | 441.8 ± 61.7 | NS |
| Cmax, ng/mL | 5.9 ± 0.74 | 5.1 ± 0.47 | NS |
| Tmax, h | 0.875 (0.25–2.0) | 0.875 (0.25–1.5) | NS |
| Cmin, ng/mL | 0.84 ± 0.09 | 0.93 ± 0.13 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 75.4 ± 10.5 | 60.8 ± 8.9 | NS |
| Cl/F, L/h | 255.1 ± 36.4 | 305.5 ± 36.3 | NS |
| Cmax, ng/mL | 5.8 ± 0.81 | 3.5 ± 0.49 | .04 |
| Tmax, h | 1.25 (0.25–8.0) | 2.5 (0.25–24.0) | .05 |
| Cmin, ng/mL | 2.1 ± 0.32 | 1.8 ± 0.24 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 53.5 ± 21.1 | 55.4 ± 27.2 | NS |
| Cl/F, L/h | 540.3 ± 89.3 | 740.1 ± 190.6 | NS |
| Cmax, ng/mL | 15.7 ± 27.0 | 6.4 ± 2.8 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 288.1 ± 37.1 | 344.5 ± 56.0 | NS |
| Cl/F, L/h | 63.1 ± 6.8 | 59.4 ± 10.1 | NS |
| Cmax, ng/mL | 23.3 ± 3.5 | 23.1 ± 3.5 | NS |
| Tmax, h | 2.3 (1.0–8.0) | 3.4 (1.0–8.0) | .05 |
NOTE. Data are mean ± SEfor 10 participants who participated in both sessions,with the exception oftime of maximum concentration(Tmax), for which dataare median (range). Student's paired t test wasused to determine Pvalues for all parameters except Tmax, for which the Wilcoxon test was used. AUC0–24, 24-h areaunder the concentration-time curve;Cmax, maximum concentration inplasma; Cmin, minimum concentrationin plasma; Cl/F, clearance; NS, not significant.
Effect of nelfinavir (NFV) on buprenorphine and buprenorphine metabolites.
| Substance, pharmacokinetic parameter | Value before NFV administration | Value after NFV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.5 ± 3.3 | 41.2 ± 4.3 | NS |
| Cl/F, L/h | 428.8 ± 33.0 | 441.8 ± 61.7 | NS |
| Cmax, ng/mL | 5.9 ± 0.74 | 5.1 ± 0.47 | NS |
| Tmax, h | 0.875 (0.25–2.0) | 0.875 (0.25–1.5) | NS |
| Cmin, ng/mL | 0.84 ± 0.09 | 0.93 ± 0.13 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 75.4 ± 10.5 | 60.8 ± 8.9 | NS |
| Cl/F, L/h | 255.1 ± 36.4 | 305.5 ± 36.3 | NS |
| Cmax, ng/mL | 5.8 ± 0.81 | 3.5 ± 0.49 | .04 |
| Tmax, h | 1.25 (0.25–8.0) | 2.5 (0.25–24.0) | .05 |
| Cmin, ng/mL | 2.1 ± 0.32 | 1.8 ± 0.24 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 53.5 ± 21.1 | 55.4 ± 27.2 | NS |
| Cl/F, L/h | 540.3 ± 89.3 | 740.1 ± 190.6 | NS |
| Cmax, ng/mL | 15.7 ± 27.0 | 6.4 ± 2.8 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 288.1 ± 37.1 | 344.5 ± 56.0 | NS |
| Cl/F, L/h | 63.1 ± 6.8 | 59.4 ± 10.1 | NS |
| Cmax, ng/mL | 23.3 ± 3.5 | 23.1 ± 3.5 | NS |
| Tmax, h | 2.3 (1.0–8.0) | 3.4 (1.0–8.0) | .05 |
| Substance, pharmacokinetic parameter | Value before NFV administration | Value after NFV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.5 ± 3.3 | 41.2 ± 4.3 | NS |
| Cl/F, L/h | 428.8 ± 33.0 | 441.8 ± 61.7 | NS |
| Cmax, ng/mL | 5.9 ± 0.74 | 5.1 ± 0.47 | NS |
| Tmax, h | 0.875 (0.25–2.0) | 0.875 (0.25–1.5) | NS |
| Cmin, ng/mL | 0.84 ± 0.09 | 0.93 ± 0.13 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 75.4 ± 10.5 | 60.8 ± 8.9 | NS |
| Cl/F, L/h | 255.1 ± 36.4 | 305.5 ± 36.3 | NS |
| Cmax, ng/mL | 5.8 ± 0.81 | 3.5 ± 0.49 | .04 |
| Tmax, h | 1.25 (0.25–8.0) | 2.5 (0.25–24.0) | .05 |
| Cmin, ng/mL | 2.1 ± 0.32 | 1.8 ± 0.24 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 53.5 ± 21.1 | 55.4 ± 27.2 | NS |
| Cl/F, L/h | 540.3 ± 89.3 | 740.1 ± 190.6 | NS |
| Cmax, ng/mL | 15.7 ± 27.0 | 6.4 ± 2.8 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 288.1 ± 37.1 | 344.5 ± 56.0 | NS |
| Cl/F, L/h | 63.1 ± 6.8 | 59.4 ± 10.1 | NS |
| Cmax, ng/mL | 23.3 ± 3.5 | 23.1 ± 3.5 | NS |
| Tmax, h | 2.3 (1.0–8.0) | 3.4 (1.0–8.0) | .05 |
NOTE. Data are mean ± SEfor 10 participants who participated in both sessions,with the exception oftime of maximum concentration(Tmax), for which dataare median (range). Student's paired t test wasused to determine Pvalues for all parameters except Tmax, for which the Wilcoxon test was used. AUC0–24, 24-h areaunder the concentration-time curve;Cmax, maximum concentration inplasma; Cmin, minimum concentrationin plasma; Cl/F, clearance; NS, not significant.
Effect of nelfinavir on buprenorphine (A), norbuprenorphine (B), buprenorphine-3-glucuronide (C), and norbuprenorphine-3-glucuronide (D)
Buprenorphine/naloxone-maintained participants showed no evidence of opiate withdrawal symptoms with NFV coadministration (mean Objective Opiate Withdrawal Scale score ± SE: before NFV administration, 0 ± 0.1; after NFV administration, 0.2 ± 0.2; P value not significant), and no cognitive deficits were detected (mean Mini-Mental State Examination score ± SE: before NFV administration, 29.3 ± 0.2; after NFV administration, 29.5 ± 0.2). NFV administration to study participants (buprenorphine/naloxone-maintained or control participants) had no significant effect on hepatic enzyme activity (aspartate aminotransferase or alanine aminotransferase) (table 1). Adverse symptoms were infrequent and minimal in intensity (buprenorphine/naloxone-maintained group: before NFV administration, 7.5 ± 1.7; after NFV administration, 10.3 ± 3.1; control group: before NFV administration, 4.3 ± 0.9; after NFV administration, 5.2 ± 1.0 [data are mean ASC score ± SE; scale range, 0–87]). The most frequently reported adverse symptoms in buprenorphine/naloxone-maintained individuals were constipation and increased appetite, each of which nonsignificantly decreased with NFV administration. Symptoms that nonsignificantly increased with NFV administration included headache, nausea, drowsiness, urination frequency, sweating, and delayed orgasm. Control participants reported a significant increase in diarrhea (mean ASC score ± SE: before NFV administration, 0.07; after NFV administration, 1.0 ± 0.9; P = .001) and a nonsignificant increase in dry mouth, whereas the symptom of “poor memory” nonsignificantly decreased after NFV administration.
Effect of RTV on buprenorphine
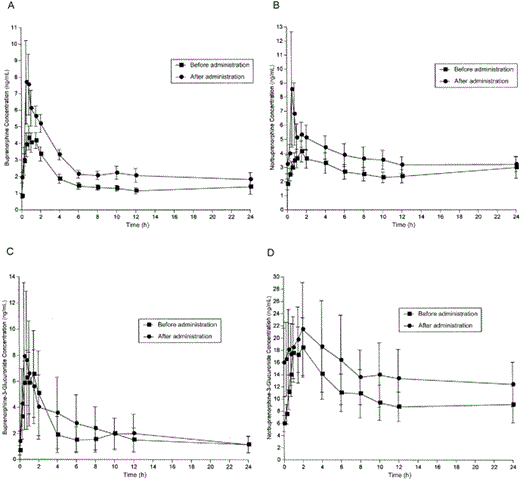
RTV administration to buprenorphine/naloxone-maintained study participants was associated with a significant increase in the buprenorphine AUC0–24 (P = .02), representing an ∼57% increase in the average buprenorphine AUC0–24 (figure 2 and table 3). Correspondingly, the clearance of buprenorphine significantly decreased (P = .008), with a trend for increased Cmax, from a mean of 5.4 ng/mL to 9.6 ng/mL (P = .09). The norbuprenorphine AUC0–24 also significantly increased (P = .05), whereas clearance significantly decreased (P = .05). RTV had no significant effect on buprenorphine-3-glucuronide or norbuprenorphine-3-glucuronide concentration. RTV produced increases in the amounts of buprenorphine and all of its metabolites that were recovered in the urine, with the largest increase occurring in the recovery of buprenorphine; however, none of the increases reached statistical significance (data not shown).
Effect of ritonavir on buprenorphine (A), norbuprenorphine (B), buprenorphine-3-glucuronide (C), and norbuprenorphine-3-glucuronide (D)
Effect of ritonavir (RTV) on buprenorphine and buprenorphine metabolites.
| Substance, pharmacokinetic parameter | Value before RTV administration | Value after RTV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.0 ± 5.7 | 61.4 ± 8.5 | .02 |
| Cl/F, L/h | 495.0 ± 71.5 | 303.3 ± 37.3 | .008 |
| Cmax, ng/mL | 5.4 ± 0.84 | 9.6 ± 2.3 | NS |
| Tmax, h | 1.0 (0.25–2.0) | 1.0 (0.25–2.0) | NS |
| Cmin, ng/mL | 0.78 ± 0.13 | 1.31 ± 0.13 | .002 |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 66.6 ± 12.9 | 88.5 ± 15.0 | .05 |
| Cl/F, L/h | 334.8 ± 71.1 | 221.1 ± 32.5 | .05 |
| Cmax, ng/mL | 4.7 ± 0.87 | 9.8 ± 4.0 | NS |
| Tmax, h | 1.75 (0.25–8.0) | 1.5 (0.25–2.0) | .05 |
| Cmin, ng/mL | 1.67 ± 0.35 | 2.48 ± 0.49 | .002 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 48.1 ± 26.6 | 58.5 ± 38.8 | NS |
| Cl/F, L/h | 1073.5 ± 341.9 | 1135.7 ± 341.9 | NS |
| Cmax, ng/mL | 8.7 ± 3.1 | 8.9 ± 5.5 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–2.0) | NS |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 253.7 ± 73.2 | 351.8 ± 121.4 | NS |
| Cl/F, L/h | 105.1 ± 21.5 | 84.5 ± 18.0 | NS |
| Cmax, ng/mL | 19.6 ± 5.1 | 23.6 ± 7.5 | NS |
| Tmax, h | 2.0 (0.5–4.0) | 1.75 (0.5–24.0) | NS |
| Substance, pharmacokinetic parameter | Value before RTV administration | Value after RTV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.0 ± 5.7 | 61.4 ± 8.5 | .02 |
| Cl/F, L/h | 495.0 ± 71.5 | 303.3 ± 37.3 | .008 |
| Cmax, ng/mL | 5.4 ± 0.84 | 9.6 ± 2.3 | NS |
| Tmax, h | 1.0 (0.25–2.0) | 1.0 (0.25–2.0) | NS |
| Cmin, ng/mL | 0.78 ± 0.13 | 1.31 ± 0.13 | .002 |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 66.6 ± 12.9 | 88.5 ± 15.0 | .05 |
| Cl/F, L/h | 334.8 ± 71.1 | 221.1 ± 32.5 | .05 |
| Cmax, ng/mL | 4.7 ± 0.87 | 9.8 ± 4.0 | NS |
| Tmax, h | 1.75 (0.25–8.0) | 1.5 (0.25–2.0) | .05 |
| Cmin, ng/mL | 1.67 ± 0.35 | 2.48 ± 0.49 | .002 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 48.1 ± 26.6 | 58.5 ± 38.8 | NS |
| Cl/F, L/h | 1073.5 ± 341.9 | 1135.7 ± 341.9 | NS |
| Cmax, ng/mL | 8.7 ± 3.1 | 8.9 ± 5.5 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–2.0) | NS |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 253.7 ± 73.2 | 351.8 ± 121.4 | NS |
| Cl/F, L/h | 105.1 ± 21.5 | 84.5 ± 18.0 | NS |
| Cmax, ng/mL | 19.6 ± 5.1 | 23.6 ± 7.5 | NS |
| Tmax, h | 2.0 (0.5–4.0) | 1.75 (0.5–24.0) | NS |
NOTE. Data are mean ± SE for 10 participants who participated in both sessions, with the exception of time of maximum concentration (Tmax), for which data are median (range). Student's paired t test was used todetermine P values forall parameters except Tmax,for which the Wilcoxon test was used. AUC0–24, 24-h area under theconcentration-time curve; Cmax, maximumcon centration in plasma; Cmin, minimum concentration in plasma; Cl/F, clearance; NS, not significant.
Effect of ritonavir (RTV) on buprenorphine and buprenorphine metabolites.
| Substance, pharmacokinetic parameter | Value before RTV administration | Value after RTV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.0 ± 5.7 | 61.4 ± 8.5 | .02 |
| Cl/F, L/h | 495.0 ± 71.5 | 303.3 ± 37.3 | .008 |
| Cmax, ng/mL | 5.4 ± 0.84 | 9.6 ± 2.3 | NS |
| Tmax, h | 1.0 (0.25–2.0) | 1.0 (0.25–2.0) | NS |
| Cmin, ng/mL | 0.78 ± 0.13 | 1.31 ± 0.13 | .002 |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 66.6 ± 12.9 | 88.5 ± 15.0 | .05 |
| Cl/F, L/h | 334.8 ± 71.1 | 221.1 ± 32.5 | .05 |
| Cmax, ng/mL | 4.7 ± 0.87 | 9.8 ± 4.0 | NS |
| Tmax, h | 1.75 (0.25–8.0) | 1.5 (0.25–2.0) | .05 |
| Cmin, ng/mL | 1.67 ± 0.35 | 2.48 ± 0.49 | .002 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 48.1 ± 26.6 | 58.5 ± 38.8 | NS |
| Cl/F, L/h | 1073.5 ± 341.9 | 1135.7 ± 341.9 | NS |
| Cmax, ng/mL | 8.7 ± 3.1 | 8.9 ± 5.5 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–2.0) | NS |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 253.7 ± 73.2 | 351.8 ± 121.4 | NS |
| Cl/F, L/h | 105.1 ± 21.5 | 84.5 ± 18.0 | NS |
| Cmax, ng/mL | 19.6 ± 5.1 | 23.6 ± 7.5 | NS |
| Tmax, h | 2.0 (0.5–4.0) | 1.75 (0.5–24.0) | NS |
| Substance, pharmacokinetic parameter | Value before RTV administration | Value after RTV administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 39.0 ± 5.7 | 61.4 ± 8.5 | .02 |
| Cl/F, L/h | 495.0 ± 71.5 | 303.3 ± 37.3 | .008 |
| Cmax, ng/mL | 5.4 ± 0.84 | 9.6 ± 2.3 | NS |
| Tmax, h | 1.0 (0.25–2.0) | 1.0 (0.25–2.0) | NS |
| Cmin, ng/mL | 0.78 ± 0.13 | 1.31 ± 0.13 | .002 |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 66.6 ± 12.9 | 88.5 ± 15.0 | .05 |
| Cl/F, L/h | 334.8 ± 71.1 | 221.1 ± 32.5 | .05 |
| Cmax, ng/mL | 4.7 ± 0.87 | 9.8 ± 4.0 | NS |
| Tmax, h | 1.75 (0.25–8.0) | 1.5 (0.25–2.0) | .05 |
| Cmin, ng/mL | 1.67 ± 0.35 | 2.48 ± 0.49 | .002 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 48.1 ± 26.6 | 58.5 ± 38.8 | NS |
| Cl/F, L/h | 1073.5 ± 341.9 | 1135.7 ± 341.9 | NS |
| Cmax, ng/mL | 8.7 ± 3.1 | 8.9 ± 5.5 | NS |
| Tmax, h | 0.875 (0.25–1.5) | 1.25 (0.25–2.0) | NS |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 253.7 ± 73.2 | 351.8 ± 121.4 | NS |
| Cl/F, L/h | 105.1 ± 21.5 | 84.5 ± 18.0 | NS |
| Cmax, ng/mL | 19.6 ± 5.1 | 23.6 ± 7.5 | NS |
| Tmax, h | 2.0 (0.5–4.0) | 1.75 (0.5–24.0) | NS |
NOTE. Data are mean ± SE for 10 participants who participated in both sessions, with the exception of time of maximum concentration (Tmax), for which data are median (range). Student's paired t test was used todetermine P values forall parameters except Tmax,for which the Wilcoxon test was used. AUC0–24, 24-h area under theconcentration-time curve; Cmax, maximumcon centration in plasma; Cmin, minimum concentration in plasma; Cl/F, clearance; NS, not significant.
RTV administration in buprenorphine/naloxone-maintained study participants was not associated with opiate withdrawal symptoms (mean Objective Opiate Withdrawal Scale score: before RTV administration, 0; after RTV administration, 0) or impaired cognition (mean Mini-Mental State Examination score ± SE: before RTV administration, 29.1 ± 0.3; after RTV administration, 29.5 ± 0.2). RTV administration had no significant effect on hepatic enzyme activity (aspartate aminotransferase or alanine aminotransferase) in buprenorphine/naloxone-maintained participants or control participants (table 1). The adverse symptoms experienced were few and of low severity (buprenorphine/naloxone-maintained group: before RTV administration, 8.1 ± 2.7; after RTV administration, 7.6 ± 2.2; control group: before RTV administration, 1.6 ± 0.6; after RTV administration, 1.3 ± 0.6 [data are mean ASC score ± SE]). For buprenorphine/naloxone-maintained individuals, the most frequently reported adverse symptoms were increased appetite and constipation, neither of which changed significantly with RTV administration. Among control participants administered RTV, very few adverse symptoms were reported. Those that were reported included headache, diarrhea, muscle stiffness, and increased appetite, but the severity of these symptoms was minimal. There were no statistically significant changes in severity of any adverse symptom.
Effect of LPV/R on buprenorphine
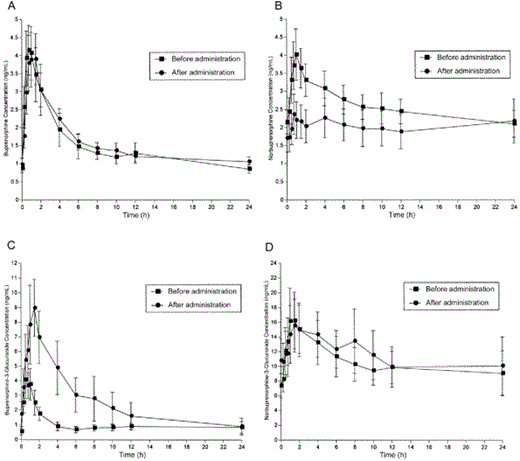
LPV/R administration to buprenorphine/naloxone-maintained study participants produced no significant changes in the pharmacokinetics of buprenorphine, except for an increase in Tmax of no clinical importance (figure 3A and table 4). LPV/R administration was associated with a significant decrease in norbuprenorphine Cmax, a nonsignificant decrease in the norbuprenorphine AUC0–24, and a corresponding increase in norbuprenorphine Cl/F (figure 3B and table 4). The mean AUC0–24 of buprenorphine-3-glucuronide more than doubled (figure 3C and table 4) (although the effect was not statistically significant, because of the large SD in the post-LPV/R results). At the same time, Cl/F decreased by 42% (P = .004), and the Cmax for buprenorphine-3-glucuronide approximately doubled (P = .02). Norbuprenorphine-3-glucuronide concentrations were not significantly altered after LPV/R administration. As was true with RTV alone, urinary recovery was increased for buprenorphine and all of its metabolites after LPV/R administration. Only the increase in buprenorphine-3-glucuronide was statistically significant (before LPV/R administration, 0.947 ± 0.137 ng/mL; after LPV/R administration, 1.65 ± 0.23 ng/mL; P = .004).
Effect of lopinavir/ritonavir on buprenorphine (A), norbuprenorphine (B), buprenorphine-3-glucuronide (C), and norbuprenorphine-3-glucuronide (D).
Effect of lopinavir/ritonavir (LPV/R) on buprenorphine and buprenorphine metabolites.
| Substance, pharmacokinetic parameter | Value before LPV/R administration | Value after LPV/R administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 35.7 ± 4.5 | 37.4 ± 4.7 | NS |
| Cl/F, L/h | 558.3 ± 110.4 | 522.5 ± 91.6 | NS |
| Cmax, ng/mL | 5.0 ± 0.70 | 4.3 ± 0.76 | NS |
| Tmax, h | 1.0 (0.5–12.0) | 1.25 (0.5–2.0) | .05 |
| Cmin, ng/mL | 0.75 ± 0.12 | 0.81 ± 0.11 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 61.6 ± 8.8 | 48.9 ± 12.0 | NS |
| Cl/F, L/h | 415 ± 161 | 496 ± 94 | NS |
| Cmax, ng/mL | 4.2 ± 0.68 | 2.9 ± 0.57 | .022 |
| Tmax, h | 1.0 (0.0–8.0) | 2.75 (0.5–24.0) | .05 |
| Cmin, ng/mL | 1.84 ± 0.32 | 1.40 ± 0.39 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 25.6 ± 6.3 | 62.4 ± 42.0 | NS |
| Cl/F, L/h | 911 ± 183 | 526 ± 144 | .004 |
| Cmax, ng/mL | 5.5 ± 1.2 | 11.3 ± 2.6 | .02 |
| Tmax, h | 1.0 (0.5–12.0) | 1.5 (0.75–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 254.5 ± 60.0 | 274.9 ± 76.1 | NS |
| Cl/F, L/h | 101 ± 26 | 99 ± 21 | NS |
| Cmax, ng/mL | 19.2 ± 4.4 | 18.3 ± 4.7 | NS |
| Tmax, h | 1.75 (0.75–24.0) | 4.0 (1.5–8.0) | NS |
| Substance, pharmacokinetic parameter | Value before LPV/R administration | Value after LPV/R administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 35.7 ± 4.5 | 37.4 ± 4.7 | NS |
| Cl/F, L/h | 558.3 ± 110.4 | 522.5 ± 91.6 | NS |
| Cmax, ng/mL | 5.0 ± 0.70 | 4.3 ± 0.76 | NS |
| Tmax, h | 1.0 (0.5–12.0) | 1.25 (0.5–2.0) | .05 |
| Cmin, ng/mL | 0.75 ± 0.12 | 0.81 ± 0.11 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 61.6 ± 8.8 | 48.9 ± 12.0 | NS |
| Cl/F, L/h | 415 ± 161 | 496 ± 94 | NS |
| Cmax, ng/mL | 4.2 ± 0.68 | 2.9 ± 0.57 | .022 |
| Tmax, h | 1.0 (0.0–8.0) | 2.75 (0.5–24.0) | .05 |
| Cmin, ng/mL | 1.84 ± 0.32 | 1.40 ± 0.39 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 25.6 ± 6.3 | 62.4 ± 42.0 | NS |
| Cl/F, L/h | 911 ± 183 | 526 ± 144 | .004 |
| Cmax, ng/mL | 5.5 ± 1.2 | 11.3 ± 2.6 | .02 |
| Tmax, h | 1.0 (0.5–12.0) | 1.5 (0.75–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 254.5 ± 60.0 | 274.9 ± 76.1 | NS |
| Cl/F, L/h | 101 ± 26 | 99 ± 21 | NS |
| Cmax, ng/mL | 19.2 ± 4.4 | 18.3 ± 4.7 | NS |
| Tmax, h | 1.75 (0.75–24.0) | 4.0 (1.5–8.0) | NS |
NOTE. Data are mean ± SE for 10 participants who participated in both sessions, with the exception oftime of maximum concentration(Tmax), for which data are median (range). Student's paired t test wasused to determine P values for all parameters except Tmax, for which the Wilcoxon test was used. AUC0–24, 24-h area under the concentration-time curve; Cmax, maximum concentration inplasma; Cmin, minimum concentrationin plasma; Cl/F, clearance; NS, not significant.
Effect of lopinavir/ritonavir (LPV/R) on buprenorphine and buprenorphine metabolites.
| Substance, pharmacokinetic parameter | Value before LPV/R administration | Value after LPV/R administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 35.7 ± 4.5 | 37.4 ± 4.7 | NS |
| Cl/F, L/h | 558.3 ± 110.4 | 522.5 ± 91.6 | NS |
| Cmax, ng/mL | 5.0 ± 0.70 | 4.3 ± 0.76 | NS |
| Tmax, h | 1.0 (0.5–12.0) | 1.25 (0.5–2.0) | .05 |
| Cmin, ng/mL | 0.75 ± 0.12 | 0.81 ± 0.11 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 61.6 ± 8.8 | 48.9 ± 12.0 | NS |
| Cl/F, L/h | 415 ± 161 | 496 ± 94 | NS |
| Cmax, ng/mL | 4.2 ± 0.68 | 2.9 ± 0.57 | .022 |
| Tmax, h | 1.0 (0.0–8.0) | 2.75 (0.5–24.0) | .05 |
| Cmin, ng/mL | 1.84 ± 0.32 | 1.40 ± 0.39 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 25.6 ± 6.3 | 62.4 ± 42.0 | NS |
| Cl/F, L/h | 911 ± 183 | 526 ± 144 | .004 |
| Cmax, ng/mL | 5.5 ± 1.2 | 11.3 ± 2.6 | .02 |
| Tmax, h | 1.0 (0.5–12.0) | 1.5 (0.75–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 254.5 ± 60.0 | 274.9 ± 76.1 | NS |
| Cl/F, L/h | 101 ± 26 | 99 ± 21 | NS |
| Cmax, ng/mL | 19.2 ± 4.4 | 18.3 ± 4.7 | NS |
| Tmax, h | 1.75 (0.75–24.0) | 4.0 (1.5–8.0) | NS |
| Substance, pharmacokinetic parameter | Value before LPV/R administration | Value after LPV/R administration | P |
| Buprenorphine | |||
| AUC0–24, ng·h/mL | 35.7 ± 4.5 | 37.4 ± 4.7 | NS |
| Cl/F, L/h | 558.3 ± 110.4 | 522.5 ± 91.6 | NS |
| Cmax, ng/mL | 5.0 ± 0.70 | 4.3 ± 0.76 | NS |
| Tmax, h | 1.0 (0.5–12.0) | 1.25 (0.5–2.0) | .05 |
| Cmin, ng/mL | 0.75 ± 0.12 | 0.81 ± 0.11 | NS |
| Norbuprenorphine | |||
| AUC0–24, ng·h/mL | 61.6 ± 8.8 | 48.9 ± 12.0 | NS |
| Cl/F, L/h | 415 ± 161 | 496 ± 94 | NS |
| Cmax, ng/mL | 4.2 ± 0.68 | 2.9 ± 0.57 | .022 |
| Tmax, h | 1.0 (0.0–8.0) | 2.75 (0.5–24.0) | .05 |
| Cmin, ng/mL | 1.84 ± 0.32 | 1.40 ± 0.39 | NS |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 25.6 ± 6.3 | 62.4 ± 42.0 | NS |
| Cl/F, L/h | 911 ± 183 | 526 ± 144 | .004 |
| Cmax, ng/mL | 5.5 ± 1.2 | 11.3 ± 2.6 | .02 |
| Tmax, h | 1.0 (0.5–12.0) | 1.5 (0.75–4.0) | .05 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, ng·h/mL | 254.5 ± 60.0 | 274.9 ± 76.1 | NS |
| Cl/F, L/h | 101 ± 26 | 99 ± 21 | NS |
| Cmax, ng/mL | 19.2 ± 4.4 | 18.3 ± 4.7 | NS |
| Tmax, h | 1.75 (0.75–24.0) | 4.0 (1.5–8.0) | NS |
NOTE. Data are mean ± SE for 10 participants who participated in both sessions, with the exception oftime of maximum concentration(Tmax), for which data are median (range). Student's paired t test wasused to determine P values for all parameters except Tmax, for which the Wilcoxon test was used. AUC0–24, 24-h area under the concentration-time curve; Cmax, maximum concentration inplasma; Cmin, minimum concentrationin plasma; Cl/F, clearance; NS, not significant.
LPV/R administration in buprenorphine/naloxone-maintained study participants was not associated with opiate withdrawal symptoms (mean Objective Opiate Withdrawal Scale score: before LPV/R administration, 0; after LPV/R administration, 0) or impaired cognition (mean Mini-Mental State Examination score ± SE: before LPV/R administration, 29.4 ± 0.2; after LPV/R administration, 29.8 ± 0.1). LPV/R administration had no significant effect on hepatic enzyme activity (aspartate aminotransferase or alanine aminotransferase) in buprenorphine/naloxone-maintained participants, and control participants showed a statistically significant but clinically unimportant decrease in the concentrations of aspartate aminotransferase (before LPV/R administration, 23 ± 1.9 U/L; after LPV/R administration, 18 ± 1.3 U/L; P = .04) and alanine aminotransferase (before LPV/R administration, 28 ± 1.3 U/L; after LPV/R administration, 18 ± 1.9 U/L; P = .01; all values were within the normal range for these indexes of hepatic function) (table 1). Adverse event occurrence and severity were low for all participants (buprenorphine/naloxone group: before LPV/R administration, 7.6 ± 2.4; after LPV/R administration, 4.9 ± 2.0; control group: before LPV/R administration, 2.1 ± 1.4; after LPV/R administration, 5.7 ± 1.2 [data are mean ASC score ± SE]). For buprenorphine/naloxone-maintained participants, the most frequently reported adverse symptoms were headache, increased appetite, constipation, nightmares, and waking early, all of which decreased after LPV/R administration, with a statistically significant decrease in waking early (P = .025). Similarly, in control participants, few adverse symptoms were reported, but those that were included headache, diarrhea, nausea, dry mouth, and increased appetite. Minimal but significant increases in headache (mean ASC score ± SE: before LPV/R administration, 0; after LPV/R administration, 0.53 ± 0.52; P = .001) and dry mouth (mean ASC score ± SE: before LPV/R administration, 0.07 ± 0.26; after LPV/R administration, 0.67 ± 0.90; P = .023) were reported by control participants.
Effects of buprenorphine on PI pharmacokinetics
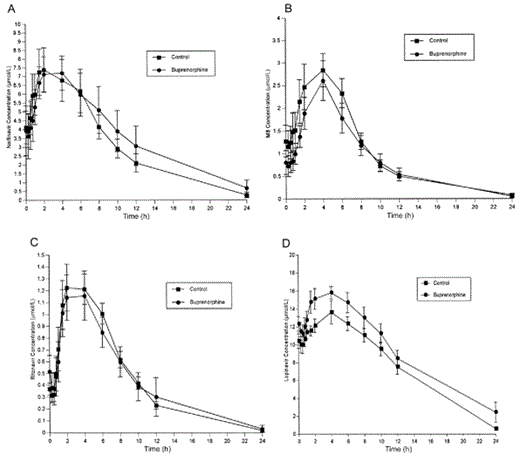
NFV and M8 concentrations were measured over a 24-h dosing interval in buprenorphine/naloxone-maintained individuals. No statistically significant changes in NFV or M8 pharmacokinetics were observed in comparison with those in control participants, except for a decrease in Cmax (figure 4A and 4B and table 5). Buprenorphine/naloxone administration had no effect on the disposition of RTV (figure 4C and table 5) but was associated with a significant increase in the LPV AUC0–12 and a decrease in LPV clearance (figure 4D and table 5). Concentrations of all 3 PIs remained in their therapeutic ranges during buprenorphine/naloxone treatment.
Effect of buprenorphine on nelfinavir (A), M8 (B), ritonavir (C), and lopinavir/ritonavir (D)
Effect of buprenorphine on nelfinavir, M8 metabolite, ritonavir, and lopinavir concentrations.
| Substance, pharmacokinetic parameter | Control group | Buprenorphine/naloxone group | P |
| Nelfinavir | |||
| AUC0–12, µmol/L·h | 91.9 ± 17.4 | 114.8 ± 23.1 | NS |
| Cl/F, L/h | 26.0 ± 9.4 | 36.0 ± 6.0 | NS |
| Cmax, µmol/L | 14.1 ± 2.3 | 8.5 ± 1.1 | .01 |
| Tmax, h | 2.0 ± 0.4 | 3.6 ± 0.6 | NS |
| Cmin, µmol/L | 2.1 ± 0.9 | 3.0 ± 1.1 | NS |
| M8 | |||
| AUC0–12, µmol/L·h | 30.9 ± 4.8 | 29.8 ± 5.3 | NS |
| Cmax, µmol/L | 5.1 ± 0.7 | 2.7 ± 0.3 | .01 |
| Tmax, h | 3.3 ± 0.4 | 3.8 ± 0.4 | NS |
| Cmin, µmol/L | 0.50 ± 0.3 | 0.55 ± 0.1 | NS |
| Ritonavir | |||
| AUC0–12, µmol/L·h | 9.43 ± 0.8 | 9.61 ± 1.2 | NS |
| Cl/F, L/h | 13.9 ± 1.3 | 13.7 ± 2.1 | NS |
| Cmax, µmol/L | 1.62 ± 0.1 | 1.58 ± 0.1 | NS |
| Tmax, h | 3.21 ± 0.4 | 4.04 ± 1.1 | NS |
| Cmin, µmol/L | 0.22 ± 0.03 | 0.37 ± 0.1 | NS |
| Lopinavir | |||
| AUC0–12, µmol/L·h | 135.1 ± 8.3 | 160.0 ± 7.8 | .04 |
| Cl/F, L/h | 5.0 ± 0.3 | 4.1 ± 0.2 | .03 |
| Cmax, µmol/L | 15.6 ± 0.9 | 17.9 ± 0.7 | NS |
| Tmax, h | 3.5 ± 0.4 | 2.9 ± 0.5 | NS |
| Cmin, µmol/L | 10.4 ± 0.8 | 12.4 ± 0.8 | NS |
| Substance, pharmacokinetic parameter | Control group | Buprenorphine/naloxone group | P |
| Nelfinavir | |||
| AUC0–12, µmol/L·h | 91.9 ± 17.4 | 114.8 ± 23.1 | NS |
| Cl/F, L/h | 26.0 ± 9.4 | 36.0 ± 6.0 | NS |
| Cmax, µmol/L | 14.1 ± 2.3 | 8.5 ± 1.1 | .01 |
| Tmax, h | 2.0 ± 0.4 | 3.6 ± 0.6 | NS |
| Cmin, µmol/L | 2.1 ± 0.9 | 3.0 ± 1.1 | NS |
| M8 | |||
| AUC0–12, µmol/L·h | 30.9 ± 4.8 | 29.8 ± 5.3 | NS |
| Cmax, µmol/L | 5.1 ± 0.7 | 2.7 ± 0.3 | .01 |
| Tmax, h | 3.3 ± 0.4 | 3.8 ± 0.4 | NS |
| Cmin, µmol/L | 0.50 ± 0.3 | 0.55 ± 0.1 | NS |
| Ritonavir | |||
| AUC0–12, µmol/L·h | 9.43 ± 0.8 | 9.61 ± 1.2 | NS |
| Cl/F, L/h | 13.9 ± 1.3 | 13.7 ± 2.1 | NS |
| Cmax, µmol/L | 1.62 ± 0.1 | 1.58 ± 0.1 | NS |
| Tmax, h | 3.21 ± 0.4 | 4.04 ± 1.1 | NS |
| Cmin, µmol/L | 0.22 ± 0.03 | 0.37 ± 0.1 | NS |
| Lopinavir | |||
| AUC0–12, µmol/L·h | 135.1 ± 8.3 | 160.0 ± 7.8 | .04 |
| Cl/F, L/h | 5.0 ± 0.3 | 4.1 ± 0.2 | .03 |
| Cmax, µmol/L | 15.6 ± 0.9 | 17.9 ± 0.7 | NS |
| Tmax, h | 3.5 ± 0.4 | 2.9 ± 0.5 | NS |
| Cmin, µmol/L | 10.4 ± 0.8 | 12.4 ± 0.8 | NS |
NOTE. Data are mean ± SE. AUC0–12, 12-h area under the concentrationtime curve; Cmax, maximum concentrationin plasma; Cmin, minimum concentration in plasma; Cl/F, clearance; NS, not significant.
Effect of buprenorphine on nelfinavir, M8 metabolite, ritonavir, and lopinavir concentrations.
| Substance, pharmacokinetic parameter | Control group | Buprenorphine/naloxone group | P |
| Nelfinavir | |||
| AUC0–12, µmol/L·h | 91.9 ± 17.4 | 114.8 ± 23.1 | NS |
| Cl/F, L/h | 26.0 ± 9.4 | 36.0 ± 6.0 | NS |
| Cmax, µmol/L | 14.1 ± 2.3 | 8.5 ± 1.1 | .01 |
| Tmax, h | 2.0 ± 0.4 | 3.6 ± 0.6 | NS |
| Cmin, µmol/L | 2.1 ± 0.9 | 3.0 ± 1.1 | NS |
| M8 | |||
| AUC0–12, µmol/L·h | 30.9 ± 4.8 | 29.8 ± 5.3 | NS |
| Cmax, µmol/L | 5.1 ± 0.7 | 2.7 ± 0.3 | .01 |
| Tmax, h | 3.3 ± 0.4 | 3.8 ± 0.4 | NS |
| Cmin, µmol/L | 0.50 ± 0.3 | 0.55 ± 0.1 | NS |
| Ritonavir | |||
| AUC0–12, µmol/L·h | 9.43 ± 0.8 | 9.61 ± 1.2 | NS |
| Cl/F, L/h | 13.9 ± 1.3 | 13.7 ± 2.1 | NS |
| Cmax, µmol/L | 1.62 ± 0.1 | 1.58 ± 0.1 | NS |
| Tmax, h | 3.21 ± 0.4 | 4.04 ± 1.1 | NS |
| Cmin, µmol/L | 0.22 ± 0.03 | 0.37 ± 0.1 | NS |
| Lopinavir | |||
| AUC0–12, µmol/L·h | 135.1 ± 8.3 | 160.0 ± 7.8 | .04 |
| Cl/F, L/h | 5.0 ± 0.3 | 4.1 ± 0.2 | .03 |
| Cmax, µmol/L | 15.6 ± 0.9 | 17.9 ± 0.7 | NS |
| Tmax, h | 3.5 ± 0.4 | 2.9 ± 0.5 | NS |
| Cmin, µmol/L | 10.4 ± 0.8 | 12.4 ± 0.8 | NS |
| Substance, pharmacokinetic parameter | Control group | Buprenorphine/naloxone group | P |
| Nelfinavir | |||
| AUC0–12, µmol/L·h | 91.9 ± 17.4 | 114.8 ± 23.1 | NS |
| Cl/F, L/h | 26.0 ± 9.4 | 36.0 ± 6.0 | NS |
| Cmax, µmol/L | 14.1 ± 2.3 | 8.5 ± 1.1 | .01 |
| Tmax, h | 2.0 ± 0.4 | 3.6 ± 0.6 | NS |
| Cmin, µmol/L | 2.1 ± 0.9 | 3.0 ± 1.1 | NS |
| M8 | |||
| AUC0–12, µmol/L·h | 30.9 ± 4.8 | 29.8 ± 5.3 | NS |
| Cmax, µmol/L | 5.1 ± 0.7 | 2.7 ± 0.3 | .01 |
| Tmax, h | 3.3 ± 0.4 | 3.8 ± 0.4 | NS |
| Cmin, µmol/L | 0.50 ± 0.3 | 0.55 ± 0.1 | NS |
| Ritonavir | |||
| AUC0–12, µmol/L·h | 9.43 ± 0.8 | 9.61 ± 1.2 | NS |
| Cl/F, L/h | 13.9 ± 1.3 | 13.7 ± 2.1 | NS |
| Cmax, µmol/L | 1.62 ± 0.1 | 1.58 ± 0.1 | NS |
| Tmax, h | 3.21 ± 0.4 | 4.04 ± 1.1 | NS |
| Cmin, µmol/L | 0.22 ± 0.03 | 0.37 ± 0.1 | NS |
| Lopinavir | |||
| AUC0–12, µmol/L·h | 135.1 ± 8.3 | 160.0 ± 7.8 | .04 |
| Cl/F, L/h | 5.0 ± 0.3 | 4.1 ± 0.2 | .03 |
| Cmax, µmol/L | 15.6 ± 0.9 | 17.9 ± 0.7 | NS |
| Tmax, h | 3.5 ± 0.4 | 2.9 ± 0.5 | NS |
| Cmin, µmol/L | 10.4 ± 0.8 | 12.4 ± 0.8 | NS |
NOTE. Data are mean ± SE. AUC0–12, 12-h area under the concentrationtime curve; Cmax, maximum concentrationin plasma; Cmin, minimum concentration in plasma; Cl/F, clearance; NS, not significant.
Discussion
The findings of this study indicate that the standard doses of the PIs NFV, RTV, and LPV/R that are regularly used in the clinical care of patients with HIV disease may be given to opioid-dependent individuals receiving maintenance treatment with typical doses of buprenorphine/naloxone, without the occurrence of clinically significant drug interactions. Pharmacokinetic interactions of significance were restricted to increased buprenorphine AUC0–24 after administration of RTV, whereas administration of NFV and LPV/R had little effect on buprenorphine pharmacokinetics. No pharmacodynamic effects were observed in relation to any of the drugs tested in combination (i.e., buprenorphine in combination with NFV, LPV/R, or RTV). Furthermore, NFV and RTV plasma concentration profiles in buprenorphine/naloxone-maintained participants did not differ significantly from those in control participants, whereas a higher LPV AUC0–12 was observed. The clinical importance of the increased LPV AUC0–12 will require additional clinical experience with the use of this combination, although the ∼15% increase in the AUC0–12 is unlikely to produce antiretroviral toxicity. These findings indicate that these PIs may be administered at standard clinical dosages in opioid-dependent patients with HIV disease who are receiving buprenorphine/naloxone.
RTV consistently increased the mean AUC0–24 and Cmax values for buprenorphine and all 3 metabolites. At the same time, mean Cl/F values decreased for buprenorphine, norbuprenorphine, and norbuprenorphine-3-glucuronide while remaining essentially unchanged for buprenorphine-3-glucuronide. Consistent with these observations were increases in urinary recovery for all 4 species. Although none of the increases in urine recovery reached statistical significance when considered individually, the consistent increases for all 4 species in both plasma and urine strongly suggest an increase in buprenorphine bioavailability as the most probable explanation.
Buprenorphine is administered sublingually, because of poor bioavailability when it is administered orally. RTV is both an inhibitor and an inducer of cytochrome P450 (CYP) 3A, as well as of the efflux transporter P-glycoprotein. Initially, inhibition predominates, but the onset of induction may reduce or even reverse the inhibitory effects as the duration of therapy increases [22]. By inhibiting intestinal P-glycoprotein as well as first-pass metabolism by intestinal and hepatic CYP3A, RTV could increase the bioavailability of buprenorphine that is swallowed rather than absorbed through the oral mucosa. The increased bioavailability of buprenorphine would produce downstream effects to increase each of the metabolites as well. This could explain the increases in the AUC0–24 of norbuprenorphine and norbuprenorphine-3-glucuronide despite inhibition of the metabolic path that produces norbuprenorphine. The increased urinary recovery of all 4 compounds is also consistent with an increase in the amount of buprenorphine absorbed.
No signs of opioid toxicity were observed in buprenorphine/naloxone-maintained participants administered RTV; however, the increase in buprenorphine and norbuprenorphine AUC0–24 with RTV administration was moderate, at 36% and 25%, respectively. The relatively modest increases produced by the powerful CYP3A inhibitor RTV may result from compensatory induction, which should be significantly developed after 7 days. It could also reflect concomitant metabolism by CYP2C8. This enzyme has been reported to have a significant role in the metabolism of buprenorphine [23] but is not inhibited by RTV.
The interaction of buprenorphine and RTV contrasts with that of methadone and RTV; RTV did not significantly increase methadone exposure [14]. In vitro experiments have shown that methadone is principally metabolized via N-demethylation by CYP3A enzymes, but a number of other enzymes, including CYP2B6, CYP2D6, CYP2C8, CYP2C9, and CYP2C19, have also been suggested to play significant roles in methadone disposition in vivo, some possibly involving a metabolic pathway other than N-demethylation [24, 25]. The existence of alternate pathways to CYP3A4 metabolism may be one explanation for the lack of a significant effect of RTV on methadone exposure.
Neither NFV nor LPV/R showed a significant interaction with buprenorphine, whereas both have been shown to have pharmacokinetic interactions with methadone that significantly lower methadone concentrations and may produce opiate withdrawal [13, 14]. NFV is principally metabolized by CYP3A4 and CYP2C19, but CYP2C9 and CYP2D6 also participate. NFV is thought to induce its own metabolism and can act as an inducer or an inhibitor of the CYP-mediated metabolism of other drugs [26]. In vitro studies have shown that NFV at therapeutic concentrations is a competitive inhibitor of CYP3A4, but it does not inhibit other CYP isoforms [27]. In the present study, NFV appeared to moderately decrease conversion of buprenorphine to norbuprenorphine, although the change failed to reach statistical significance and there was no observable effect on buprenorphine concentrations. The failure of NFV to significantly affect buprenorphine concentrations may have resulted from the interplay of simultaneous inhibition and induction of buprenorphine metabolism.
LPV has been shown to inhibit CYP3A and CYP2D6 in vitro, but less so than does RTV [28]. A novel effect of LPV/R in this study was the apparent induction of buprenorphine glucuronidation. Because this effect was not seen with RTV, it may be an effect of LPV. In a drug interaction study examining LPV/R and methadone administration, plasma concentrations of methadone were significantly reduced, and some study participants reported opiate withdrawal symptoms, indicating that the LPV/R combination induces methadone metabolism. This result was probably related to the effects of LPV, because administration of RTV alone at the dosage found in the LPV/R combination drug had little effect on methadone concentrations [14].
A potential contributor to reduced methadone concentrations after LPV/R coadministration is the induction of intestinal CYP enzymes and, possibly, P-glycoprotein, with a concomitant reduction in methadone bioavailability. The lack of an LPV/R effect on buprenorphine metabolism may be reflective of the sublingual route of administration, in which the majority of the drug is absorbed into the oral mucosa and then directly into the circulation. Because any swallowed buprenorphine is already poorly bioavailable, further reduction of intestinal bioavailability would have little impact. This could represent a distinct advantage to the use of buprenorphine/naloxone in the treatment of opioid-dependent patients with HIV disease. Alternatively, RTV inhibition of N-dealkylation by CYP3A may have been balanced by LPV induction of buprenorphine glucuronidation (a pathway not available for methadone clearance), resulting in no net change in overall buprenorphine clearance and AUC0–24.
No adverse interactions were detected when buprenorphine/naloxone was concomitantly administered with NFV, RTV, or LPV/R. Objective measures demonstrated that there were no signs of opiate withdrawal, nor were there signs of impaired cognition. No other overt toxic effects were observed, nor were there clinically significant changes in observed side effects. There were only 2 combinations that produced significant alterations in the pharmacokinetics of either drug. RTV alone increased buprenorphine exposure, and buprenorphine increased LPV concentrations. These changes were apparently not large enough to be clinically meaningful. All of these medications may be dosed per usual clinical practice in the treatment of HIV disease in this population.
Methadone treatment in combination with antiretroviral therapy has been associated with multiple adverse drug interactions [8, 12–14, 29, 30]. Our studies of antiretroviral drug interactions with buprenorphine/naloxone have included efavirenz and delavirdine [21], as well as NFV, RTV and LPV/R in the present study. No interactions were observed, with combinations of any of these drugs, that required a change in dose of any medication. The apparently reduced potential for clinically important interactions may favor buprenorphine over methadone for treatment of opioid dependence in those receiving these antiretroviral medications for HIV disease.
The findings of these studies have relevance to clinical interventions aimed at opioid-dependent individuals with HIV disease and have implications for containment of viral transmission by injection drug users. Injection drug use is an important route of transmission of bloodborne viral infections and is responsible for 33% of AIDS cases in the United States [31]. Globally, and particularly in many developing nations, injection drug use represents a principal vector of HIV transmission [32]. Comprehensive treatment of opioid addiction with cessation of injection drug use and effective treatment of HIV disease is an important clinical objective in this population. Unlike methadone, which is available only through specialized treatment programs and is unavailable in some areas, buprenorphine is available by prescription, enabling a single physician to provide care for both HIV disease and opioid addiction [16].
Others have shown that colocation of health care services for those with comorbid conditions improves clinical outcomes [33]. Specifically, the colocation of substance abuse treatment services in primary care clinics and of medical care in substance abuse treatment programs has been associated with positive clinical outcomes [6, 7]. Buprenorphine/naloxone pharmacotherapy for opioid dependence lends itself to both clinical settings [16]. The lack of adverse drug interactions between buprenorphine and several antiretroviral medications that can produce significant interactions when administered to methadone-maintained individuals is potentially an important advantage of buprenorphine/naloxone treatment of opioid dependence. Furthermore, the demonstration of antiretroviral drug concentrations that remain in the therapeutic range during simultaneous buprenorphine/naloxone administration when standard clinical doses of these medications are administered make treatment of opioid dependence with buprenorphine/naloxone (or, when clinically indicated, buprenorphine alone) simpler, safer, and more likely to be effective in the treatment of patients with HIV disease.
Acknowledgments
Financial support
National Institute on Drug Abuse/National Institutes of Health (NIH) (grants RO1 DA 13004 and KO2 DA00478 to E.M.K. and grant RO1 DA 10100 to D.E.M.; General Clinical Research Center at Virginia Commonwealth University (grant M01RR00065 from the National Center for Research Resources/NIH).
Supplement sponsorship
This article was published as part of a supplement entitled “Buprenorphine and HIV Primary Care: New Opportunities for Integrated Treatment,” sponsored by the National Institute on Drug Abuse, National Institutes of Health, Public Health Service, US Department of Health and Human Services.
Potential conflicts of interest
All authors: no conflicts.





Comments