-
PDF
- Split View
-
Views
-
Cite
Cite
Kakuri M. Omari, Gareth R. John, Stuart C. Sealfon, Cedric S. Raine, CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis, Brain, Volume 128, Issue 5, May 2005, Pages 1003–1015, https://doi.org/10.1093/brain/awh479
Close - Share Icon Share
Abstract
Subsequent to demyelination in multiple sclerosis, myelin repair occurs but, as lesions age, the ability to remyelinate diminishes. Molecular pathways underlying oligodendrocyte behaviour during CNS remyelination remain to be elucidated. In this study, we report for the first time constitutive expression of the CXC/α chemokine receptors, CXCR1, CXCR2 and CXCR3, on oligodendrocytes in normal adult human CNS tissue, the levels of which were upregulated in multiple sclerosis and other neurological diseases (OND). In addition, both immature (A2B5+/O4+) and more mature (CNPase+) human oligodendrocytes in vitro expressed the same three receptors. The respective ligands to CXCR1, CXCR2 and CXCR3 [i.e. CXCL8/IL-8, CXCL1/GRO-α and CXCL10/IP-10), were absent in CNS tissue from normals and subjects with OND, but were present at high levels on hypertrophic (reactive) astrocytes at the edge of active (but not silent) multiple sclerosis lesions. Astrocytes in vitro could be induced to express chemokines following stimulation with pro-inflammatory cytokines. CXCL8 and CXCL1 production by human astrocytes at both the RNA and protein levels could be induced by interleukin (IL)-1β, while CXCL10 was induced by both IL-1β and interferon-γ. Since these cytokines are integral to inflammatory events occurring at the margins of active multiple sclerosis lesions, their upregulation in these regions may underlie the dynamics of chemokine expression observed herein. The simultaneous expression of different CXC chemokine receptors on oligodendrocytes, and their ligands on astrocytes around multiple sclerosis lesions, may bespeak novel functional roles for these immune system molecules in the recruitment of oligodendrocytes and remyelination.
Introduction
Chemokines are small, 8–14 kDa, basic proteins classified into four groups, CXC/α, CC/β, C/γ and CX3C/δ, based on the N-terminal amino acid sequence. Through interactions with seven transmembrane G protein-coupled receptors, chemokines have the ability to attract specific leukocyte cell types and play a role in a number of inflammatory conditions, including atherosclerosis, psoriasis and allergic responses (Murdoch and Finn, 2000; Zlotnik and Yoshie, 2000). Studies on multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis (EAE), have suggested that chemokines may be involved in establishment of disease. Th1-type effector cells expressing the chemokine receptors, CCR5 and CXCR3, appear to be recruited into CNS parenchyma by the chemokines, CCL3/MIP-1α, CCL5/RANTES and CXCL10/IP-10 (Karpus et al., 1995; Balashov et al., 1999; Tran et al., 2000; Fife et al., 2001; Teleshova et al., 2002), while CCL2/MCP-1, CCL3 and CCL5 are important in recruitment of macrophages via interactions with CCR1, CCR2 and CCR5 (Fife et al., 2000; Izikson et al., 2000; Huang et al., 2001; Trebst et al., 2001). It has also become evident that in addition to infiltrating cells of the immune system, resident CNS cells can function as a source of chemokines. In this regard, astrocytes, microglia and endothelial cells have been shown to produce a wide range of CXC and CC chemokines (Cole et al., 1998; Janabi et al., 1999; Hua and Lee, 2000; Shukaliak and Dorovini-Zis, 2000; Rezaie et al., 2002; Salmaggi et al., 2002; Filipovic et al., 2003; Meeuwsen et al., 2003; Omari et al., 2004). With regard to chemokine receptors, such molecules have also been localized on resident CNS cell types, specifically astrocytes, microglia and neurons (Lavi et al., 1997; Coughlan et al., 2000; Simpson et al., 2000a,b; Goldberg et al., 2001; Biber et al., 2002; Rezaie et al., 2002; Filipovic et al., 2003; Flynn et al., 2003).
Why resident CNS cells express chemokine receptors is not entirely understood. Chemokine receptor activation results in a number of downstream events including gene expression, cell adhesion, cell polarization and chemotaxis. Data on molecular pathways responsible for chemokine receptor signalling point to JAK/STAT proteins having an integral role (Mellado et al., 2001). In the rat, oligodendrocytes, the myelinating cells of the CNS, have been shown to express at least two chemokine receptors, CXCR1 and CXCR2 (Nguyen and Stangel, 2001). CXCL1/GRO-α, one of the ligands to CXCR2 (others include CXCL2/GRO-β, CXCL3/GRO-γ, CXCL5/ENA78, CXCL7/NAP-2 and CXCL8/IL-8), has been shown to play an important role in oligodendroglial development in that these cells proliferate in vitro in response to CXCL1 (Robinson et al., 1998). Moreover, oligodendrocyte precursor cell migration is halted in the presence of the same chemokine in vivo (Tsai et al., 2002). CXCL8, the ligand to CXCR1 chemokine receptor, along with CXCL6/GCP-2, has not been shown to affect oligodendrocytes, but is known to induce proliferation of microvascular endothelium and tumour cells (Heidemann et al., 2003; Mockenhaupt et al., 2003; Zhu et al., 2004). The chemokine, CXCL10, which shares the receptor CXCR3 with CXCL9/MIG and CXCL11/I-TAC, has been widely implicated to play an important role in the recruitment of leukocytes into the CNS (Balashov et al., 1999; Fife et al., 2001; Sorensen et al., 2002). Flynn et al. (2003) have also reported on the ability of CXCL10 to induce proliferation of human adult astrocytes in vitro. To date, there have been no studies on the expression and role of chemokine receptors on human oligodendrocytes.
Oligodendrocytes are a primary target in multiple sclerosis and are severely depleted during the course of the disease (Raine, 1997). However, mechanisms underlying the demise of oligodendrocytes remain in question (D'Souza et al., 1996; Bonetti and Raine, 1997; Lucchinetti et al., 2000; Barnett and Prineas, 2004). Interestingly, around some active lesions, oligodendrocytes are not only preserved, but may exist in increased numbers, indicating oligodendroglial hyperplasia (Raine et al., 1981; Prineas et al., 1989). It remains to be determined whether oligodendrocyte precursor cells (OPCs), or surviving cells, may be the source of new oligodendrocytes. Despite the presence of OPCs (Scolding et al., 1998; Chang et al., 2000; Maeda et al., 2001; Wolswijk, 2002), remyelination in multiple sclerosis is usually limited in extent (Prineas et al., 1993; Raine and Wu, 1993; Franklin, 2002). Related to this is evidence showing that oligodendrocyte maturation may be blocked via inhibitory signals (Wang et al., 1998; John et al., 2002). Other pathways influencing oligodendrocyte behaviour during the course of multiple sclerosis probably exist but remain to be elucidated. Perhaps relevant to this is the presence of receptors normally associated with the immune system on human oligodendrocytes, suggesting that this cell type may respond to other inflammatory signals (D'Souza et al., 1996; Bonetti and Raine, 1997; Cannella and Raine, 2004).
In view of current interest in chemokines and other factors that may influence oligodendroglial recruitment in multiple sclerosis, we have investigated expression of CXC chemokine receptors and a subset of their ligands during the course of the disease. Here, we demonstrate expression of the three receptors, CXCR1, CXCR2 and CXCR3, on oligodendrocytes in CNS samples from normal human, multiple sclerosis and other neurological disease (OND) cases, and on fetal human oligodendrocytes in vitro. In contrast, the respective ligands, CXCL8, CXCL1 and CXCL10, were not found in normal and OND samples, but were detected at high levels on hypertrophic astrocytes around active multiple sclerosis lesions. Interestingly, human astrocytes in vitro could be induced to produce high levels of these CXC chemokines after stimulation with proinflammatory cytokines, the latter known to be important in multiple sclerosis (Benvenuto et al., 1992; Link et al., 1994; Cannella and Raine, 1995). Taken in concert, our results suggest that oligodendrocyte behaviour during the course of multiple sclerosis may be influenced by astrocyte-derived CXC chemokines.
Material and methods
Human tissue samples
Cryostat sections were prepared from seven cases of multiple sclerosis, three primary progressive and four secondary progressive, containing chronic active and silent lesions. Control sections came from cerebral white matter from OND cases [olivopontocerebellar degeneration (OPCD), amyotrophic lateral sclerosis (ALS) and stroke], in addition to tissue from three neurologically normal subjects (Table 1). For work in vitro, human fetal glial cells were isolated from forebrain and spinal cord tissue, obtained from the Einstein Human Fetal Tissue Repository (New York, NY). Use of human tissue was conducted in accordance with approved Internal Review Board protocols.
Summary of cases utilized for immunohistochemistry
| Case no. . | Diagnosis . | Disease duration (years) . | Sex/age (years) . | Cause of death . | Post-mortem interval (h) . |
|---|---|---|---|---|---|
| 1 | PPMS | 11 | F/31 | Respiratory failure | 3 |
| 2 | PPMS | 17 | F/37 | Respiratory failure | 6 |
| 3 | PPMS | 4 | F/32 | Bronchopneumonia | 12 |
| 4 | SPMS | 9 | F/45 | Cardiac arrest | 11 |
| 5 | SPMS | 10 | F/38 | Bronchopneumonia | 8 |
| 6 | SPMS | 21 | F/56 | Cardiac arrest | 2 |
| 7 | SPMS | 15 | M/46 | Cardiac arrest | 8 |
| 8 | OPCD | 4 | M/31 | Bronchopneumonia | 4 |
| 9 | ALS | 5 | F/49 | Bronchopneumonia | 8 |
| 10 | Stroke | 12 h | F/80 | Stroke | 5 |
| 11 | Normal | NA | M/19 | Cardiac arrest/obesity | 9 |
| 12 | Normal | NA | M/40 | Adult respiratory distress | 8.5 |
| 13 | Normal | NA | F/80 | Metastatic cancer | 6 |
| Case no. . | Diagnosis . | Disease duration (years) . | Sex/age (years) . | Cause of death . | Post-mortem interval (h) . |
|---|---|---|---|---|---|
| 1 | PPMS | 11 | F/31 | Respiratory failure | 3 |
| 2 | PPMS | 17 | F/37 | Respiratory failure | 6 |
| 3 | PPMS | 4 | F/32 | Bronchopneumonia | 12 |
| 4 | SPMS | 9 | F/45 | Cardiac arrest | 11 |
| 5 | SPMS | 10 | F/38 | Bronchopneumonia | 8 |
| 6 | SPMS | 21 | F/56 | Cardiac arrest | 2 |
| 7 | SPMS | 15 | M/46 | Cardiac arrest | 8 |
| 8 | OPCD | 4 | M/31 | Bronchopneumonia | 4 |
| 9 | ALS | 5 | F/49 | Bronchopneumonia | 8 |
| 10 | Stroke | 12 h | F/80 | Stroke | 5 |
| 11 | Normal | NA | M/19 | Cardiac arrest/obesity | 9 |
| 12 | Normal | NA | M/40 | Adult respiratory distress | 8.5 |
| 13 | Normal | NA | F/80 | Metastatic cancer | 6 |
PPMS = primary progressive multiple sclerosis; SPMS = secondary progressive multiple sclerosis; OPCD = olivopontocerebellar degeneration; ALS = amyotrophic lateral sclerosis; NA = not applicable.
Summary of cases utilized for immunohistochemistry
| Case no. . | Diagnosis . | Disease duration (years) . | Sex/age (years) . | Cause of death . | Post-mortem interval (h) . |
|---|---|---|---|---|---|
| 1 | PPMS | 11 | F/31 | Respiratory failure | 3 |
| 2 | PPMS | 17 | F/37 | Respiratory failure | 6 |
| 3 | PPMS | 4 | F/32 | Bronchopneumonia | 12 |
| 4 | SPMS | 9 | F/45 | Cardiac arrest | 11 |
| 5 | SPMS | 10 | F/38 | Bronchopneumonia | 8 |
| 6 | SPMS | 21 | F/56 | Cardiac arrest | 2 |
| 7 | SPMS | 15 | M/46 | Cardiac arrest | 8 |
| 8 | OPCD | 4 | M/31 | Bronchopneumonia | 4 |
| 9 | ALS | 5 | F/49 | Bronchopneumonia | 8 |
| 10 | Stroke | 12 h | F/80 | Stroke | 5 |
| 11 | Normal | NA | M/19 | Cardiac arrest/obesity | 9 |
| 12 | Normal | NA | M/40 | Adult respiratory distress | 8.5 |
| 13 | Normal | NA | F/80 | Metastatic cancer | 6 |
| Case no. . | Diagnosis . | Disease duration (years) . | Sex/age (years) . | Cause of death . | Post-mortem interval (h) . |
|---|---|---|---|---|---|
| 1 | PPMS | 11 | F/31 | Respiratory failure | 3 |
| 2 | PPMS | 17 | F/37 | Respiratory failure | 6 |
| 3 | PPMS | 4 | F/32 | Bronchopneumonia | 12 |
| 4 | SPMS | 9 | F/45 | Cardiac arrest | 11 |
| 5 | SPMS | 10 | F/38 | Bronchopneumonia | 8 |
| 6 | SPMS | 21 | F/56 | Cardiac arrest | 2 |
| 7 | SPMS | 15 | M/46 | Cardiac arrest | 8 |
| 8 | OPCD | 4 | M/31 | Bronchopneumonia | 4 |
| 9 | ALS | 5 | F/49 | Bronchopneumonia | 8 |
| 10 | Stroke | 12 h | F/80 | Stroke | 5 |
| 11 | Normal | NA | M/19 | Cardiac arrest/obesity | 9 |
| 12 | Normal | NA | M/40 | Adult respiratory distress | 8.5 |
| 13 | Normal | NA | F/80 | Metastatic cancer | 6 |
PPMS = primary progressive multiple sclerosis; SPMS = secondary progressive multiple sclerosis; OPCD = olivopontocerebellar degeneration; ALS = amyotrophic lateral sclerosis; NA = not applicable.
Immunohistochemistry
Frozen sections (10 µm) fixed in acetone were quenched for endogenous peroxidase activity and blocked for 1 h with 10% normal goat or rabbit sera. Sections were then incubated with primary antibodies against CXCR1 (IgG2a; 1 : 100), CXCR2 (IgG2a; 1 : 50) and CXCR3 (IgG1; 1 : 100) (R&D Systems, Minneapolis, MN); or their ligands CXCL8, CXCL1 and CXCL10 (goat polyclonals; 1 : 10; R&D Systems), diluted in 2% normal goat or rabbit serum for 36 h at 4°C. Immunoreacted sections were then developed by incubation with secondary biotinylated antibodies, followed by the Vectastain avidin–biotin complex (ABC) solution (Vector Laboratories, Burlingame, CA), and 3,3′-diaminobenzidine (DAB) solution (KPL, Gaithersburg, MD). Two-colour immunohistochemistry was performed by re-incubating CXCL1-labelled slides with primary anti-CXCR2 antibody, followed by biotinylated anti-mouse IgG2a antibody (SouthernBiotech, Birmingham, AL), ABC solution and then Vector VIP peroxidase chromogen (Vector). To prevent cross-reactivity, slides were treated with a biotin/avidin blocking reagent (Vector) prior to incubation with the second primary antibody. As negative controls, sections were incubated with isotype-matched irrelevant antibodies (monoclonal IgG2a and IgG1; BD Biosciences, San Diego, CA), at the same concentration as the primary antibody, or with carrier buffer alone. To rule out non-specific staining further, the anti-CXCL1 polyclonal antibody was adsorbed using soluble human recombinant CXCL1 (100 ng/ml; R&D Systems) for 1 h at room temperature, then applied to multiple sclerosis tissue sections and developed as described above.
For immunofluorescent staining, following fixation, sections were incubated with 0.1% glycine for 20 min to quench autofluorescence, and were then blocked with normal goat or rabbit serum. Oligodendroglial identity was confirmed with antibodies to 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase; IgG1; 1 : 300; Sigma, St Louis, MO), and astroglial identity with S-100 (IgG1; 1 : 200; Sigma) and glial fibrillary acidic protein (GFAP; IgG1; 1 : 200; Sigma). These phenotypic markers were used in combination with CXCR and CXCL antibodies for purposes of double labelling (Table 2). Slides were then incubated with a combination of secondary antibodies: biotinylated goat anti-mouse IgG2a (1:100)/Texas red-conjugated goat anti-mouse IgG1 (1:100; SouthernBiotech) or biotinylated rabbit anti-goat IgG (1:300; Vector)/Texas red-conjugated goat anti-mouse IgG1 (1:100), followed by 5 mg/ml streptavidin conjugated to fluorescein isothiocyante (FITC; Jackson ImmunoResearch, West Grove, PA).
Antibody combinations used for immunofluorescence staining
| CXC receptor/ligand . | Phenotypic maker . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | A2B5 . | O4 . | CNPase . | S-100 . | |||
| CXCR1 | C | C | C, T | – | |||
| CXCR2 | C | C | C, T | – | |||
| CXCR3 | C | C | –* | – | |||
| CXCL1 | – | – | – | T | |||
| CXCL8 | – | – | – | T | |||
| CXCL10 | – | – | – | T | |||
| CXC receptor/ligand . | Phenotypic maker . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | A2B5 . | O4 . | CNPase . | S-100 . | |||
| CXCR1 | C | C | C, T | – | |||
| CXCR2 | C | C | C, T | – | |||
| CXCR3 | C | C | –* | – | |||
| CXCL1 | – | – | – | T | |||
| CXCL8 | – | – | – | T | |||
| CXCL10 | – | – | – | T | |||
C = cultured cells; T = tissue slides; – = not tested.
Combination not tested since both CXCR3 and CNPase antibodies are mouse IgG1.
Antibody combinations used for immunofluorescence staining
| CXC receptor/ligand . | Phenotypic maker . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | A2B5 . | O4 . | CNPase . | S-100 . | |||
| CXCR1 | C | C | C, T | – | |||
| CXCR2 | C | C | C, T | – | |||
| CXCR3 | C | C | –* | – | |||
| CXCL1 | – | – | – | T | |||
| CXCL8 | – | – | – | T | |||
| CXCL10 | – | – | – | T | |||
| CXC receptor/ligand . | Phenotypic maker . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | A2B5 . | O4 . | CNPase . | S-100 . | |||
| CXCR1 | C | C | C, T | – | |||
| CXCR2 | C | C | C, T | – | |||
| CXCR3 | C | C | –* | – | |||
| CXCL1 | – | – | – | T | |||
| CXCL8 | – | – | – | T | |||
| CXCL10 | – | – | – | T | |||
C = cultured cells; T = tissue slides; – = not tested.
Combination not tested since both CXCR3 and CNPase antibodies are mouse IgG1.
Isolation and culture of human glial cells
Primary cultures of human oligodendrocytes were established from 18–22 week gestation fetal spinal cords as described previously (Wilson et al., 2003). After enzymatic digestion and overnight incubation, the oligodendroglial-enriched cell suspension was gently aspirated, centrifuged and the oligodendrocytes resuspended in N2B3 medium, i.e. Dulbeco's modified Eagles medium (Sigma) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.25 µg/ml amphotericin B (all from Gibco BRL, Carlsbad, CA), 0.5 µg/ml insulin, 0.1 µg/ml holo-transferrin, 16 µg/ml putrescine, 0.4 µg/ml thyroxine (T4), 0.3 µg/ml tri-iodothyronine (T3), 0.06 µg/ml progesterone, 0.04 µg/ml sodium selenite (all from from Sigma) and 0.1 mg/ml bovine serum albumin path-o-cyte 4 (ICN Biomedicals, Aurora, OH). Cells were plated onto glass coverslips coated with 5 µg/ml poly-l-lysine (Sigma), at a density of 1–3 × 104 cells per coverslip. Oligodendrocyte cultures were maintained in N2B3 medium alone, or supplemented with 10 ng/ml platelet-derived growth factor (PDGF)-AA (Sigma), and medium was replenished every other day for up to 6 days.
Astrocytes were isolated from human fetal forebrain and spinal cord, using methods similar to those described by Lee et al. (1992). After removing the oligodendroglial-enriched supernatant fluid, cells attached to the 25 cm2 tissue culture flask were re-incubated in RPMI-1640 medium supplemented with 10% fetal calf serum (both from Gibco), 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 0.25 µ/ml amphotericin B for 10–14 days without agitation. To obtain a pure population of astrocytes, microglia were removed by shaking and the remaining astrocytes were passaged at least twice before being plated onto 100 mm cell culture dishes or 24-well cell culture plates (Corning Incorporated, Corning, NY). Astrocytes were maintained in serum-supplemented RPMI in a 37°C/7% CO2 incubator, with medium change twice a week. Upon reaching confluence, cells were treated for 6–48 h with interleukin (IL)-1β, interferon (IFN)-γ (both from PeproTech, Rocky Hill, NJ) or bacterial lipopolysaccharide (LPS, Sigma), at a final concentration of 0.1–100 ng/ml. After stimulation, supernatant fluid was collected and stored at −20°C.
Immunocytochemistry
Oligodendrocytes maintained in culture were processed for immunocytochemistry as previously described (Robinson et al., 1998; Wilson et al., 2003). Cells fixed with 2% paraformaldehyde (Sigma) were incubated with primary antibodies against CXCR1 (IgG2a; 1 : 100), CXCR2 (IgG2a; 1 : 50) or CXCR3 (IgG1; 1 : 100), in combination with either A2B5 (IgM; 1 : 200; Chemicon, Temecula, CA) or CNPase (IgG1; 1 : 100), diluted in 2% normal goat serum for 1 h at 37°C. After washing, oligodendrocytes were incubated for 1 h at 37°C with biotinylated goat anti-mouse IgG2a (1:100), or biotinylated goat anti-mouse IgG1 (1 : 100; SouthernBiotech), plus Texas red-conjugated goat anti-mouse IgM (1 : 100; Vector) or Texas red-conjugated goat anti-mouse IgG1 (1 : 200). Live cell staining was conducted to co-localize CXC receptors with the early oligodendrocyte marker, O4 (IgM; 1 : 200; Chemicon). Cells were incubated with chemokine receptor antibodies together with the marker O4, then incubated with biotinylated goat anti-mouse IgG2a/IgG1 plus Texas red-conjugated goat anti-mouse IgM, prior to fixation. Oligodendrocytes were then incubated with fluorescein-conjugated streptavidin (5 µg/ml) for 45 min at room temperature. Table 2 summarizes the combinations of antibodies used for immunofluorescent staining. As a control, oligodendrocytes were incubated with isotype-matched irrelevant antibody (monoclonal IgG2a and IgG1), at the same concentration as the primary antibody.
Enzyme-linked immunosorbent assays (ELISA)
Production of CXC chemokines by astrocytes following treatment with cytokines or LPS was determined using Quantikine® ELISA kits specific for CXCL8, CXCL1 and CXCL10 according to the manufacturer's instructions (R&D Systems). Briefly, cell culture supernatant fluid and standards were added to wells pre-coated with capture antibodies specific for each chemokine, and incubated for 1.5–2 h at room temperature. Wells were then incubated for 1–2 h with horseradish peroxidase (HRP)-conjugated antibodies at room temperature for CXCL8 and CXCL10, or 4°C for CXCL1, followed by addition of substrate (tetramethylbenzidine; TMB). Colour development was allowed to occur for 30 min at room temperature before introduction of 1 M sulfuric acid. Absorbance was measured at 450 nm with correction at 540 nm on a SpectraMax Plus microplate spectrophotometer (Molecular Devices Corp., Sunnyvale, CA). Subsequently, amounts of chemokines in cell culture supernatant fluid were calculated using SoftMax Pro v2.1.1 (Molecular Devices Corp.). Baseline chemokine production was determined by analysing supernatant fluid from astrocytes maintained in serum-supplemented RPMI in the absence of cytokines or LPS. Chemokine production was tested in duplicate wells and repeated using two different astrocyte cultures.
Quantitative polymerase chain reaction (PCR)
With a previously described protocol (Yuen et al., 2002a), each transcript in each sample was assayed five times and the median detection threshold (CT) values were used to calculate the Fp values (fold-change ratios between experimental and control samples for each gene), used in the analysis. Amplicon size and reaction specificity were confirmed by agarose gel electrophoresis. Data validity by modelling of reaction efficiency and analysis of measurement precision have been described elsewhere (Yuen et al., 2002b). Primers used for the detection of CXC chemokines were as follows: CXCL8F 5′-GGC CAA GAG AAT ATC CGA AC-3′, CXCL8R 5′-AGG CAC AGT GGA ACA AGG AC-3′; CXCL1F 5′-TGA GAT CAT TGT GAA GGC AG-3′, CXCL1R 5′-GAG AAA TGT TGA CCA CAC AC-3′; CXCL10F 5′-TCC CAT CAC TTC CCT ACA TG-3′, and CXCL10R 5′-TGA AGC AGG GTC AGA ACA TC-3′.
Data acquisition and analysis
Digital images of tissue sections and cultured cells were acquired on a Zeiss Axioskop epifluorescent microscope (Carl Zeiss, Thornwood, NY) or a BioRad Radiance 2000 Scanning Laser Confocal microscope (Bio-Rad, Hercules, CA). All data were subjected to analysis of variance (ANOVA) to determine differences between treatment groups. Where differences were found, the Student's t test was applied to analyse individual differences against control groups. The Mann–Whitney rank sum test was applied to data that were not normally distributed. Data were analysed using SigmaStat v2.03 and graphs were generated on SigmaPlot 2001 (SPSS Science, Chicago, IL).
Results
Human oligodendrocytes express CXC chemokine receptors
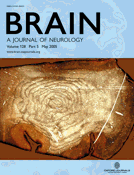
Immunostaining of normal white matter showed low level constitutive expression of CXCR1 and CXCR2 on small rounded cells with the size and distribution of interfascicular oligodendrocytes (Fig. 1A and E). In multiple sclerosis sections, higher level immunoreactivity for CXCR1 (Fig. 1B and C) and CXCR2 (Fig. 1F and G) was detected on interfascicular oligodendrocytes around active and silent lesions. Similarly, oligodendrocytes in all sections from OND cases displayed an increase in reactivity for CXCR1 and CXCR2 over normal levels (Fig. 1D and H). Low magnification across active multiple sclerosis lesion margins revealed increased immunoreactivity for CXCR2, which was readily observed in areas displaying an increase in oligodendrocyte numbers (oligodendrocyte hyperplasia), regions flanked by normal-appearing white matter (NAWM) and demyelinated plaque (Fig. 1M). Immunofluorescent staining was performed to confirm identification of the cells expressing CXC chemokine receptors further. Sections were stained for CXCR1 and CXCR2 in combination with the oligodendrocyte marker, CNPase. Similar to immunoperoxidase staining, confocal microscopy demonstrated that interfascicular oligodendrocytes expressing CXCR1 or CXCR2 were also positive for CNPase (Fig. 1N and O). The results of the immunohistochemistry analysis are summarized in Table 3.
Human oligodendrocytes express CXCR1 (A–D), CXCR2 (E–H) and CXCR3 (I–L) chemokine receptors in situ. Note the weak constitutive expression of CXCR1 (A), CXCR2 (E) and CXCR3 (I) on small rounded cells, typical of interfascicular oligodendrocytes, in normal human white matter. In contrast, upregulated CXCR1 (B–D) and CXCR2 (F–H) expression was observed on oligodendrocytes in frozen sections from multiple sclerosis and OND, while CXCR3 (J–L) staining intensity was consistently low in all cases tested. On occasion, astrocytes were strongly positive for CXCR3 in multiple sclerosis tissue (K), but not in normals or OND. In M, CXCR2+ oligodendrocytes are seen between the demyelinated plaque (*) on the right and the normal-appearing white matter (NAWM) to the left, a prominent zone of oligodendrocyte hyperplasia. In N and O, the oligodendroglial nature of cells positive for CXC receptors was confirmed by immunofluorescence and phenotypic staining. In multiple sclerosis, the oligodendrocyte marker, CNPase, co-localized with cells bearing CXCR1 (N) and CXCR2 (O) chemokine receptors. The merged images on the right confirm double labelling. Scale bars = 7.5 µm (A–L, N and O); 94 µm (M).
CXC receptor and ligand expression in CNS tissue
| CNS tissue . | CXCR1 . | CXCL8 . | CXCR2 . | CXCL1 . | CXCR3 . | CXCL10 . |
|---|---|---|---|---|---|---|
| Active multiple sclerosis | A+/−; OL+ | A+/++ | A+/−; OL+/++ | A++ | A++; OL+/− | A+/++ |
| Silent multiple sclerosis | A+/−; OL+ | A+/− | A+/−; OL+ | A+/− | A++; OL+/− | A+ |
| OND* | OL+ | ND | OL+ | ND | A+; OL+/− | ND |
| Normal | OL+/− | ND | OL+ | ND | A+; OL+/− | ND |
| CNS tissue . | CXCR1 . | CXCL8 . | CXCR2 . | CXCL1 . | CXCR3 . | CXCL10 . |
|---|---|---|---|---|---|---|
| Active multiple sclerosis | A+/−; OL+ | A+/++ | A+/−; OL+/++ | A++ | A++; OL+/− | A+/++ |
| Silent multiple sclerosis | A+/−; OL+ | A+/− | A+/−; OL+ | A+/− | A++; OL+/− | A+ |
| OND* | OL+ | ND | OL+ | ND | A+; OL+/− | ND |
| Normal | OL+/− | ND | OL+ | ND | A+; OL+/− | ND |
OND = other neurological disease; OL = oligodendrocyte; A = astrocyte. Scoring scale: +/− = weak; + = moderate; ++ = strong; +++ = very strong; ND = none detected.
OPCD, ALS and stroke.
CXC receptor and ligand expression in CNS tissue
| CNS tissue . | CXCR1 . | CXCL8 . | CXCR2 . | CXCL1 . | CXCR3 . | CXCL10 . |
|---|---|---|---|---|---|---|
| Active multiple sclerosis | A+/−; OL+ | A+/++ | A+/−; OL+/++ | A++ | A++; OL+/− | A+/++ |
| Silent multiple sclerosis | A+/−; OL+ | A+/− | A+/−; OL+ | A+/− | A++; OL+/− | A+ |
| OND* | OL+ | ND | OL+ | ND | A+; OL+/− | ND |
| Normal | OL+/− | ND | OL+ | ND | A+; OL+/− | ND |
| CNS tissue . | CXCR1 . | CXCL8 . | CXCR2 . | CXCL1 . | CXCR3 . | CXCL10 . |
|---|---|---|---|---|---|---|
| Active multiple sclerosis | A+/−; OL+ | A+/++ | A+/−; OL+/++ | A++ | A++; OL+/− | A+/++ |
| Silent multiple sclerosis | A+/−; OL+ | A+/− | A+/−; OL+ | A+/− | A++; OL+/− | A+ |
| OND* | OL+ | ND | OL+ | ND | A+; OL+/− | ND |
| Normal | OL+/− | ND | OL+ | ND | A+; OL+/− | ND |
OND = other neurological disease; OL = oligodendrocyte; A = astrocyte. Scoring scale: +/− = weak; + = moderate; ++ = strong; +++ = very strong; ND = none detected.
OPCD, ALS and stroke.
In contrast to CXCR1 and CXCR2, CXCR3 was present at low levels on oligodendrocytes in both normal and disease groups (Fig. 1I–L). Moreover, in multiple sclerosis tissue, astrocytes displayed more pronounced staining for CXCR3 compared with astrocytes in OND and normal controls. Recognizing that astrocytes and microglia in vitro have been reported to express CXC chemokine receptors (Biber et al., 2002; Rezaie et al., 2002; Flynn et al., 2003), we paid particular attention to these cells. In multiple sclerosis samples only, hypertrophic astrocytes were occasionally weakly reactive for CXCR1 and CXCR2, and neurons in grey matter also displayed immunoreactivity (data not shown). We cannot speculate on the expression of CXCR1 or CXCR2 on microglia as the antibodies applied were of the IgG2a isotype, reagents well known for binding non-specifically to Fc receptors (Ulvestad et al., 1994). This was confirmed when tissue sections from both normal and diseased CNS tissue were incubated with an IgG2a isotype-matched irrelevant antibody, which gave positive staining for microglia. Substitution of the anti-CXCR3 antibody with an IgG1 irrelevant antibody showed lack of non-specific binding by the primary antibody.
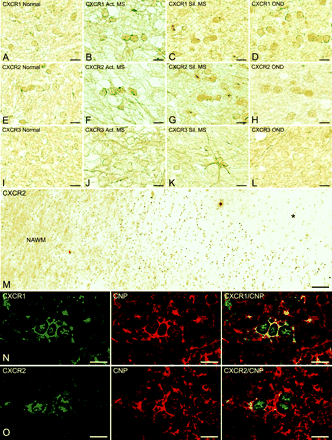
In addition to expression in situ, CXC chemokine receptors were examined in vitro in cultures of human oligodendrocytes. Immature A2B5+ oligodendrocytes expressed CXCR1, CXCR2 and CXCR3 (Fig. 2A–C). Similarly, O4+ oligodendrocytes also stained positively for CXCR1 and CXCR2 (Fig. 2D and E). Oligodendrocytes expressing O4 displayed less elaborate staining for CXCR3 (data not shown), an expected finding in view of the low expression of CXCR3 detected in situ. Developmentally more mature oligodendrocytes, being highly branched and positive for CNPase, displayed CXCR1 and CXCR2 (Fig. 2E and G). This finding was in agreement with the immunohistochemistry of CNS tissue, which showed CXCR1- and CXCR2-bearing oligodendrocytes double labelled with CNPase. All three CXC chemokine receptors appeared to be present on the surface of oligodendrocytes, especially in cultures double labelled with the oligodendrocyte marker, O4, where the antibodies were applied on unfixed cells. In instances where cells were fixed prior to double labelling with antibodies to chemokine receptors and oligodendroglial markers (A2B5 and CNPase), intracellular staining was observed in addition to surface expression. To rule out non-specific staining by CXCR-specific antibodies, oligodendrocytes were incubated with isotype-matched irrelevant control antibodies and these yielded negative results (Fig. 2H). Thus, human oligodendrocytes consistently showed chemokine receptor expression, the levels of some of which were elevated in multiple sclerosis and OND samples.
Human oligodendrocytes display surface expression of CXC chemokine receptors in vitro. In A–C, immature A2B5+ oligodendrocytes co-express CXCR1 (A), CXCR2 (B) and CXCR3 (C) receptors. Similarly, in D and E, cells positive for the early oligodendroglial marker, O4, also labelled with CXCR1 (D) and CXCR2 (E). F and G show mature, highly branched oligodendrocytes bearing the marker CNPase, double staining with antibodies against CXCR1 (F) and CXCR2 (G). Non-specific binding was assessed by incubating cells with isotype-matched irrelevant antibodies, resulting in negative staining (H). Scale bars = 15 µm (A–H).
CXC ligands are upregulated on hypertrophic astrocytes in active multiple sclerosis
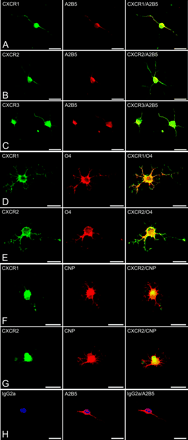
Immunoperoxidase staining for the respective ligands for CXCR1, CXCR2 and CXCR3 chemokine receptors, i.e. CXCL8/IL-8, CXCL1/GRO-α and CXCL10/IP-10, displayed labelling of astrocytes only. In particular, hypertrophic astrocytes in active multiple sclerosis lesions reacted strongly for CXCL8 and CXCL1 (Fig. 3A and B). Moreover, at the edge of some lesions, CXCL1-expressing astrocytes occurred adjacent to oligodendroglial cells positive for CXCR2 (Fig. 3C). In contrast to active lesions, in silent multiple sclerosis, astrocytes displayed diminished expression of CXCL8 and CXCL1 (Fig. 3D and E). To check for specificity of the staining pattern, we found that adsorption of the anti-CXCL1 polyclonal antibody with recombinant human CXCL1 protein prior to incubation abolished immunoreactivity (Fig. 3F). Also, minimal DAB product was observed on tissue sections where the primary antibody was omitted (data not shown). In the case of CXCL10, strong staining was detected on hypertrophic astrocytes in active multiple sclerosis (Fig. 3G), and reduced expression was present on astrocytes in silent multiple sclerosis lesions. To confirm the astroglial phenotype of cells expressing CXCL8 and CXCL1, multiple sclerosis tissue was double stained with an antibody against a marker for astrocytes, S-100 (Fig. 3H and I). Again, only reactive astrocytes around active multiple sclerosis lesions expressed high levels of chemokines.
Frozen sections displaying expression of CXC ligands on hypertrophic astrocytes in multiple sclerosis lesions. Immunoperoxidase staining revealed high levels of CXCL8 (A) and CXCL1 (B) on hypertrophic astrocytes in active multiple sclerosis lesions. In C, CXCL1-(brown) expressing astrocytes were found in close proximity to CXCR2- (purple) positive oligodendrocytes at the edge of an active lesion. In contrast, limited staining of CXCL8 and CXCL1 was observed on astrocytes in silent multiple sclerosis (D and E). Lack of non-specific staining is demonstrated by adsorption of the CXCL1 antibody with recombinant human CXCL1 (F). Reactive astrocytes in active multiple sclerosis cases were also strongly positive for CXCL10 (G). The astroglial nature of cells producing CXCL8 (H) and CXCL1 (I) in active multiple sclerosis lesions was confirmed by double immunofluorescence staining with S-100, a marker for reactive astrocytes. Scale bars = 7.5 µm (A, B, D–I); 15 µm (C).
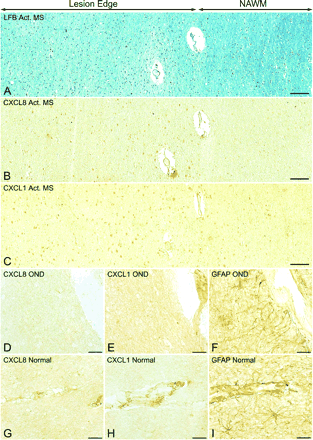
A key finding was the expression pattern of CXC ligands in active multiple sclerosis lesions. Hypertrophic astrocytes at the lesion edge (Fig. 4A) labelled positively with both CXCL8 (Fig. 4B) and CXCL1 (Fig. 4C). Blood vessel elements also appeared to stain with antibodies against CXCL8 and CXCL1. However, no staining for either chemokine was detected in adjacent regions of NAWM. Equally significant was the observation that in OND and normal control white matter, no reaction for any of the above chemokines was observed on astrocytes. In adjacent sections, the same cell type stained intensely for the astrocytic marker GFAP, proving that astrocytes were present in the tissue (Fig. 3D–I). Therefore, expression of CXC ligands was limited to hypertrophic astrocytes in active stages of disease and was absent in control tissue.
CXC ligands are selectively expressed by hypertrophic astrocytes in active multiple sclerosis lesions. Hypertrophic astrocytes present at the edge of active multiple sclerosis lesions, demonstrated in sections stained by the Luxol fast blue method (A), label positively for CXCL8 (B) and CXCL1 (C). CXC ligands are specifically expressed in regions near the lesion, while there is a lack of CXCL8 and CXCL1 staining in areas of NAWM. In contrast, other than staining blood vessel elements, CXCL8 and CXCL1 were absent in OND (D and E) and normal (G and H) control tissue, despite the presence of GFAP-expressing astrocytes (F and I). Scale bars = 94 µm (A–C); 22.5 µm (D–I).
IL-1β and IFN-γ regulate CXC chemokine synthesis by astrocytes in vitro
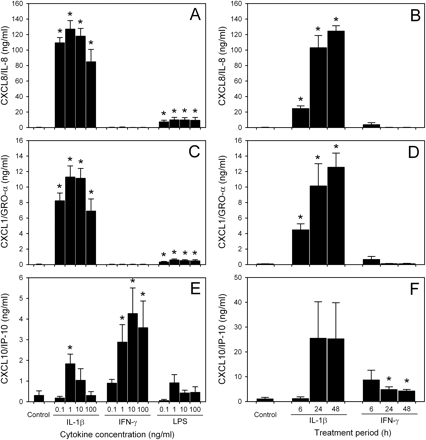
To date, the factors involved in the production of CXC chemokines by glial cells have not been entirely defined. Several studies have shown that astrocytes can be stimulated to produce CXC chemokines (Cole et al., 1998; Janabi et al., 1999; Salmaggi et al., 2002; Meeuwsen et al., 2003). To elucidate further the mechanisms by which astrocytes may be induced to produce CXC chemokines, we measured secretion of CXCL8, CXCL1 and CXCL10 by sandwich ELISAs following incubation of human astrocytes in vitro with the proinflammatory cytokines, IL-1β and IFN-γ, as well as LPS. Astrocytes maintained in the absence of cytokine stimulation produced minimal amounts of CXCL8 (144–450 pg/ml), CXCL1 (8.5–81 pg/ml) or CXCL10 (306–1098 pg/ml). Treatment of astrocytes with IL-1β resulted in dramatic upregulation of CXCL8 (54- to 882-fold) and CXCL1 (55- to 1328-fold). At the range tested, cytokine concentration had little effect on chemokine level. However, the increase in CXCL8 and CXCL1 production was time dependent, and the highest levels were detected in supernatant fluid from astrocytes stimulated for 24–48 h (Fig. 5A–D). Stimulation with LPS resulted in a minor upregulation in CXCL8 and CXCL1 secretion compared with IL-1β (63- and 55-fold average increase, respectively), while IFN-γ had no effect (Fig. 5A and C). Unlike CXCL8 and CXCL1, CXCL10 secretion appeared to be consistently upregulated following incubation with IFN-γ (2- to 13-fold increase; Fig. 5E and F). Peak CXCL10 levels were present in supernatant fluid from astrocytes treated for 24 h with 10–100 ng/ml IFN-γ. In most instances, there was minimal CXCL10 production (<5-fold increase) by spinal cord-derived astrocytes treated with IL-1β (Fig. 5E). In experiments looking at the effects of time on CXC chemokine production, one set of cultures used was derived from forebrains. In the case of CXCL10, we observed a large increase in chemokine secretion by forebrain-derived astrocytes in response to 10 ng/ml IL-1β. When combined with spinal cord-derived astrocytes, this resulted in an overall large amount of CXCL10 production (15-fold average increase; Fig. 5F). A possible explanation for this observation could be the recent finding that IL-1 induces the interferon regulatory factor-3 in human forebrain astrocytes, and thus activates genes typically induced by IFN-γ (Rivieccio et al., 2004). Our results might suggest differences between spinal cord- and forebrain-derived astrocytes in response to IL-1β stimulation. Astrocytes incubated with LPS produced substantially lower amounts of CXCL10 (1.0-fold average increase; Fig. 5E).
Induced CXC chemokine production and secretion by astrocytes in vitro. Levels of CXCL8 (A and B), CXCL1 (C and D) and CXCL10 (E and F) were detected by sandwich ELISA in supernatant fluid collected from astrocyte cultures after 6–48 h treatment with proinflammatory cytokines or LPS. IL-1β induced significant release of CXCL8 and CXCL1 by human astrocytes in a time-dependent manner, while IFN-γ had no effect. In contrast, both IL-1β and IFN-γ treatment resulted in induced CXCL10 production by astrocytes. Limited amounts of CXCL8, CXCL1 and CXCL10 were detected in astrocyte supernatant fluid following stimulation with LPS. Concentration effects in A, C and E were tested in supernatant fluid collected after 24 h incubation. Graph bars represent mean chemokine secretion ± SEM of astrocyte cultures from two different fetuses (n = 4). (*)P < 0.03 compared with control untreated astrocytes.
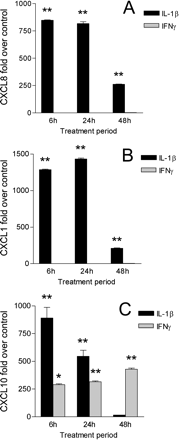
Induction of CXC chemokines by astrocytes was analysed further at the RNA level from forebrain-derived cultures. In agreement with the results obtained by sandwich ELISAs, quantitative PCR experiments showed that stimulation with 10 ng/ml IL-1β resulted in upregulation of CXCL8 and CXCL1 RNA over control levels. Maximal amounts of CXCL8 and CXCL1 RNA were detected after 6–24 h stimulation, which preceded the highest level of chemokine protein present in supernatant fluid collected after 48 h treatment with IL-1β (Fig. 6A and B). Similarly, stimulation with IL-1β resulted in a rapid induction of CXCL10 RNA in astrocytes after 6 h, which diminished over time. In contrast, astrocytes treated with 10 ng/ml IFN-γ showed sustained upregulation of CXCL10 RNA over the 48 h test period (Fig. 6C). Thus, astrocyte production of CXCL8, CXCL1 and CXCL10 in vitro appeared to be under the control of proinflammatory cytokines.
Quantitative PCR analysis of CXCL8 (A), CXCL1 (B) and CXCL10 (C) gene induction in forebrain-derived astrocytes. A significant increase in CXCL8 and CXCL1 gene expression was detected following 6–24 h stimulation with 10 ng/ml IL-1β, while no effect was observed after treatment with IFN-γ. IL-1β also induced CXCL10 in astrocytes after 6–24 h stimulation. In contrast, incubation with IFN-γ resulted in sustained CXCL10 gene expression during the 48 h test period. Data points represent mean fold induction ± SD of duplicate cultures from different forebrains. (*)P < 0.01; (**)P < 0.001 compared with control untreated samples.
Discussion
In the present study, we demonstrate for the first time on human oligodendrocytes the expression of CXCR1, CXCR2 and CXCR3, chemokine receptors commonly found on granulocytes, monocytes, mast cells and lymphocytes (Murdoch and Finn, 2000; Murphy et al., 2000). While human oligodendrocytes constitutively expressed all three chemokine receptors, higher levels of CXCR1 and CXCR2 were seen in multiple sclerosis, and to a lesser extent in OND. On the other hand, CXCR3 reactivity was low in all conditions examined. Parallel experiments on human oligodendrocytes in vitro revealed that both immature (A2B5+/O4+) and more mature (CNPase+) oligodendrocytes displayed the same three chemokine receptors. In view of the known hyperplasia of oligodendrocytes around active multiple sclerosis lesions (Raine et al., 1981; Prineas et al., 1989; also see Fig. 1M), we propose that the above expression pattern may be significant to oligodendrocyte behaviour during the course of disease. However, whether CXC chemokine receptor signalling leads to the proliferation of human oligodendrocytes or prevents their migration (both of which would culminate in increased cell numbers) remains to be determined.
Interestingly, the ligands to CXCR1, CXCR2 and CXCR3 (CXCL8, CXCL1 and CXCL10, respectively) were only present on hypertrophic astrocytes around active multiple sclerosis lesions, being totally absent in normal and OND samples. Double staining revealed the presence of chemokine-positive (CXCL1) reactive astrocytes associated with chemokine receptor-expressing (CXCR2) oligodendrocytes at the active lesion margin, implying a functional relationship. Since few reports exist to date on CXC chemokines and human astrocytes (Cole et al., 1998; Janabi et al., 1999; Simpson et al., 2000b; Salmaggi et al., 2002; Meeuwsen et al., 2003), we extended our study to the in vitro level and demonstrated that the proinflammatory cytokine IL-1β induced production by astrocytes of significantly higher amounts of CXCL8 and CXCL1 than controls, while both IL-1β and IFN-γ induced high levels of CXCL10.
The latter observations further underscore the role played by cytokines during the course of multiple sclerosis. Microglia and infiltrating T lymphocytes are major cytokine-producing cell types in the CNS, expressing both IL-1β and IFN-γ (Benvenuto et al., 1992; Link et al., 1994; Cannella and Raine, 1995). Peak cytokine levels are detected in active multiple sclerosis, with lower levels in silent cases, and cytokines are generally absent in non-inflammatory OND samples. This paradigm offers a possible mechanism underlying the observed CXC chemokine patterns in multiple sclerosis lesions. The presence of high levels of IL-1β and IFN-γ in early stages of the disease could lead to prominent expression of CXCL8, CXCL1 and CXCL10, which subsequently diminish as cytokine production wanes. Moreover, the absence of proinflammatory cytokines in OND and normals might correspond to the observed lack of chemokines in CNS samples from such cases. Therefore, CXC chemokine receptors constitutively expressed on oligodendrocytes will only be triggered by their specific ligands in active stages of multiple sclerosis when cytokine, and consequently chemokine, production is maximal.
Understanding of events leading to the accumulation or recruitment of oligodendrocytes at the lesion margin might be relevant to lesion repair. Such mechanisms could involve molecules such as chemokines which are known to drive cell migration and proliferation. In this regard, leading candidates might be CXCR/CXCL pathways, whereby CXC receptor-bearing oligodendrocytes might proliferate and accumulate at the lesion margin in response to CXC ligand produced by local astrocytes. Precedence for this might lie in studies in rats where it has been shown that immature CXCR2-positive oligodendrocytes proliferate in response to CXCL1 (from astrocytes) in combination with PDGF-AA (Robinson et al., 1998). Moreover, elevated levels of CXCL1 in the jimpy mutant correlate with increased proliferation of NG2+ oligodendrocyte progenitors (Wu et al., 2000). CXCL1 has also been shown to provide a migratory stop signal for OPCs, thus influencing positioning of cells of the oligodendrocyte lineage during CNS development (Tsai et al., 2002). Although no effect has been described on oligodendrocytes, CXCL8 and CXCL10 have been shown to induce proliferation of other cell types, including endothelial cells and astrocytes (Heidemann et al., 2003; Mockenhaupt et al., 2003; Zhu et al., 2004).
Our finding that CXC chemokine receptors are consistently present on human oligodendrocytes is in accord with the work in rodents. In addition, the presence of the corresponding ligands, CXCL8, CXCL1 and CXCL10, on hypertrophic astrocytes (seen here primarily in active multiple sclerosis lesions) suggests potential cross-talk with CXCR1, CXCR2 and CXCR3 on oligodendrocytes. Ongoing work is designed to determine whether the chemokine/chemokine receptor pairs, studied individually in vitro, are capable of inducing human oligodendrocyte proliferation and downstream signalling. On the other hand, it is equally possible that CXC chemokines might be preventing oligodendrocyte migration past the lesion edge, and consequently disrupt repair processes. Together with cytokines which drive chemokine production, we propose that interactions between CXC chemokines and their receptors may play a key role in oligodendrocyte recruitment around active multiple sclerosis lesions. Exploration of these pathways may offer novel therapeutic avenues to enhance the limited remyelination typically seen in multiple sclerosis.
We wish to thank Miriam Pakingan for expert technical assistance; Dr B. Poulos of the Einstein Fetal Tissue Repository, for the human fetal tissue; Dr B. Cannella for valuable advice and discussion; and Dr C. Petito, University of Miami Brain Bank (HD 83284), and Dr S. Morgello, Manhattan HIV Brain Bank (MH 59724), for providing normal CNS samples. This work was supported in part by grants from the National Multiple Sclerosis Society (FG 1422-A-1 to K.M.O.; RG 1001-J-10 to C.S.R.); and the National Institutes of Health (NS07098, NS08952 and NS11920 to C.S.R.; NS046620 to G.R.J.; DK046943 to S.C.S.). K.M.O. is a Postdoctoral Fellow of the National Multiple Sclerosis Society; G.R.J. is supported by the Jayne and Harvey Beker Research Program in Multiple Sclerosis; and C.S.R. is the Wollowick Family Professor of Multiple Sclerosis Research at the Albert Einstein College of Medicine.
References
Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions.
Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion.
Benvenuto R, Paroli M, Buttinelli C, Franco A, Barnaba V, Fieschi C, et al. Tumor necrosis factor-alpha and interferon-gamma synthesis by cerebrospinal fluid-derived T cell clones in multiple sclerosis.
Biber K, Dijkstra I, Trebst C, De Groot CJ, Ransohoff RM, Boddeke HW. Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia.
Bonetti B, Raine CS. Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis.
Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions.
Cannella B, Raine CS. Multiple sclerosis: cytokine receptors on oligodendrocytes predict innate regulation.
Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions.
Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3.
Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, et al. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons.
D'Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, et al. Multiple sclerosis: Fas signaling in oligodendrocyte cell death.
Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis.
Fife BT, Kennedy KJ, Paniagua MC, Lukacs NW, Kunkel SL, Luster AD, et al. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis.
Filipovic R, Jakovcevski I, Zecevic N. GRO-alpha and CXCR2 in the human fetal brain and multiple sclerosis lesions.
Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes.
Goldberg SH, van der Meer P, Hesselgesser J, Jaffer S, Kolson DL, Albright AV, et al. CXCR3 expression in human central nervous system diseases.
Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2.
Hua LL, Lee SC. Distinct patterns of stimulus-inducible chemokine mRNA accumulation in human fetal astrocytes and microglia.
Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis.
Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2.
Janabi N, Hau I, Tardieu M. Negative feedback between prostaglandin and alpha- and beta-chemokine synthesis in human microglial cells and astrocytes.
John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation.
Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis.
Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, et al. CXCR-4 (fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons.
Lee SC, Liu W, Brosnan CF, Dickson DW. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia.
Link J, Soderstrom M, Kostulas V, Olsson T, Hojeberg B, Ljungdahl A, et al. Optic neuritis is associated with myelin basic protein and proteolipid protein reactive cells producing interferon-gamma, interleukin-4 and transforming growth factor-beta.
Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination.
Maeda Y, Solanky M, Menonna J, Chapin J, Li W, Dowling P. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions.
Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli.
Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation.
Mockenhaupt M, Peters F, Schwenk-Davoine I, Herouy Y, Schraufstatter I, Elsner P, et al. Evidence of involvement of CXC-chemokines in proliferation of cultivated human melanocytes.
Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases.
Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors.
Nguyen D, Stangel M. Expression of the chemokine receptors CXCR1 and CXCR2 in rat oligodendroglial cells.
Omari KM, Chui R, Dorovini-Zis K. Induction of beta-chemokine secretion by human brain microvessel endothelial cells via CD40/CD40L interactions.
Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions.
Prineas JW, Kwon EE, Goldenberg PZ, Ilyas AA, Quarles RH, Benjamins JA, et al. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions.
Raine CS. The neuropathology of multiple sclerosis. In: Raine CS, McFarland HF, Tourtellotte WW, editors. Multiple sclerosis: clinical and pathogenetic basis. London: Chapman & Hall;
Raine CS, Wu E. Multiple sclerosis: remyelination in acute lesions.
Raine CS, Scheinberg L, Waltz JM. Multiple sclerosis. Oligodendrocyte survival and proliferation in an active established lesion.
Rezaie P, Trillo-Pazos G, Everall IP, Male DK. Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: potential role of chemokines in the developing CNS.
Rivieccio MA, John GR, Suh HS, Song X, Lee SC, Brosnan CF. IL-1 activates interferon regulatory factor-3: implications for an anti-viral response in human primary astrocytes (abstract).
Robinson S, Tani M, Strieter RM, Ransohoff RM, Miller RH. The chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation.
Salmaggi A, Gelati M, Dufour A, Corsini E, Pagano S, Baccalini R, et al. Expression and modulation of IFN-gamma-inducible chemokines (IP-10, Mig, and I-TAC) in human brain endothelium and astrocytes: possible relevance for the immune invasion of the central nervous system and the pathogenesis of multiple sclerosis.
Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis.
Shukaliak JA, Dorovini-Zis K. Expression of the beta-chemokines RANTES and MIP-1 beta by human brain microvessel endothelial cells in primary culture.
Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue.
Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions.
Sorensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system.
Teleshova N, Pashenkov M, Huang YM, Soderstrom M, Kivisakk P, Kostulas V, et al. Multiple sclerosis and optic neuritis: CCR5 and CXCR3 expressing T cells are augmented in blood and cerebrospinal fluid.
Tran EH, Kuziel WA, Owens T. Induction of experimental autoimmune encephalomyelitis in C57BL/6 mice deficient in either the chemokine macrophage inflammatory protein-1alpha or its CCR5 receptor.
Trebst C, Sorensen TL, Kivisakk P, Cathcart MK, Hesselgesser J, Horuk R, et al. CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis.
Tsai HH, Frost E, To V, Robinson S, Ffrench-Constant C, Geertman R, et al. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration.
Ulvestad E, Williams K, Vedeler C, Antel J, Nyland H, Mork S, et al. Reactive microglia in multiple sclerosis lesions have an increased expression of receptors for the Fc part of IgG.
Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, et al. Notch receptor activation inhibits oligodendrocyte differentiation.
Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors.
Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord.
Wu Q, Miller RH, Ransohoff RM, Robinson S, Bu J, Nishiyama A. Elevated levels of the chemokine GRO-1 correlate with elevated oligodendrocyte progenitor proliferation in the jimpy mutant.
Yuen T, Zhang W, Ebersole BJ, Sealfon SC. Monitoring G-protein-coupled receptor signaling with DNA microarrays and real-time polymerase chain reaction.
Yuen T, Wurmbach E, Pfeffer RL, Ebersole BJ, Sealfon SC. Accuracy and calibration of commercial oligonucleotide and custom cDNA microarrays.
Zhu YM, Webster SJ, Flower D, Woll PJ. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells.
Author notes
Departments of 1Pathology (Neuropathology), 2Neurology and 3Neuroscience, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, 4Corinne Goldsmith Dickinson Center for Multiple Sclerosis and 5Department of Neurology, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY, USA