-
PDF
- Split View
-
Views
-
Cite
Cite
Shinichiro Takahashi, Kanji Nagai, Norio Saito, Masaru Konishi, Toshio Nakagohri, Naoto Gotohda, Mitsuyo Nishimura, Junji Yoshida, Taira Kinoshita, Multiple Resections for Hepatic and Pulmonary Metastases of Colorectal Carcinoma, Japanese Journal of Clinical Oncology, Volume 37, Issue 3, March 2007, Pages 186–192, https://doi.org/10.1093/jjco/hym006
Close - Share Icon Share
Abstract
Resections are effective for some patients with both hepatic and pulmonary metastases of colorectal cancer, but the best selection criteria for the resections and effective treatment for recurrence after the resections have not been determined.
A retrospective analysis was performed for 30 consecutive patients who received aggressive multiple resections for both hepatic and pulmonary metastases of colorectal cancer. Recurrences after resections were surgically treated whenever resectable.
For the 30 patients, 45 hepatectomies and 40 pulmonary resections were performed and 17 patients received three or more resections. No mortality was observed. Overall survival after the first metastasectomy for the second organ (liver or lung) was 58% and nine 5-year survivors were observed. Multivariate analyses revealed that primary colon cancer, stage IV in TNM classification and maximum size of hepatic tumor >3 cm at initial hepatectomy were poor prognostic factors, but several long-term survivors were observed even among patients with those factors.
Multiple resections for hepatic and pulmonary metastases of colorectal cancer are safe and effective. No single factor is considered to be a contraindication for the resections. For recurrence after the resections, surgical resection is also recommended if resectable.
INTRODUCTION
The liver and lung are the most common sites of distant metastases for colorectal carcinoma (1). Hepatic and pulmonary metastases may be detected sequentially or simultaneously in patients with colorectal carcinoma. Efficacy of resections for these two distant metastases has been reported in several studies (2–14). However, the criteria to select patients for those resections are still obscure.
In addition, although recurrence after those resections is one of the major problems of the strategy, further surgical approaches for recurrence after those resections are controversial.
The purpose of this study was to evaluate the efficacy of aggressive multiple resections for hepatic and pulmonary metastases of colorectal carcinoma and to find prognostic factors that might elucidate who would benefit most from hepatic and pulmonary resections for colorectal metastases.
PATIENTS AND METHODS
Two hundred and sixty-seven patients who had undergone hepatic resection and 98 patients who had undergone pulmonary resection, as the first treatment for colorectal metastasis at the National Cancer Center Hospital East between September 1992 and June 2005 were examined retrospectively. Eight patients had undergone surgical resections for both hepatic and pulmonary metastases as the first treatment for colorectal metastases. Metastases were synchronous with primary colorectal carcinoma in one of the eight patients. In the remaining 259 patients who had undergone hepatic resection as the first treatment for colorectal metastasis, 83 had the second recurrence in the liver, 29 in the lung, 12 in both liver and lung and 52 in the other organs. Sixteen of the 29 patients with pulmonary recurrence and one of the 12 patients with both hepatic and pulmonary recurrences were treated surgically. Two patients had undergone resections for both hepatic and pulmonary recurrences after more than two hepatic metastasectomies. In the remaining 90 patients who had undergone pulmonary resection as the first treatment for colorectal metastasis, three had the second recurrence in the liver, 27 in the lung, four in both liver and lung and 16 in other organs. All three patients with hepatic recurrence were treated surgically. However, all four patients with both hepatic and pulmonary recurrences underwent systemic chemotherapy as the second treatment.
As a result, 30 patients underwent both hepatic and pulmonary resections for colorectal metastasis. The patients consisted of 19 men and 11 women, ranging in age from 24 to 75 years with a mean of 59 years. Two of the patients had received adjuvant chemotherapy (tegafur/uracil and 5-fluorouracil/leucovorin) after primary colorectal resection and one patient had received preoperative chemoradiation for rectal cancer.
The criteria for hepatectomy were as follows: (1) metastatic lesions are confined to the liver and technically resectable, (2) no extrahepatic metastases except resectable pulmonary metastasis are detected, and (3) liver function is equal to complete resection of all hepatic tumors. The criteria for pulmonary resection were as follows: (1) metastatic lesions are confined to the lung and technically resectable, (2) no extrathoracic metastases except resectable hepatic metastasis are detected, and (3) cardiorespiratory function is equal to complete resection of all pulmonary tumors. The timing of the detection of hepatic and pulmonary metastases or the number of prior resections for metastases did not affect these criteria, so the selection criteria for further resections for recurrences after hepatic and pulmonary resections are the same as above.
At hepatectomy, intraoperative ultrasonography was performed to confirm tumor location and size of the lesions in all patients, and all of the resections were ultrasound-guided procedures. Hepatic resection was performed by the forceps fracture method under inflow occlusion (Pringle's maneuver). At pulmonary resection, hilar or mediastinal lymph node dissection was used to sample lymph nodes of most patients who had a lobectomy.
When hepatic and pulmonary metastases were detected simultaneously, hepatic resection was carried out first, followed by pulmonary resection.
No patient received adjuvant chemotherapy after hepatectomy or pulmonary resection.
After hepatic or pulmonary resection, patients were closely followed with diagnostic imaging [chest X-ray and abdominal computed tomography (CT)] and measurement of serum carcinoembryonic antigen (CEA) levels every 3 months; they also underwent an annual colonoscopy to detect any tumor recurrence. The median follow-up of survivors was 53 months.
Morphological Investigations
The resected specimens of colon or rectum, liver and lung were fixed in 10% phosphate-buffered formalin, cut at intervals of 5 mm and embedded in paraffin. Serial sections of 3-µm thickness were stained with hematoxylin and eosin for morphological examination. Each case was histologically classified according to the histological type, tumor size, location, number of metastases, presence of serosal invasion, nodal status and margin status. Histological diagnosis was performed according to the World Health Organization intestinal tumor classification (15).
Statistical Analysis
The student t-test was used to compare data between subgroups by the location of the primary tumor. The Mann–Whitney's U test was used to compare serum CEA levels between subgroups. Analyses of survival rates were performed using the Kaplan–Meier method (16) and differences between the curves were tested using the log-rank test. Factors related to survival were analyzed with the Cox proportional hazards regression model (17). A P value of less than 0.05 was considered to denote significance.
RESULTS
Clinicopathological Features of Primary and Metastatic Tumors
The primary tumors were staged as I (n = 1), II (n = 10), III (n = 15) and IV (n = 4) according to TNM classification (Table 1). All patients at stage IV had hepatic metastasis at resection of the primary tumor.
Correlation between clinicopathologic factors and overall survival in patients with resected hepatic and pulmonary metastases from colorectal cancer
| . | No. . | Median survival (mo) . | P value . | . | No. . | Median survival (mo) . | P value . |
|---|---|---|---|---|---|---|---|
| Primary colorectal lesion | Pulmonary metastases | ||||||
| Location | First pulmonary resection | ||||||
| rectum | 13 | 52.7 | 0.03 | Number of tumors | |||
| colon | 17 | 38.6 | 1 | 18 | 47.9 | 0.31 | |
| TNM classification | ≥2 | 12 | 27.1 | ||||
| I | 1 | 88.9 | 0.02* | Maximum size of the tumor (cm) | |||
| II | 10 | 48.9 | <3 | 21 | 34.8 | 0.69 | |
| III | 15 | 38.8 | ≥3 | 9 | 38.8 | ||
| IV | 4 | 14.6 | Distribution of metastases | ||||
| Lymph node metastasis | unilobar | 24 | 42.1 | 0.68 | |||
| absent | 11 | 54.8 | 0.64 | bilobar | 6 | 27.1 | |
| present | 19 | 32.8 | Hilar or mediastinal lymph node | ||||
| Histological type of adenocarcinoma | negative | 28 | 36.7 | 0.89 | |||
| well or moderately differentiated | 28 | 38.7 | 0.77 | positive | 2 | 43.6 | |
| poorly differentiated and others | 2 | 41.7 | All pulmonary resections | ||||
| Number of tumors | |||||||
| Hepatic metastases | <3 | 22 | 38.7 | 0.92 | |||
| First hepatectomy | ≥3 | 8 | 44.8 | ||||
| Number of tumors | Maximum size of the tumor (cm) | ||||||
| 1 | 18 | 40.8 | 0.26 | <3 | 19 | 34.8 | 0.93 |
| ≥2 | 12 | 36.8 | ≥3 | 11 | 38.8 | ||
| Maximum size of the tumor (cm) | Distribution of metastases | ||||||
| <3 | 14 | 40.0 | 0.03 | unilobar | 21 | 41.1 | 0.97 |
| ≥3 | 16 | 35.8 | bilobar | 9 | 30.8 | ||
| Distribution of metastases | |||||||
| unilobar | 20 | 40.8 | 0.36 | CEA level at initial recurrence (ng/ml) | |||
| bilobar | 10 | 36.8 | <50 | 25 | 38.7 | 0.34 | |
| Lymph node of hepatoduodenal ligament | ≥50 | 5 | 33.0 | ||||
| negative | 29 | 38.8 | 0.02 | Disease-free interval from resection of primary tumor | |||
| positive | 1 | 13.9 | <1 year | 19 | 38.8 | 0.23 | |
| All hepatectomies | ≥1 year | 11 | 38.6 | ||||
| Total number of tumors | Simultaneous detection of hepatic and pulmonary recurrences | ||||||
| <3 | 19 | 38.6 | 0.79 | yes | 11 | 34.8 | 0.35 |
| ≥3 | 11 | 38.8 | no | 19 | 38.8 | ||
| Maximum size of the tumor (cm) | Initial metastasis in the lung | ||||||
| <3 | 13 | 38.8 | 0.08 | yes | 3 | 54.8 | 0.72 |
| ≥3 | 17 | 38.6 | no | 27 | 38.6 | ||
| Distribution of metastases | Total number of liver and lung resections | ||||||
| unilobar | 17 | 43.0 | 0.49 | 2 | 13 | 33.0 | 0.50 |
| bilobar | 13 | 34.8 | ≥3 | 17 | 54.3 |
| . | No. . | Median survival (mo) . | P value . | . | No. . | Median survival (mo) . | P value . |
|---|---|---|---|---|---|---|---|
| Primary colorectal lesion | Pulmonary metastases | ||||||
| Location | First pulmonary resection | ||||||
| rectum | 13 | 52.7 | 0.03 | Number of tumors | |||
| colon | 17 | 38.6 | 1 | 18 | 47.9 | 0.31 | |
| TNM classification | ≥2 | 12 | 27.1 | ||||
| I | 1 | 88.9 | 0.02* | Maximum size of the tumor (cm) | |||
| II | 10 | 48.9 | <3 | 21 | 34.8 | 0.69 | |
| III | 15 | 38.8 | ≥3 | 9 | 38.8 | ||
| IV | 4 | 14.6 | Distribution of metastases | ||||
| Lymph node metastasis | unilobar | 24 | 42.1 | 0.68 | |||
| absent | 11 | 54.8 | 0.64 | bilobar | 6 | 27.1 | |
| present | 19 | 32.8 | Hilar or mediastinal lymph node | ||||
| Histological type of adenocarcinoma | negative | 28 | 36.7 | 0.89 | |||
| well or moderately differentiated | 28 | 38.7 | 0.77 | positive | 2 | 43.6 | |
| poorly differentiated and others | 2 | 41.7 | All pulmonary resections | ||||
| Number of tumors | |||||||
| Hepatic metastases | <3 | 22 | 38.7 | 0.92 | |||
| First hepatectomy | ≥3 | 8 | 44.8 | ||||
| Number of tumors | Maximum size of the tumor (cm) | ||||||
| 1 | 18 | 40.8 | 0.26 | <3 | 19 | 34.8 | 0.93 |
| ≥2 | 12 | 36.8 | ≥3 | 11 | 38.8 | ||
| Maximum size of the tumor (cm) | Distribution of metastases | ||||||
| <3 | 14 | 40.0 | 0.03 | unilobar | 21 | 41.1 | 0.97 |
| ≥3 | 16 | 35.8 | bilobar | 9 | 30.8 | ||
| Distribution of metastases | |||||||
| unilobar | 20 | 40.8 | 0.36 | CEA level at initial recurrence (ng/ml) | |||
| bilobar | 10 | 36.8 | <50 | 25 | 38.7 | 0.34 | |
| Lymph node of hepatoduodenal ligament | ≥50 | 5 | 33.0 | ||||
| negative | 29 | 38.8 | 0.02 | Disease-free interval from resection of primary tumor | |||
| positive | 1 | 13.9 | <1 year | 19 | 38.8 | 0.23 | |
| All hepatectomies | ≥1 year | 11 | 38.6 | ||||
| Total number of tumors | Simultaneous detection of hepatic and pulmonary recurrences | ||||||
| <3 | 19 | 38.6 | 0.79 | yes | 11 | 34.8 | 0.35 |
| ≥3 | 11 | 38.8 | no | 19 | 38.8 | ||
| Maximum size of the tumor (cm) | Initial metastasis in the lung | ||||||
| <3 | 13 | 38.8 | 0.08 | yes | 3 | 54.8 | 0.72 |
| ≥3 | 17 | 38.6 | no | 27 | 38.6 | ||
| Distribution of metastases | Total number of liver and lung resections | ||||||
| unilobar | 17 | 43.0 | 0.49 | 2 | 13 | 33.0 | 0.50 |
| bilobar | 13 | 34.8 | ≥3 | 17 | 54.3 |
CEA, carcinoembryonic antigen.
*Stage I, II or III versus Stage IV.
Correlation between clinicopathologic factors and overall survival in patients with resected hepatic and pulmonary metastases from colorectal cancer
| . | No. . | Median survival (mo) . | P value . | . | No. . | Median survival (mo) . | P value . |
|---|---|---|---|---|---|---|---|
| Primary colorectal lesion | Pulmonary metastases | ||||||
| Location | First pulmonary resection | ||||||
| rectum | 13 | 52.7 | 0.03 | Number of tumors | |||
| colon | 17 | 38.6 | 1 | 18 | 47.9 | 0.31 | |
| TNM classification | ≥2 | 12 | 27.1 | ||||
| I | 1 | 88.9 | 0.02* | Maximum size of the tumor (cm) | |||
| II | 10 | 48.9 | <3 | 21 | 34.8 | 0.69 | |
| III | 15 | 38.8 | ≥3 | 9 | 38.8 | ||
| IV | 4 | 14.6 | Distribution of metastases | ||||
| Lymph node metastasis | unilobar | 24 | 42.1 | 0.68 | |||
| absent | 11 | 54.8 | 0.64 | bilobar | 6 | 27.1 | |
| present | 19 | 32.8 | Hilar or mediastinal lymph node | ||||
| Histological type of adenocarcinoma | negative | 28 | 36.7 | 0.89 | |||
| well or moderately differentiated | 28 | 38.7 | 0.77 | positive | 2 | 43.6 | |
| poorly differentiated and others | 2 | 41.7 | All pulmonary resections | ||||
| Number of tumors | |||||||
| Hepatic metastases | <3 | 22 | 38.7 | 0.92 | |||
| First hepatectomy | ≥3 | 8 | 44.8 | ||||
| Number of tumors | Maximum size of the tumor (cm) | ||||||
| 1 | 18 | 40.8 | 0.26 | <3 | 19 | 34.8 | 0.93 |
| ≥2 | 12 | 36.8 | ≥3 | 11 | 38.8 | ||
| Maximum size of the tumor (cm) | Distribution of metastases | ||||||
| <3 | 14 | 40.0 | 0.03 | unilobar | 21 | 41.1 | 0.97 |
| ≥3 | 16 | 35.8 | bilobar | 9 | 30.8 | ||
| Distribution of metastases | |||||||
| unilobar | 20 | 40.8 | 0.36 | CEA level at initial recurrence (ng/ml) | |||
| bilobar | 10 | 36.8 | <50 | 25 | 38.7 | 0.34 | |
| Lymph node of hepatoduodenal ligament | ≥50 | 5 | 33.0 | ||||
| negative | 29 | 38.8 | 0.02 | Disease-free interval from resection of primary tumor | |||
| positive | 1 | 13.9 | <1 year | 19 | 38.8 | 0.23 | |
| All hepatectomies | ≥1 year | 11 | 38.6 | ||||
| Total number of tumors | Simultaneous detection of hepatic and pulmonary recurrences | ||||||
| <3 | 19 | 38.6 | 0.79 | yes | 11 | 34.8 | 0.35 |
| ≥3 | 11 | 38.8 | no | 19 | 38.8 | ||
| Maximum size of the tumor (cm) | Initial metastasis in the lung | ||||||
| <3 | 13 | 38.8 | 0.08 | yes | 3 | 54.8 | 0.72 |
| ≥3 | 17 | 38.6 | no | 27 | 38.6 | ||
| Distribution of metastases | Total number of liver and lung resections | ||||||
| unilobar | 17 | 43.0 | 0.49 | 2 | 13 | 33.0 | 0.50 |
| bilobar | 13 | 34.8 | ≥3 | 17 | 54.3 |
| . | No. . | Median survival (mo) . | P value . | . | No. . | Median survival (mo) . | P value . |
|---|---|---|---|---|---|---|---|
| Primary colorectal lesion | Pulmonary metastases | ||||||
| Location | First pulmonary resection | ||||||
| rectum | 13 | 52.7 | 0.03 | Number of tumors | |||
| colon | 17 | 38.6 | 1 | 18 | 47.9 | 0.31 | |
| TNM classification | ≥2 | 12 | 27.1 | ||||
| I | 1 | 88.9 | 0.02* | Maximum size of the tumor (cm) | |||
| II | 10 | 48.9 | <3 | 21 | 34.8 | 0.69 | |
| III | 15 | 38.8 | ≥3 | 9 | 38.8 | ||
| IV | 4 | 14.6 | Distribution of metastases | ||||
| Lymph node metastasis | unilobar | 24 | 42.1 | 0.68 | |||
| absent | 11 | 54.8 | 0.64 | bilobar | 6 | 27.1 | |
| present | 19 | 32.8 | Hilar or mediastinal lymph node | ||||
| Histological type of adenocarcinoma | negative | 28 | 36.7 | 0.89 | |||
| well or moderately differentiated | 28 | 38.7 | 0.77 | positive | 2 | 43.6 | |
| poorly differentiated and others | 2 | 41.7 | All pulmonary resections | ||||
| Number of tumors | |||||||
| Hepatic metastases | <3 | 22 | 38.7 | 0.92 | |||
| First hepatectomy | ≥3 | 8 | 44.8 | ||||
| Number of tumors | Maximum size of the tumor (cm) | ||||||
| 1 | 18 | 40.8 | 0.26 | <3 | 19 | 34.8 | 0.93 |
| ≥2 | 12 | 36.8 | ≥3 | 11 | 38.8 | ||
| Maximum size of the tumor (cm) | Distribution of metastases | ||||||
| <3 | 14 | 40.0 | 0.03 | unilobar | 21 | 41.1 | 0.97 |
| ≥3 | 16 | 35.8 | bilobar | 9 | 30.8 | ||
| Distribution of metastases | |||||||
| unilobar | 20 | 40.8 | 0.36 | CEA level at initial recurrence (ng/ml) | |||
| bilobar | 10 | 36.8 | <50 | 25 | 38.7 | 0.34 | |
| Lymph node of hepatoduodenal ligament | ≥50 | 5 | 33.0 | ||||
| negative | 29 | 38.8 | 0.02 | Disease-free interval from resection of primary tumor | |||
| positive | 1 | 13.9 | <1 year | 19 | 38.8 | 0.23 | |
| All hepatectomies | ≥1 year | 11 | 38.6 | ||||
| Total number of tumors | Simultaneous detection of hepatic and pulmonary recurrences | ||||||
| <3 | 19 | 38.6 | 0.79 | yes | 11 | 34.8 | 0.35 |
| ≥3 | 11 | 38.8 | no | 19 | 38.8 | ||
| Maximum size of the tumor (cm) | Initial metastasis in the lung | ||||||
| <3 | 13 | 38.8 | 0.08 | yes | 3 | 54.8 | 0.72 |
| ≥3 | 17 | 38.6 | no | 27 | 38.6 | ||
| Distribution of metastases | Total number of liver and lung resections | ||||||
| unilobar | 17 | 43.0 | 0.49 | 2 | 13 | 33.0 | 0.50 |
| bilobar | 13 | 34.8 | ≥3 | 17 | 54.3 |
CEA, carcinoembryonic antigen.
*Stage I, II or III versus Stage IV.
At the initial hepatectomy, the average number of hepatic tumors was 2.1 (range, 1–12), the average maximum size was 3.2 cm (range, 0.3–9 cm) and the average preoperative CEA level was 19.9 ng/ml (range, 0.8–68.5 ng/ml). In all hepatectomies, the average number of hepatic tumors was 2.8 and the average maximum size was 3.3 cm. Lymph node metastasis at the hepatoduodenal ligament was shown in one patient.
Regarding pulmonary metastases, the average number of pulmonary tumors was 1.8 (range, 1–5), the average maximum size was 2.2 cm (range, 0.7–6.7 cm) and the average prethoracotomy CEA level was 12.4 ng/ml (range, 1.0–66.7 ng/ml) at initial pulmonary resection. In all pulmonary resections, the average number of pulmonary tumors was 2.1 and the average maximum size was 2.5 cm. Hilar lymph node metastasis of the lung was shown in two patients.
Surgical Resections for Hepatic and Pulmonary Metastases
Forty-five hepatectomies (30 partial resections, four subsegmentectomies, seven segmentectomies and four lobectomies according to Couinaud's anatomical classification (18)) and 40 pulmonary resections (32 partial resections, seven lobectomies and one pneumonectomy) were performed on the 30 patients. The average number of operations performed for hepatic or pulmonary metastases per patient was 2.8. Three operations were performed on 11 patients, four operations on four patients each and five operations on two patients each.
There was no perioperative mortality. Five complications were observed: two cases of biliary leak and one case each of portal vein thrombosis after hepatectomy, wound infection and air leak after pulmonary resection.
The location of initial metastasis was lung in three patients, liver in 19, and both liver and lung in eight. Eleven patients experienced hepatic and pulmonary metastases detected simultaneously.
Recurrence after Surgical Resections for Hepatic and Pulmonary Metastases
Among 30 patients who underwent surgical resections for hepatic and pulmonary metastases, 25 developed recurrences when recurrence was defined as the first recurrent disease after at least one resection each for hepatic and pulmonary metastases. Locations of recurrences were as follows: lung in 11 patients, liver and lymph node in four each, both liver and lung in three, peritoneum, local recurrence and brain in one each. Re-resection could be performed in 15 of the 25 patients. Of the remaining 10 patients, eight received systemic chemotherapy, one each received radiation therapy and best supportive care.
Survival
Survival time was calculated from the date of the first metastasectomy for the second organ metastasized (liver or lung).
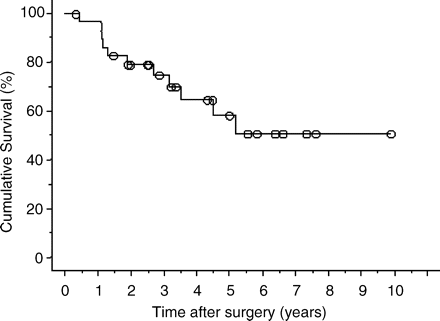
Actuarial overall survival was 58% at 5 years with a median survival of 39 months (Fig. 1). Disease-free survival was 56% at 1 year and 8% at 3 years, with a median recurrence-free survival of 13 months. Nine 5-year survivors were observed and eight of the nine patients are still alive without disease. Of the nine 5-year survivors, six had undergone three operations and one had undergone four operations.
Cumulative survival curves for 30 patients who underwent resections for both hepatic and pulmonary metastases of colorectal cancer.
When survival time was calculated from the date of the first metastasectomy for the first organ, actuarial overall survival was 70% at 5 years with a median survival of 60 months.
Correlation between Clinicopathologic Factors and Overall Survival
To find prognostic factors for survival after resection of hepatic and pulmonary metastases, clinicopathologic factors and overall survival calculated from the date of the first metastasectomy for the second organ were analyzed in 30 patients (Table 1). Primary colon carcinoma (P = 0.03), stage IV in TNM classification (P = 0.02), maximum size of hepatic tumor >3 cm at initial hepatectomy (P = 0.03), and lymph node metastasis of the hepatoduodenal ligament (P = 0.02) were significantly associated with poor overall survival. Whether hepatic and pulmonary metastases were detected simultaneously or sequentially was not correlated with survival (P = 0.35). Neither a disease-free interval of less than 1 year from resection of the primary tumor nor initial metastasis in the lung affected survival.
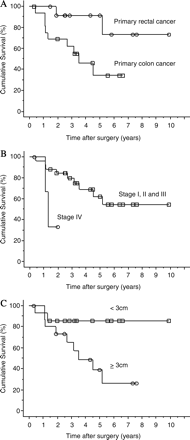
We examined the independent predictive value of the aforementioned factors on overall survival (Table 2). Lymph node metastasis of the hepatoduodenal ligament was excluded from the analysis because only one of the 30 patients had the factor. Primary colon carcinoma (Fig. 2A), stage IV in TNM classification (Fig. 2B), and maximum size of hepatic tumor >3 cm at initial hepatectomy (Fig. 2C) had predictive value for decreased overall survival after resection of hepatic and pulmonary metastases from colorectal cancer.
Cumulative survival curves after resections for hepatic and pulmonary metastases of colorectal cancer according to (A) location of primary tumor, (B) stage in TNM classification, and (C) maximum size of hepatic tumor at initial.
Multivariate analyses of factors affecting overall survival in patients with resected hepatic and pulmonary metastases from colorectal cancer
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Location of primary tumor | ||
| Rectum | — | 0.01 |
| Colon | 8.74 (1.53—49.91) | |
| TNM classification of primary tumor | ||
| I, II, III | — | 0.03 |
| IV | 11.37 (1.34—96.53) | |
| Maximum size of tumor at first hepatectomy (cm) | ||
| <3 | — | <0.01 |
| ≥3 | 14.47 (2.33–89.85) | |
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Location of primary tumor | ||
| Rectum | — | 0.01 |
| Colon | 8.74 (1.53—49.91) | |
| TNM classification of primary tumor | ||
| I, II, III | — | 0.03 |
| IV | 11.37 (1.34—96.53) | |
| Maximum size of tumor at first hepatectomy (cm) | ||
| <3 | — | <0.01 |
| ≥3 | 14.47 (2.33–89.85) | |
CI, confidence interval; CEA, carcinoembryonic antigen.
Multivariate analyses of factors affecting overall survival in patients with resected hepatic and pulmonary metastases from colorectal cancer
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Location of primary tumor | ||
| Rectum | — | 0.01 |
| Colon | 8.74 (1.53—49.91) | |
| TNM classification of primary tumor | ||
| I, II, III | — | 0.03 |
| IV | 11.37 (1.34—96.53) | |
| Maximum size of tumor at first hepatectomy (cm) | ||
| <3 | — | <0.01 |
| ≥3 | 14.47 (2.33–89.85) | |
| . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Location of primary tumor | ||
| Rectum | — | 0.01 |
| Colon | 8.74 (1.53—49.91) | |
| TNM classification of primary tumor | ||
| I, II, III | — | 0.03 |
| IV | 11.37 (1.34—96.53) | |
| Maximum size of tumor at first hepatectomy (cm) | ||
| <3 | — | <0.01 |
| ≥3 | 14.47 (2.33–89.85) | |
CI, confidence interval; CEA, carcinoembryonic antigen.
Comparing clinicopathological factors of patients with primary colon carcinoma and those of patients with primary rectal carcinoma, maximum size of pulmonary tumors (2.6 ± 1.6 cm versus 1.7 ± 0.7 cm) was significantly larger and prethoracotomy CEA level (18.2 ± 23.8 ng/ml versus 5.3 ± 5.4 ng/ml) was significantly higher in patients with primary colon carcinoma. The interval from primary resection to the first pulmonary resection tended to be longer in patients with primary colon carcinoma than in patients with primary rectal carcinoma (25.7 months versus 17.1 months, median).
DISCUSSION
Results of this study indicate that aggressive multiple resections for hepatic and pulmonary metastases of colorectal carcinoma are safe and contribute to long-term survival in some patients.
Hepatic and pulmonary metastases may be detected sequentially or simultaneously in patients with colorectal carcinoma. Although two distant organs are affected by the disease, several studies have demonstrated the efficacy of resections for both hepatic and pulmonary metastases (2–14). However, because of the frequent recurrences after resections, the best selection criteria for resection have not been established.
Lenhart et al. reported a disease-free survival of only 24% at 2 years in patients who underwent sequential hepatic and pulmonary resections for colorectal metastases (9). In the present study, the 2-year disease-free survival rate after the first metastasectomy for the second organ was also 24% with a median disease-free survival of only 13 months. The best treatment strategy for the recurrences after hepatic and pulmonary resections is obscure. However, only surgical removal of metastases offers a chance of cure. Aggressive repeat metastasectomy has been applied for recurrences after hepatic and pulmonary resections in our institution.
For the 30 patients of the present study, 45 hepatectomies and 40 pulmonary resections were performed and 17 patients received three or more resections with a maximum of five resections. Overall survival after the first metastasectomy for the second organ was 58% and nine 5-year survivors were observed. Surprisingly, seven of the nine 5-year survivors had undergone three or more resections. When survival time was calculated from the date of the first metastasectomy for the first metastasized organ, overall survival reached 70% at 5 years with a median survival of 60 months in the present study. Little is available on the result of repeat metastasectomy for recurrences after hepatic and pulmonary resections. Our results of long-term survival after hepatic and pulmonary resections in spite of frequent recurrences support the view that patients who can undergo resections for both hepatic and pulmonary metastases of colorectal cancer are in a selected population but can sometimes survive a long time with multiple metastasectomies. Interestingly, a recent study by Shah et al. also reported 74% 5-year survival rate after multidisciplinary surgical metastasectomies for colorectal cancer (19). The strategy and results of Shah et al. were similar to ours. However, while a majority of the patients received adjuvant chemotherapy after metastasectomies in Shah's study, no patient underwent adjuvant chemotherapy in the present study. These results indicate that the strategy of aggressive multiple metastasectomies count more than postoperative chemotherapies in the treatment for very restricted population of patients.
We found three factors for poor prognosis: size of hepatic tumor >3 cm at the first hepatectomy, primary colon carcinoma and stage IV tumor.
Maximum size of the hepatic tumor has been reported to be one of the important prognostic factors after hepatic resections for colorectal hepatic metastasis (20,21). This factor could affect prognosis in this population.
The reason for poor prognosis in patients with primary colon cancer is unknown. Patients with primary colon cancer had larger pulmonary tumors, higher CEA levels at the first pulmonary resection and relatively longer intervals from primary resection to the first pulmonary resection than patients with primary rectal cancer. A higher prethoracotomy CEA level was a factor of poor prognosis after hepatic and pulmonary resections in several studies (6,11). However, the reason why patients with primary colon cancer had more advanced pulmonary tumors than those with primary rectal cancer was unclear. A ‘cascade’ hypothesis based on the anatomy of the draining veins from the colon and rectum suggests that pulmonary metastasis in patients with primary colon carcinoma might come from hepatic metastasis with progressive site-induced change; however, pulmonary metastasis in patients with primary rectal carcinoma might come directly from the primary tumor, which seemed to be compatible with our results (22–24). However, the prognostic power of primary tumor location has not been demonstrated yet in patients with resected colorectal pulmonary metastasis (25–27); further examinations are needed to verify the hypothesis.
Neither the large size of the hepatic tumor nor primary colon carcinoma might influence the selection criteria for hepatic and pulmonary resections, because several long-term survivors were observed, even among patients with those factors.
Patients with stage IV disease had a poorer prognosis and showed no long-term survival. However, stage IV itself should not be considered as a contraindication for resections because the follow-up duration of patients with stage IV was short and the poor prognosis in stage IV was not consistent with the result that the disease-free interval from primary resection showed no correlation with prognosis.
Other factors such as synchronous metastasis (5), bilateral or multiple lung metastases (5,7), multiple liver metastases (8), short disease-free interval (8), simultaneous liver and lung metastases (10), mediastinal nodes involvement (11), primary histology (12) and high levels of both CEA and CA19-9 before metastasectomy (13) have been reported as prognostic factors after hepatic and pulmonary metastasectomy of colorectal cancer. Among those factors, whether the timing of the detection of hepatic and pulmonary metastases influences prognosis after resections has been an issue. In the present study, none of the aforementioned factors, including the timing of the detection of the metastases, showed any prognostic value. Based on our results, no single factor that contraindicated resections for hepatic and pulmonary metastases of colorectal cancer was identified. Thus, surgical resections might be the best option when both hepatic and pulmonary metastases are resectable in colorectal cancer. However, treatment for patients with several poor prognostic factors for multiple resections is still unknown.
The reason for the high survival rate 5 years after resections for hepatic and pulmonary metastases in our study might be partly explained by precise intrathoracic and abdominal examinations using helical computed tomography (28,29). However, it can not be denied that patients who can undergo both hepatic and pulmonary metastasectomy for colon cancer might have unique characteristics in some factors. For example, there may be some unique host-tumor interaction, considering the rare possibility of both hepatic and pulmonary resections for colorectal metastases and the surprisingly high survival rate after the metastasecomies in spite of multiple, multiphase and multi-organ metastases. The aforementioned hypothesis is supported by the fact that excellent survival in the present study was achieved, unexpectedly, without any help of adjuvant chemotherapy, although adjuvant chemotherapy after pulmonary or hepatic metastasectomy is a potential treatment for improving the prognosis of patients with colorectal cancer. Further investigation to clarify the reason for the good prognosis of this population might elucidate the mechanisms of metastases in colorectal cancer.
A limitation of our study is the relatively small population, because patients who can undergo resections for both hepatic and pulmonary metastases of colorectal carcinoma are rare. There is some possibility that correlations between several clinicopathological factors such as positive lymph nodes of the hepatoduodenal ligament, hilus pulmonis, or mediastinum and survival after resections could not be sufficiently validated because of the small cohort. A large multi-institutional study is recommended to verify the correlation.
In conclusion, multiple resections for hepatic and pulmonary metastases of colorectal cancer are safe and effective. Surgical resections could be the best option for resectable hepatic and pulmonary metastases in colorectal cancer.
Acknowledgments
This work was supported in part by grants from Ministry of Health, Labour and Welfare.
Conflict of interest statement
None declared.