Abstract
Due to the high atomic number of gold nanoparticles (GNPs), they are known as new radiosensitizer agents for enhancing the efficiency of superficial radiotherapy techniques by increasing the dose absorbed in tumor cells wherein they can be accumulated selectively. The aim of this study was to compare the effect of various common low energy levels of orthovoltage x-rays and megavoltage γ-rays (Co-60) on enhancing the therapeutic efficiency of HeLa cancer cells in the presence of conjugated folate and non-conjugated (pegylated) GNPs. To achieve this, GNPs with an average diameter of 52 nm were synthesized and conjugated to folic acid molecules. Pegylated GNPs with an average diameter of 47 nm were also synthesized and used as non-conjugated folate GNPs. Cytotoxicity assay of the synthesized folate-conjugated and pegylated GNPs was performed using different levels of nanoparticle concentration incubated with HeLa cells for 24 h. The radiosensitizing effect of both the conjugated and pegylated GNPs on the cells at a concentration of 50 µM was compared using MTT as well as clonogenic assays after exposing them to 2 Gy ionizing radiation produced by an orthovoltage x-ray machine at four different kVps and γ-rays of a Co-60 unit. Significant differences were noted among various irradiated groups with and without the folate conjugation, with an average dose enhancement factor (DEF) of 1.64 ± 0.05 and 1.35 ± 0.05 for the folate-conjugated and pegylated GNPs, respectively. The maximum DEF was obtained with the 180 kVp x-ray beam for both of the GNPs. Folate-conjugated GNPs can significantly enhance the cell killing potential of orthovoltage x-ray energies (especially at 180 kVp) in folate receptor-expressing cancer cells, such as HeLa, in superficial radiotherapy techniques.
Export citation and abstract BibTeX RIS
Introduction
More than half of cancer patients will go through radiotherapy procedures during their treatment period (Brun et al 2009). Despite improvements made in radiotherapy efficiency, the side effects of ionizing radiation in normal tissues are the dose-limiting factors restricting higher doses in tumors (Drouet and Lagrange 2010). Nevertheless, increasing the absorbed dose to target cells alone could considerably increase the tumor control probability. One way to enhance the radiation dose only to cancerous cells can be made by using radiosensitization agents accumulated preferably in such cells. Study of the radiosensitization effect of high atomic number materials was initiated more than 30 years ago by investigating the radioenhancing factor of iodine (Matsudaira et al 1980). However, owing to the recent achievements of nanotechnology, nowadays it seems increasingly possible to selectively accumulate gold nanoparticles (GNPs) with high atomic number nanomaterials as radioenhancing agents in cancerous cells (Brun et al 2009). In recent years, the use of GNPs in biological research has increased rapidly (Jain et al 2011). GNPs have special properties such as nanoscale size, biocompatibility, comfortable synthesis and bioconjugation, intense absorbing and scattering properties. Due to these properties, GNPs have provided various applications in targeted drug delivery (Yang et al 2005), cell imaging (Shukla et al 2005, Chen et al 2005) and cancer therapy (Hainfeld et al 2004, Chen et al 2007). The biocompatibility and low toxicity of GNPs has nominated it as the best candidate to be used for radioenhancing purposes (Shukla et al 2005, Lewinski et al 2008).
The radiosensitizing effect of GNPs in radiotherapy has been mainly reported by Hainfeld et al (2004). More recently, the enhancement of cell radiation sensitivity by naked GNPs (Rahman et al 2009) and pegylated GNPs (Liu et al 2010) was also investigated using various radiation beam modalities, among which superficial kilovoltage x-ray was reported to be the most efficient modality. In addition, Butterworth et al (2012) reviewed the physical basis and biological mechanisms of the GNP radiosensitization effect. Contradictory results of the GNP-induced dose enhancement factor (DEF) with kilovoltage x-ray energy irradiations have been reviewed in the latter study, indicating a significant variation of the reported results from one study to another. In accordance with the above study, for example, in an in vitro study (Rahman et al 2009) using the same cell type (BAEC cells), GNP size (1.9 nm) and concentration (1 mM), the reported DEFs had more than ten times variation (from 24.6 to 2.2 for 80 and 150 kVp x-rays, respectively). However, the incident photon energies for creating the photoelectric effect must be higher than the electron binding energy of the target atoms (Attix 2004). Therefore, for the gold K-shell electrons (having an 80.7 keV binding energy), it is expected that the probability of the photoelectric effect would be higher for the 150 kVp polyenergetic x-ray beams compared to 80 kVp as it provides more photons, with the energies close to the gold K-shell binding energy. Moreover, a disparity is claimed (Butterworth et al 2012) to exist between the predicted physical and observed experimental DEFs in the literature, revealing the effect of biological mechanisms on GNP radiosensitization, such as the surface functionalization of these nanoparticles. Some studies have focused on the specific delivery of GNPs to cancer target cells using surface-modified GNPs (Roa et al 2009, Kong et al 2008). The over-expression of folate receptors (FR) on some cancer cells is reported to be 100 times more than in normal healthy cells and has been exploited to target folate-linked therapeutic agents specifically to FR-expressing tumors (Sega and Low 2008, Doucette and Stevens 2001). Folate also has promising characteristics such as non-immunogenicity, specificity for cancer cells, and the possibility of conjugating with GNPs (Mansoori et al 2010). These properties have made it a front-runner as a targeting moiety in many cancer treatments (Mansoori et al 2010).
HeLa cancerous cells are reported to have a high level of FR expression (Masters 2002). It is also expected that conjugating GNPs with folate enhances the killing potential of orthovoltage x-rays when irradiating HeLa cells, due to the high FR expression of these cells. Therefore, the main purpose of this in vitro study was to compare the effect of different low energy levels of orthovoltage x-rays as well as megavoltage Co-60 γ-rays in the presence of conjugated folate and pegylated (non-conjugated folate) GNPs on enhancing the therapeutic efficiency of HeLa cancerous cells.
Material and methods
Chemicals and cell lines
The main chemical materials used for the synthesis of the nanoparticles were hydrogen tetrachloroaurate (III) trihydrate (HAuCl4.3H2O), sodium borohydride (NaBH4), trisodium citrate (Na3C6H5O7), N,N'-dicyclohexylcarbodiimide (C13H22N2), 4-aminothiophenol (C6H14NS) and folic acid (C19H19N7O6), all purchased from Sigma-Aldrich (Germany); and dichloromethane (CH2Cl2), dimethylsulfoxide (DMSO), methanol (CH3OH), polyethylene glycol (PEG; molecular weight of 2 kDa), purchased from Merck (Germany). The materials used for the cell cultures and MTT assays included Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), trypan blue, penicillin/streptomycin, trypsin-EDTA 0.25% and MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) all purchased from GIBCO (Invitrogen, Germany). The HeLa cell line was provided by Bonyakhteh Co. (Tehran, Iran).
Folate-conjugated GNP synthesis
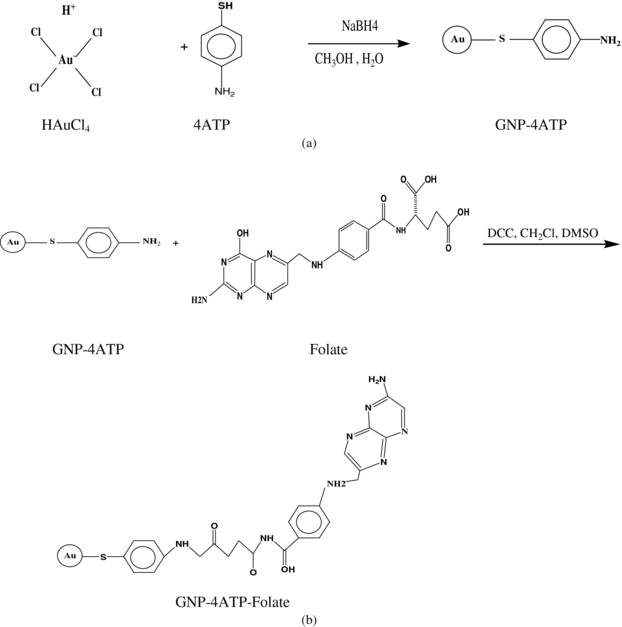
The general method for synthesizing functionalized GNPs conjugated with folate has been described before (Shakeri-Zadeh et al 2010a, 2010b). This method was used and improved by changing the weight of the reductant to obtain GNPs with an average diameter of 50 nm. The folate was bound covalently to the GNPs at two stages to form functionalized folate-capped GNPs. Firstly, the 4-aminothiophenol (4ATP) molecule used as a linker was bound to the GNPs from its –SH end during synthesis to form 4ATP-GNPs. Then, the folate was bound to 4ATP-GNPs to form stabilized folic acid 4ATP-GNPs (FA-4ATP-GNPs). The scheme of this synthesis procedure is shown in figure 1, drawn by ChemDraw Ultra software, version 7.0.3. The FT-IR measurements were recorded using KBr pellets at room temperature to verify the conjugating formation. Transmission electron microscopic (TEM) images of the nanoparticles were taken with a LEO 906 microscope (Germany) operated at 100 kV. The samples were prepared for TEM measurements by placing a droplet of colloidal solution onto a carbon-coated copper grid allowed to dry in air. Based on the TEM photographs, the distribution and average size of the GNPs were determined with the ImageTool software (ImageTool for Windows, 3.00).
Figure 1. Schematic diagram of the synthesis procedure of the GNP-4ATP (a) and the GNP-4ATP folate (b).
Download figure:
Standard image High-resolution imagePegylated GNP synthesis
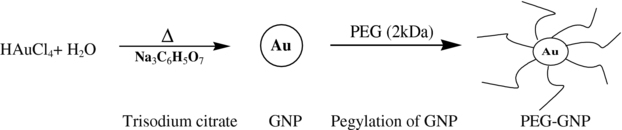
Pegylated GNPs were synthesized and used as the non-folate-conjugated GNPs. For this purpose, the method proposed by Turkevich et al (1951) was used, in which trisodiumcitrate is used as the reductant for synthesizing naked GNPs. The weight of the reductant, as the most efficient factor involved in controlling the nanoparticle size, was changed to obtain GNPs with an average diameter of 50 nm. Thereafter, PEG molecules (with a molecular weight of 2000 Da, 0.05 wt%) were added to the GNP colloid while stirring to synthesize sterically stabilized nanoparticles. The scheme of this synthesis is shown in figure 2, drawn by ChemDraw Ultra software, version 7.0.3. The TEM images of these nanoparticles were taken with the same LEO 906 microscope at 100 kV, and from these, the GNP distribution and average size were determined using the ImageTool software.
Figure 2. Schematic diagram of the synthesis procedure of the pegylated GNPs.
Download figure:
Standard image High-resolution imageCell culture
The HeLa cells were grown as a monolayer using DMEM supplemented with 10% FBS, 20 mM D-glucose, 100 UI ml−1 penicillin and 100 µg ml−1 streptomycin in 75 cm2 flasks (BD-Falcon, BD Biosciences, USA). The cell growth was carried out in a humidified atmosphere containing 5% CO2 to 95% air at 37 °C. The culture medium was changed regularly to obtain a sufficient number of cells. When the cells were at more than 70 percent confluence, they were washed with PBS and incubated with the trypsin-EDTA solution for 3 min at 37 °C to detach them from the flask. The cells were then re-suspended in the culture medium for seeding. Trypan blue staining was performed to determine the cell density and viability before seeding.
GNP uptake assays
Atomic absorption measurements of the gold concentrations in the cells were required for determining the internalization trend of nanoparticles into the cells as well as for choosing a suitable time schedule for irradiating the cells after the GNP incubation. Therefore, a number of 104 cells/well were plated in six-well plates for a period of 48 h. Afterwards, they were incubated with the folate and non-folate-conjugated (pegylated) GNPs (FA-GNPs and PEG-GNPs) over time periods of 6, 12, 24 and 48 h in triplicate groups. Subsequently, each group of cells was washed three times in the PBS solution, trypsinized, inactivated from the trypsin-EDTA by adding the FBS, manually counted, and finally centrifuged at 1200 rpm for 5 min before the supernatant was removed. Then, the sediment containing the cells was digested in aqua regia. Thereafter, the gold content internalized into the cells was determined using an AA-670G furnace atomic absorption spectroscopy instrument (Shimadzu, Japan). This procedure was made based on a standard curve obtained by the instrument using a standard solution from which the value of the gold absorption recorded at 242.8 nm was calculated and attributed to the gold concentration of each sample.
Irradiation of cells and radiobiological assays
A number of 5 × 103 cells per well were plated in 96-well plates over 48 h. Then, they were incubated with the folate-conjugated and pegylated GNPs for 24 h before irradiation. The irradiation of the various groups of the cells was done by delivering 2 Gy of γ-rays using a Co-60 machine (Theratronphonix, Canada) as well as an orthovoltage x-ray treatment machine (Stabilipan2, Siemens, Germany) operated at different kVp values ranging from 120 to 250. The orthovoltage unit had several applicator cones with different lengths and openings, enabling us to define the treatment distance and field size. However, the geometrical dimensions of the plates led us to choose an applicator with a 10 × 15 cm2 field size and a focus to surface distance of 30 cm. Air KERMA measurements of the orthovoltage machine were performed using a calibrated PTW UNIDOS-E electrometer and a Farmer ionization chamber with a sensitive volume of 0.6 cc (PTW, Germany). All the relevant parameters of the orthovoltage x-rays and megavoltage γ-rays of the radiotherapy units as well as the equivalent energies of the x-ray beams measured under the narrow-beam geometry condition (Attix 2004) are listed in table 1.
Table 1. Relevant parameters of orthovoltage x-rays and Co-60 radiotherapy units.
| Potential energy (kVp) | Filament current (mA) | Additional filtration (mm) | HVL1 (mm Al) | Equivalent energy (keV) | Dose rate (Gy min−1)a |
|---|---|---|---|---|---|
| 120 | 20 | 4 Al | 3.57 | 37.2 | 1.85 |
| 180 | 20 | 0.2 Cu | 6.49 | 48.1 | 3.43 |
| 200b | 20 | 0.5 Cu | 9.68 | 62.3 | 2.67 |
| 200c | 20 | 0.5 Cu + 4.32 Al | 11.7 | 74.7 | 1.71 |
| 250 | 15 | 1 Cu | 13.16 | 84.1 | 2.38 |
| Co-60 | – | – | – | 1250d | 1.18e |
a A fixed dose rate of 1.7 Gy min−1 was used by changing the SSD for various irradiation conditions. b Having only 0.5 mmCu additional filtration. c Having 0.5 mmCu + 4.32 mmAl additional filtration. d The common mean energy derived from the dominant γ-rays (1.17 and 1.33 meV) of Co-60 units. e Achieved at an SSD equal to 50 cm.
The cell death or proliferation as the radiation response and cytotoxicity of the nanoparticles were established with MTT and clonogenic assays performed at 48 h and 14 days after the irradiation, respectively (Kong et al 2008). The level of radiation biotoxicity on the cells represented with the relative percentage of cell survival in the presence of the GNPs to that with radiation alone was indicated as the DEF. The experiments were repeated three times. The cell survival values presented in the figures show the mean ± standard deviation. The percentage of cell survival values among different groups was compared using one-way analysis of variance (ANOVA) followed by Tukey multiple comparison tests with a 95% confidence interval.
Results
Synthesis and characterization of the GNPs
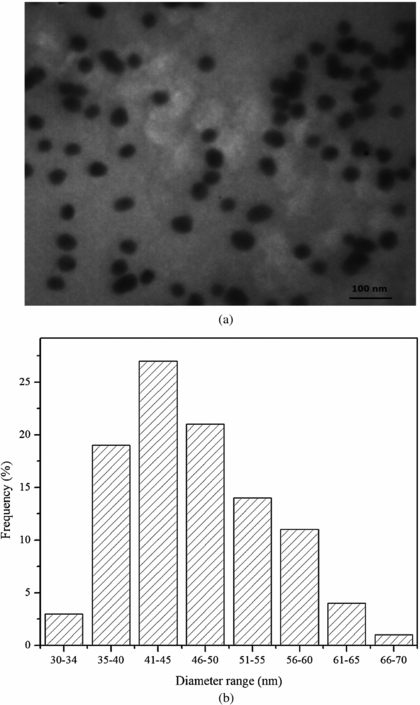
The folate-conjugated GNPs were synthesized using sodium borohydride as a reducing agent and conjugated with the 4ATP molecule, a bifunctional linker capable of binding separately with GNP and folic acid from its –SH and –NH2 ends, respectively (Shakeri-Zadeh et al 2010a, 2010b). Analyzing the FT-IR spectrums of 4ATP, 4ATP-GNP and FA-4ATP-GNP verified the formation of the final conjugation as suggested by others (Shakeri-Zadeh et al 2010a, 2010b). The spherical morphology of the GNPs was also verified by TEM microphotography. The analysis of the TEM images carried out on more than 200 nanoparticles indicated an average diameter of 52 ± 11.5 nm for the GNPs (figure 3).
Figure 3. (a) Transmission electron microscopy image of folate-conjugated gold nanoparticles (FA-GNP). (b) Distribution of the GNP diameter obtained from analyzing more than 200 GNPs using the ImageTool software.
Download figure:
Standard image High-resolution imageThe pegylated GNPs were synthesized using trisodiumcitrate through a different method to that used for the folate-conjugated GNPs. The TEM micrographs showed an almost spherical morphology for these GNPs. A similar analysis of the TEM images made on more than 200 of these GNPs indicated an average diameter of 47 ± 8.2 nm (figure 4).
Figure 4. (a) Transmission electron microscopy image of pegylated GNPs. (b) Distribution of GNP diameter obtained from analyzing more than 200 GNPs using the ImageTool software.
Download figure:
Standard image High-resolution imageUptake assays of folate- and non-folate-conjugated (pegylated) GNPs
Figure 5 shows the average number of FA-GNPs and PEG-GNPs per HeLa cell versus the incubation time period calculated from the gold concentrations (Liu et al 2007) after exposing the cells to the GNPs. The figure indicates that the internalization of the nanoparticles into the HeLa cells is increased rapidly up to 24 and 12 h after the incubation for FA-GNPs and PEG-GNPs, respectively. Therefore, we used an incubation time period of 24 h for studying the cytotoxicity assay as well as the irradiation toxicity of the cells.
Figure 5. The average number of internalized FA-GNPs and PEG-GNPs per HeLa cell based on atomic absorption measurements versus the incubation time period.
Download figure:
Standard image High-resolution imageCytotoxicity of the GNPs
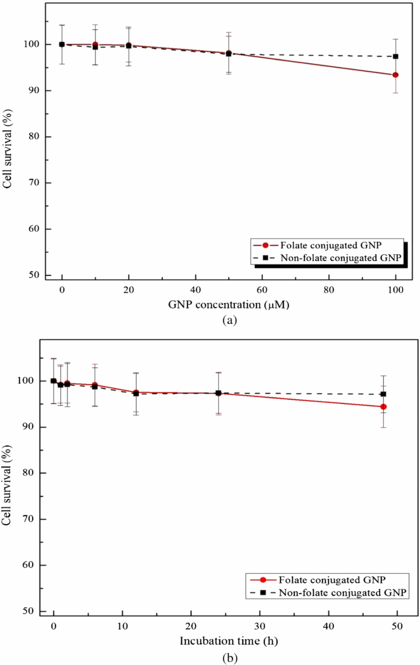
To determine the cytotoxicity of the FA-GNPs and PEG-GNPs on HeLa cells, the cells were incubated with different concentrations of GNPs (0, as the control, and 10, 20, 50 and 100 µM) for a period of 24 h. The cytotoxicity of the GNPs performed by the MTT assay showed no significant difference (p-value > 0.05) between the controls and those treated with the PEG-GNPs over the whole range of GNP concentrations, while for those treated with the FA-GNPs the non-significant difference with the controls ended at 50 µM (figure 6(a)). Hence, the incubation of the cells with a 100 µM concentration of FA-GNPs proved to be toxic (p-value < 0.05). According to previous studies (Gu et al 2009), the cytotoxicity of nanoparticles could be time-dependent. Therefore, the cells were incubated over different time periods (0, 1, 2, 6, 12, 24 and 48 h) with a fixed concentration (50 µM) of FA-GNPs as well as PEG-GNPs. The incubation of both of the GNPs showed no cytotoxicity effect on the cells at up to 24 h (p-value > 0.05). However, the cells incubated with the FA-GNPs over 48 h proved (p-value < 0.05) to be toxic (figure 6(b)).
Figure 6. Survival percentage of HeLa cells incubated at different GNP concentrations at a fixed incubation time of 24 h (a); and at various time periods with a fixed GNP concentration of 50 µM (b).
Download figure:
Standard image High-resolution imageFolate- and non-folate-conjugated GNP radiation sensitivity
When the HeLa cells incubated with the 50 µM concentration of GNPs for 24 h were exposed to 2 Gy of ionizing radiation using various orthovoltage energies of the x-ray machine, significant differences (p-value < 0.05) were noted among their survival rates compared to their control groups due to the presence of GNPs, as shown from the MTT (figure 7) and clonogenic assays (figure 8). In addition, there were significant differences (p-value < 0.05) between the survival rates of the HeLa cells incubated with folate and non-folate (pegylated) GNPs (figures 7 and 8). However, when the cells were exposed to 2 Gy of the megavoltage γ-rays of the Co-60 unit, a significant difference was noted only in the survival of HeLa cells incubated with FA-GNPs compared to its control group (figures 7 and 8).
Figure 7. Comparison of the survival percentages of the HeLa cells with the presence and absence of FA- and PEG-GNPs for the control and various treatment groups based on MTT assay done 48 h after exposing them to 2 Gy of ionizing radiation using different orthovoltage x- and γ-rays. (NB: the nanoparticle concentration in the treatment groups was 50 µM, and 200 kVpa and 200 kVpb represent the groups irradiated to 200 kVp beams having only 0.5 mmCu and 0.5 mmCu + 4.32 mmAl filtrations, respectively.)
Download figure:
Standard image High-resolution imageFigure 8. Comparison of the survival percentages of HeLa cells in the presence and absence of FA- and PEG-GNPs for the control and various treatment groups based on clonogenic assay done 14 days after exposing them to 2 Gy of ionizing radiation using different orthovoltage x- and γ-rays. (NB: the nanoparticle concentration in the treatment groups was 50 µM, and 200 kVpa and 200 kVpb represent the groups irradiated to 200 kVp beams having only 0.5 mmCu and 0.5 mmCu + 4.32 mmAl filtrations, respectively.)
Download figure:
Standard image High-resolution imageThe DEF values of all photon energies for both of the GNPs are shown in table 2. As can be noted from the table, all the orthovoltage x-ray beams were superior to the Co-60 γ-rays in enhancing the radiosensitization effect due to the GNPs (p-value < 0.05). Furthermore, the table indicates that the 180 kVp x-ray beam has the maximum DEF compared to other orthovoltage beams, although not statistically significant (p-value > 0.05). We have also introduced a parameter called the targeting factor, presented in the last column of table 2. This factor indicates the ratio of the DEF of the HeLa cells using the folate and non-folate GNPs (DEFFA-GNP/DEFPEG-GNP) based on the common MTT and clonogenic assay methods resulting from targeting the folate-conjugated GNPs internalized consequently into the HeLa cells having a significantly high level of FRs.
Table 2. DEF values resulting from combining the 50 µM FA-GNPs and 2 Gy ionizing radiation produced at different orthovoltage x-ray energies and Co-60 γ-rays for HeLa cells using MTT and clonogenic assays. The ratio of the DEF of HeLa cells with Folate-Conjugated GNPs relative to those with Pegylated GNPs (named the targeting factor; TF) is also calculated and presented for all the photon beams.
| DEF | ||||||
|---|---|---|---|---|---|---|
| Folate-conjugated GNPs | Pegylated GNPs | TF (DEFFA-GNP/DEFPEG-GNP) | ||||
| Photon | MTT | Clonogenic | MTT | Clonogenic | MTT | Clonogenic |
| beam | assay | assay | assay | assay | assay | assay |
| 120 kVp | 1.61 ± 0.09 | 1.68 ± 0.08 | 1.28 ± 0.06 | 1.40 ± 0.07 | 1.26 ± 0.09 | 1.18 ± 0.08 |
| 180 kVp | 1.67 ± 0.10 | 1.76 ± 0.09 | 1.31 ± 0.05 | 1.43 ± 0.07 | 1.30 ± 0.09 | 1.23 ± 0.09 |
| 200 kVpa | 1.62 ± 0.07 | 1.63 ± 0.05 | 1.32 ± 0.06 | 1.39 ± 0.05 | 1.23 ± 0.08 | 1.16 ± 0.06 |
| 200 kVpb | 1.59 ± 0.11 | 1.61 ± 0.07 | 1.29 ± 0.03 | 1.37 ± 0.03 | 1.24 ± 0.09 | 1.18 ± 0.06 |
| 250 kVp | 1.63 ± 0.04 | 1.58 ± 0.06 | 1.33 ± 0.05 | 1.39 ± 0.03 | 1.29 ± 0.05 | 1.13 ± 0.05 |
| Co-60 | 1.04 ± 0.02 | 1.05 ± 0.02 | 1.02 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.02 | 1.01 ± 0.02 |
a Having only 0.5 mmCu additional filtration. b Having 0.5 mmCu+4.32 mmAl additional filtration.
The FA-GNPs were superior to the PEG-GNPs in cell targeting. The results also indicated that irradiating the cells to 180 kVp orthovoltage beams is more efficient. Therefore, various doses at this energy were applied on the cells incubated with and without the FA-GNPs to draw their survival curves. The resulting survival curves of the HeLa cells in the presence and absence of the FA-GNPs are shown in figure 9. As can be noted from the figure, there was an increase in the DEF values with the increase of absorbed dose at this kVp.
Figure 9. The survival curves of HeLa cells in the presence and absence of FA-GNPs irradiated to the 180 kVp orthovoltage x-ray beam.
Download figure:
Standard image High-resolution imageDiscussion
This study focused on the modification of GNPs by conjugating them with folate to facilitate their internalization into cancerous HeLa cells. Also it focused on the impact of different photon beam energies (ranging from kilovoltage to megavoltage) on the enhancement of cell damage when irradiations were made in the presence of the nanoparticles. Based on the results, it can be concluded that all the common orthovoltage photon energies investigated in this study (from 120 to 250 kVp) enhance the death rate of the cancerous cells containing the FA-GNPs and PEG-GNPs. However, the DEFs of all the orthovoltage beams were greater than those of the Co-60 γ-rays. This can be ascribed to the increase in the photoelectric interaction cross-section of the relevant photons of the orthovoltage x-ray beams with the GNPs compared to the Co-60 megavoltage γ-rays (Van den Heuvel et al 2010). Furthermore, the DEFs of the cells conjugated with the FA-GNPs were greater than those conjugated with non-folate GNPs (PEG-GNPs) for all the photon energies used. This can be due to the high level of FRs on the HeLa cell membrane leading subsequently to the higher internalization of the folate GNPs into the cells, as noted in figure 5. Although folate-conjugated GNPs are preferred for their higher internalization into the cells compared to pegylated GNPs, it is chemically possible to synthesize folate-conjugated pegylated GNPs (FA-GNPs-PEG) to create the advantage of two different surface modifications of such a compound; namely cell targeting and good colloidal stability. According to a previous study (Chithrani et al 2006) the optimal uptake of GNPs occurs with spherical GNPs having a 50 nm diameter. Therefore, we attempted to synthesize GNPs of this size and conjugated them with the folate to have a maximal uptake. Our in vitro study showed an increase of FA-GNP accumulation in the cells. However, it was also demonstrated that the folate-conjugated nanoparticles have great potential for selectively targeting vascularized tumor cells expressing significant levels of FRs, where they are administered intravenously in in vivo situations (Leamon and Low 2001).
We also used a fixed concentration of GNPs to perform a reliable comparison between the irradiation plus FA-GNP and/or PEG-GNP groups. Previous studies (Brun et al 2009, Rahman et al 2009, Shakeri-Zadeh et al 2010a, 2010b) have reported that increasing the GNP concentration would lead to the increase of the DEF. Therefore, we used the highest concentration of GNPs having the lowest level of toxicity based on our cytotoxicity assays (figure 6(a)).
To have the most efficient radiation treatment in tumors loaded with GNPs, it is necessary to select the most efficient beam energy. Based on a conceptual approach, irradiation of such tumors with an energy equal to or just above the gold K-edge may be considered as a good option (Brun et al 2009). However, irradiation of glioma cells loaded with platinum (Pt) has shown identical results using beam energies below and above the Pt K-edge (Biston et al 2004). In a comprehensive study done by Brun et al (2009), the effect of three key parameters (GNP size and concentration and beam energy) on the GNP radiosensitization level has been investigated. The most efficient photon energy reported in that study was 50 keV among an estimated range of energies from 15 to 70 keV. To rationalize this observation, they focused on the ratio of the µen/ρ values of gold to water, which is freely available from the National Institute of Standards and Technology web site. Based on the relevant µen/ρ values of gold and water at different energies ranging from a few keVs to several meVs, the maximum ratio of µen/ρ of gold to water occurs at between 40 and 50 keVs. In our study the maximum DEF (1.67 ± 0.11) was obtained at an equivalent energy of 48.1 keV (table 1), which can be attributed to the higher ratio of µen/ρ of gold to water at this energy. In addition, we focused on the effect of targeting the GNPs on the cell damage. In a similar study (Roa et al 2009), the combination of thio-glucose-capped GNPs (Glu-GNPs) with 200 kVp x-rays resulted in 1.5 to 2 DEF within 72 h, which is in agreement with our results. However, various values of the DEFs reported by others ranged from a lower value of 1.23 to a much higher value of 24.6 (Brun et al 2009, Rahman et al 2009, Liu et al 2010, Kong et al 2008) using orthovoltage x-ray beams quite different from ours. Such a wide range of variation in the DEFs must have arisen from the differences in the GNP size, concentration and conjugation, as well as the different cell lines used in those studies. We also investigated the radiation dose enhancement effects in the presence of the GNPs using the common radiobiological methods of MTT and clonogenic assays with different end points that confirmed each other by indicating an increasing trend of the DEFs with the orthovoltage x-ray beams up to 180 kVp.
Conclusions
Based on the overall results it can be concluded that folate-conjugated GNPs can be used as a suitable radiosensitization agent for FR-expressing cancerous cells, such as HeLa, to enhance the effect of the radiation dose produced by orthovoltage x-ray radiotherapy machines or similar modalities producing polyenergetic x-ray beams at energies close to 180 kVp or monoenergetic γ-rays of 50 keV. However, our results are only based on an in vitro study. Hence, more in vivo investigations and also clinical trials are required before we can recommend our technique for clinical applications. Thereafter, and if such comprehensive in vivo studies lead to findings similar to our in vitro results, the application of this novel technique in clinical practice could enable oncologists to decrease prescribed radiation doses, while having the same damage to tumor cells as the target, as well as greater protection of normal tissues/organs at risk located close to the target. Such advantages could also be proposed for future studies to improve the treatment efficacy of intraoperative radiotherapy and brachytherapy procedures, in which similar kilovoltage photon beams can be generated and used.
Acknowledgments
This study is the result of a PhD project carried out by the first author under the supervision of the second author and with the help and advice of other co-authors as the advisers at Tarbiat Modares University with the collaboration of Imam Hossein Hospital in Tehran, Iran. Hence, we would like to express our special thanks to these institutions for providing us the financial, technical and clinical support required for carrying out this research. Our special thanks are expressed to Mrs Nafiseh Farzi and Ms Manijeh Beygi for their generous help and cooperation provided for the irradiation of the cells and dosimetry procedures.