-
PDF
- Split View
-
Views
-
Cite
Cite
P. Cabre, A. Signate, S. Olindo, H. Merle, D. Caparros-Lefebvre, O. Béra, D. Smadja, Role of return migration in the emergence of multiple sclerosis in the French West Indies, Brain, Volume 128, Issue 12, December 2005, Pages 2899–2910, https://doi.org/10.1093/brain/awh624
Close - Share Icon Share
Abstract
The emergence of multiple sclerosis in island societies has been investigated only in a few Caucasian populations living in temperate regions. The effect of human migration on the risk of developing this disease is still an open question because of possible genetic selection. We conducted an epidemiological study of the multiple sclerosis population in the French West Indies (Martinique and Guadeloupe), a population which includes large numbers of West Indians who have returned after emigrating to metropolitan France. Standardized incidence ratios (SIRs) for multiple sclerosis among migrants were calculated and their genetic characteristics were compared to those of non-migrants. The crude prevalence of multiple sclerosis was 14.8/105 on December 31, 1999 (95% CI: 11.9–17.7); and its crude mean annual incidence for the period July 1, 1999 to June 30, 2002 was 1.4/105 (95% CI: 1.0–1.8), confirming its emergence in the French West Indies. Recurrent neuromyelitis optica, which is virtually the only form of multiple sclerosis in black African populations in tropical regions, represented not >17.8% of these cases. During the 1 440 000 person-years of follow-up, 33 incidence cases were identified in migrants. Since the number of expected cases was 19.3, the overall SIR was 1.71 (95% CI: 1.19–2.38; P < 0.01) among migrants. The increase in the SIR was more marked if the stay was made before the age of 15 years (4.05, 95% CI: 2.17–6.83; P < 0.0001). European ancestry in the two migrating and non-migrating populations was similar. Martinique, which has a higher rate of return migration, has a higher prevalence of multiple sclerosis (21.0/105 versus 8.5/105) and a higher incidence (2.0/105 versus 0.7/105) than Guadeloupe. The emergence of the disease in the French West Indies is of environmental rather than genetic origin. It may be explained either through the introduction by migrants of precipitating environmental factors that operate in a critical way before the age of 15 years, and/or by the recent disappearance from the French West Indies of protective environmental factors acting before this age.
Introduction
Until the beginning of the 1990s, inflammatory demyelinating diseases of the central nervous system in the French West Indies (Martinique and Guadeloupe) consisted almost exclusively of Human-T-lymphotropic-virus-type 1 (HTLV-1) associated myelopathy (Vernant et al., 1987). Only exceptional cases of multiple sclerosis were encountered in the French West Indian population (Poser and Vernant, 1993), as in the other main West Indian islands such as Jamaica (Cruickshank and Montgomery, 1961). However, as in black South African populations (Cosnet, 1981), a clinical picture of recurrent neuromyelitis optica (RNMO), lying within the clinical spectrum of multiple sclerosis (Vernant et al., 1997), was sometimes observed. During the late 1990s, the diagnosis of typical cases of multiple sclerosis increased to the extent that Martinique no longer remained a low prevalence area (Cabre et al., 2001). The fact that West Indian people returned to the Caribbean after first emigrating to metropolitan France, where the disease is frequent, prompted us to suggest that this migration may play a causal role. The emergence of multiple sclerosis in geographically isolated island populations that are subject to environmental change is critical to the understanding of its pathogenic origin. In addition, the report of a sudden increase in the prevalence and incidence of multiple sclerosis in our West Indian community demonstrates that the racial protection afforded to black people is an environmental artifact and not the result of genetic resistance. We present here the results of a prospective population study on the incidence and prevalence of multiple sclerosis in the whole of the French West Indies, investigating the role of migration as a risk factor.
Methods
Geographic areas of study
The French West Indies comprise two islands: Martinique and Guadeloupe. Both are situated in the Caribbean basin in the arc of the lesser West Indies at latitudes between 14°30 N and 16°N. Both have a similar surface area of ∼1200 km2. Numerous geoclimatic and demographic variables affecting multiple sclerosis epidemiology are virtually identical in the two islands: tropical climate with a mean annual temperature fluctuating between 22 and 30°C, similar high population density and population structure consisting mainly of AfroCaribbeans (>90%) with some interbreeding with the Caucasian population (estimated to be <30%) (Monplaisir et al., 1985). The total population of Martinique is 343 000 and that of continental Guadeloupe 340 000, with 63% aged <40 years (according to the 1999 census). Their levels of urbanization are similar and there are central conurbations on both islands—Fort de France-Schöelcher-Le Lamentin in Martinique and Pointe à Pître-Les Abymes-Baie Mahault in Guadeloupe, which are inhabited by 40% of the population. The geological environment is identical, with volcanic mountains in the north of both islands Mount Pelee in Martinique and La Soufriere volcano in Guadeloupe. Finally, there was a major economic change in both islands during the late 20th century and traditional agriculture was abandoned in favour of tourism and service industries. Martinique and Guadeloupe are the most highly developed islands in the Caribbean, classified 14th and 18th, respectively, in terms of human development at the world level.
Return migration
During the 1950s, and in particular the 1960s, many people emigrated from Guadeloupe and Martinique, mainly to France. Several factors explain the importance of this migratory movement to the colonial power. Firstly, there was a population explosion which began in 1920 due to the general decline in mortality and concomitant stable and very high birth rates. The natural rate of increase in population peaked at ∼3% at the end of the 1950s. The second factor was mass unemployment, which was precipitated by a crisis in the plantation farming sector. At the same time, the industrial economy of France, rapidly expanding after the war, required considerable manpower. France used various means to encourage workers of West Indian origin to emigrate—financial inducements and creation of specialized bodies such as the BUMIDOM (Bureau of Migration from Départements d'Outre-Mer) founded in 1962. All these measures were aimed at encouraging and facilitating the definitive installation of West Indian migrants in France in order to decrease the demographic explosion in Martinique and Guadeloupe. Subsequently, return migration of West Indian subjects to Guadeloupe and Martinique was probably prompted by unemployment in metropolitan France and the relative economic development of the French West Indies.
Selection of MS cases
This is a prospective population study conducted from January 1, 1998 to December 31, 2004. Potential cases of patients suffering from multiple sclerosis were identified from various sources: (i) hospital neurologists, (ii) private neurologists, (iii) hospital and private ophthalmologists, (iv) general practitioners, (v) physiotherapists, (vi) health insurance files for the whole French West Indian population. The structure of the medical profession in contact with patients suffering from multiple sclerosis is strikingly similar on both islands, with five and four hospital neurologists for Martinique and Guadeloupe, respectively, practising in Martinique in a single department and in Guadeloupe in two different departments, three and four private neurologists, 249 and 256 general practitioners, 25 and 26 ophthalmologists, and two physiotherapy centres, respectively. During a 1-year preparation phase, the various health professionals involved in the study were informed during preparatory meetings and through individual letters to general practitioners indicating the date of the start and end of the study. The conduct of the study was facilitated by the installation at Fort de France on January 1, 1998 of the first MRI machine in the French West Indies.
Any patient identified as a potential case was questioned and examined by a neurologist trained in diagnosis by the Vancouver Multiple Sclerosis Clinic (P.C.). Each case was then discussed at a weekly staff meeting attended by a neuroradiologist to analyse the MRI films before final inclusion. For the diagnostic criteria of multiple sclerosis, we had the choice between Poser (Poser et al., 1983) and the more recent McDonald criteria (McDonald et al., 2001). The McDonald criteria were finally selected as they shorten the diagnosis time in certain patients presenting only a single clinical episode by longitudinal MRI analysis. General practitioners were repeatedly phoned throughout the study and annual meetings with all study participants were organized to report the preliminary results.
Epidemiological objectives
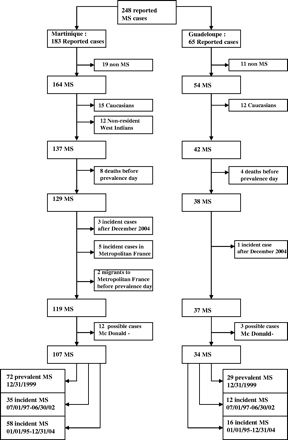
The primary objectives of the study were to determine the global prevalence of multiple sclerosis in the French West Indies, and on each island on December 31, 1999 in the West Indian population, and the mean annual incidence of the disease either for the period July 1, 1999 to June 30, 2002 for the whole population or for each island. Migration as a risk factor for multiple sclerosis was studied from all incident cases occurring in migrants between January 1, 1995 and December 31, 2004. Figure 1 shows the series of cases used to calculate these epidemiological variables. The following cases were excluded from the total of 248 cases reported by the various sources: those in which the diagnosis was not confirmed by the investigators (N = 30), cases affecting Caucasian subjects (N = 27), cases in West Indian subjects who were permanent residents of neighbouring Caribbean islands (St Lucia, Virgin Islands, Grenadine Islands) and French Guyana (N = 12). Patients who died before the prevalence day were also excluded as they suffered mainly from RNMO (N = 12). Patients who developed multiple sclerosis in metropolitan France and who subsequently migrated to the French West Indies were also excluded as they could induce bias in the epidemiological parameters if this migration was due to the patient's health (N = 5) and so were people who emigrated during the study before the prevalence day (N = 2). Four cases occurring after December 31, 2004 were not considered as incident cases. Finally, possible cases not combining all the McDonald criteria were not included (N = 15).
A standardized form was filled in for every included case specifying migration history with time and place of residence outside the Caribbean basin. A case was considered to have a history of migration if the patient had lived for at least 1 year in a temperate region of latitude above 40°N. The age of onset, delay of diagnosis, existence of familial forms, age and sex on prevalence day were also noted. Cases were classified into two classes for typical multiple sclerosis and RNMO according to the criteria of the Mayo Clinic (Wingerchük et al., 1999). Whether residence was rural or urban and the professional occupation at the time of onset of the disease were also mentioned. These variables comprised the secondary objectives of the study.
Quantitative and qualitative evaluation of West Indian migration
To evaluate the quantitative and qualitative characteristics of return migration not taken into account in the 1999 census, a representative sample of the West Indian population of 1600 individuals aged between 15 and 64 years on prevalence day December 31, 1999 was randomly selected in Martinique and Guadeloupe using the quota method. The quota method applies the same proportion of criteria such as, age, sex, socioeconomic status, and place of residence in the sample as in the general population. The exclusion of subjects aged <15 years or >64 years was not a major problem for the study as the 15–64 age range included nearly all cases needed to determine prevalence and incidence of multiple sclerosis. Data collected by four investigators between 2000 and 2001 on each island included age, sex, migration with the name of the host country where applicable specifying the town for metropolitan France, the year of arrival in a temperate region, the year of return to the French West Indies and socioeconomic class. According to the French National Institute of Economic Statistics, there are eight socioeconomic classes: (i) Higher managerial staff, (ii) Lower managerial staff, (iii) Intermediate occupations, (iv) Employees, (v) Workers, (vi) Farmers, (vii) Retirees and (viii) No professional occupation. We grouped together these socioeconomic classes as follows: classes i, ii, iii and iv high and classes v, vi, vii and viii low.
Genetic characteristics of migrants
One of the major causes of bias in studies of migrants and multiple sclerosis is the genetic non-representativity of migrants in comparison with non-migrants, as the most unidirectional human migrations may be motivated by political, ethnological or even religious reasons permitting the genetic selection of individuals and reducing the effects of environmental factors. As there was interbreeding within the West Indian population, a major question asked by this study was whether the returning West Indian migrants were as interbred as the non-migrant population. We investigated this problem by studying two distinct genetic variables in both a black African population and a white European population from which the West Indian population is derived. The first analysis was carried out as in other studies (Najim Al-Din et al., 1990) by comparing the frequency of ABO rhesus blood groups between migrants and non-migrants. There is a different distribution, in particular in the frequency of the O+ group, between black African and Caucasian populations. This information was evaluated by asking an additional question during the above-mentioned poll for which 1081/1600 answers (67.6%) were obtained. The second approach was an analysis of the frequency of alleles at the type 1 human leucocyte antigen (HLA) major histocompatibility complex (21 alleles for locus A and 27 alleles for locus B) of anthropological importance to evaluate the genetic drift of migrant and non-migrant West Indian populations in comparison with a population from Equatorial Guinea (Bera et al., 2001). One hundred Martinicans, representative of the Martinican population with a type 1 HLA genotype previously determined according to international nomenclature (Bodmer et al., 1997), were contacted and questioned about their migration history.
Statistical analysis
Ethics committee approval
The study design was approved by an ethics committee and permission was obtained from the Department of Medical Association to contact patients who were declared to have potential multiple sclerosis. The polling method was approved by the French National Commission for Data Protection and Liberties.
Role of sponsors
Study sponsors played no role in the study design, collection and analysis of results, interpretation of the data obtained, writing of the report or in the decision to submit the paper for publication.
Results
Prevalence
One hundred and one cases of multiple sclerosis were recorded in the West Indian population on December 31, 1999, giving a crude disease prevalence of 14.8/105 (95% CI: 11.9–18.7). The 2-source capture–recapture model estimated that the survey was 94% complete (six cases missing). The mean age of onset in this prevalence cohort was 31.4 years (SD: 10.7) and we noted 5.9% of familial forms. According to the McDonald classification, 72 patients combined the clinical criteria of dissemination in time and space, 20 patients presented the clinical criterion of dissemination in time and MRI criterion of dissemination in space, one patient presented the clinical criterion of dissemination in space and the MRI criterion of dissemination in time, one patient presented a clinically isolated syndrome and seven patients corresponded to the definition of primary progressive multiple sclerosis. Eighteen patients (17.8%) presented the criteria of RNMO. Seventy-two cases of multiple sclerosis were prevalent in Martinique, giving a prevalence of 21/105 (95% CI: 16.1–25.9) which was greater than that noted in Guadeloupe where there were 29 cases, giving a prevalence of 8.5/105 (95% CI: 5.4–11.6). The distribution of prevalence cases according to age and sex gave in Martinique a prevalence peak in women and in men in the age range 35–44 years and a fall in prevalence for the 45–54 year age range. The sex ratio of the disease in Martinique was high at 4.1, corresponding to 58 women and 14 men. In Guadeloupe, the distribution of prevalence cases according to age and sex gave a prevalence peak affecting women in the age range 25–34 years, which was younger than in Martinique. The prevalence in men was low for all age ranges with a female/male sex ratio of 6.2, higher than in Martinique, corresponding to 25 cases in women and only 4 cases in men. Analysis of clinical forms showed a clear majority of typical multiple sclerosis in Martinique with 64 cases compared to eight cases of RNMO. In Guadeloupe, the typical form of the disease also predominated, with 19 conventional multiple sclerosis cases for 10 cases of RNMO, a proportion lower than in Martinique. Meta analysis of the two islands showed that the prevalence of the disease in urban areas was approximately double that in rural areas in both Martinique and Guadeloupe. The prevalence was three times as high in high socioeconomic classes than in low classes.
Incidence
Forty-seven patients belonging to the West Indian population presented with their first attack of multiple sclerosis between July 1, 1997 and June 30, 2002, giving a crude mean annual incidence for this period of 1.4/105 (95% CI 0.9–1.7). According to the 2-source capture–recapture method, there would have been 51 cases during this period. The mean age of onset of incidence cases was 34 years (SD: 11.1), based on 42 women and 5 men. Among these incidence cases, 20 combined the clinical criteria of dissemination in time and space, 14 combined clinical criterion of dissemination in time and MRI criterion of dissemination in space, five combined the clinical criterion of dissemination in space and the MRI criterion of dissemination in time, seven were clinically isolated syndromes and one corresponded to the clinical form of primary progressive multiple sclerosis. During the study period, 35 incidence cases were observed in Martinique, giving a mean annual incidence of 2/105 (95% CI 1.4–2.6). The mean annual incidence for the same period was lower in Guadeloupe, with a rate of 0.7/105 (95% CI 0.6–0.7). The expected number of incidence cases in Martinicans estimated from the Guadeloupan study was 12.24 from which is calculated an SIR for Martinicans of 2.86 (95% CI 2.0–3.99; P < 0.0001). During this period, four cases of RNMO occurred, three in Guadeloupe and one in Martinique.
Influence of migration
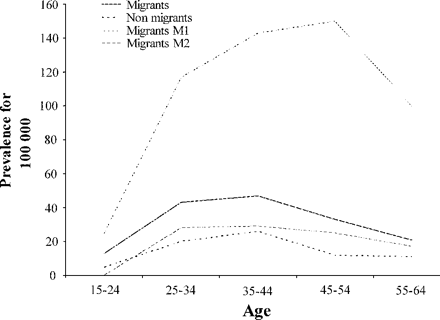
Tables 1 and 2 also show the prevalence and mean annual incidence of multiple sclerosis in the West Indian population aged 15 to 64 years on December 31, 1999, according to migration. Prevalence of the disease in this age range was 36.1/105 (95% CI: 26.3–45.9) in migrants and 15.1/105 (95% CI: 10.8–19.4) in non-migrants, giving an overall SPR of 1.98 among migrants (95% CI: 1.48–2.60; P < 0.0001). The increase of SPR was more marked if the stay was made before the age of 15 years (5.99, 95% CI: 3.79–8.98; P < 0.0001) (Table 3). A dose–response relationship between the duration of residence in a temperate region and the risk of multiple sclerosis was found (>10 years, 3.1, 95% CI: 2.01–4.47; 5–10 years, 1.83, 95% CI: 0.99–3.04; <5 years, 1.11, 95% CI: 0.55–1.99) (Table 3). Figure 2 shows that the prevalence of the disease was greater in migrants than in non-migrants and clearly greater if the stay in a temperate region was before the age of 15 years, in comparison with a stay after this age whatever age range was considered. The mean annual incidence of multiple sclerosis for the period from July 1, 1997 to June 30, 2002 in the West Indian population aged 15 to 64 years on December 31, 1999 was 2.9/105 in migrants (95% CI: 1.7–4.1), and this was greater than the incidence of 1.7/105 (95% CI: 1.1–2.3) observed in non-migrants (Table 2). Seventy patients suffering from multiple sclerosis were migrants. The mean duration of their stay was 12.3 years (SD: 9.5 years) range 1–35 years. Nearly all of them (69/70) had lived in metropolitan France and most (57/70 = 81.4%) in the Paris region, that is, north of the 45th parallel. The mean time between the arrival in metropolitan France and the development of multiple sclerosis in these cases was 19.1 years (SD: 10.3 years), and this may correspond to the incubation period of the disease.
Prevalence of MS in the French West Indies in the West Indian population aged from 15 to 64 years on 12/31/1999 according to migration to a temperate region. M1: migrants to a temperate region before the age of 15 years, M2: migrants to a temperate region after the age 15 years.
Prevalence of MS in the French West Indies
| . | No. . | Population at risk . | As on December 31, 1999 . | . | ||||
|---|---|---|---|---|---|---|---|---|
. | . | . | Prevalencea (95% CI) . | Prevalenceb (95% CI) . | ||||
| French West Indies | 101 | 683 000 | 14.8 (11.9–17.7) | 14.1 (11.4–16.8) | ||||
| Martinique | 72 | 343 000 | 21 (16.1–25.9) | 19.6 (14.9–24.3) | ||||
| Guadeloupe | 29 | 340 000 | 8.5 (5.4–11.6) | 8.8 (5.7–11.9) | ||||
| Martinique according to sex and age | ||||||||
| F 15–24 years | 2 | 23 000 | 8.7 | |||||
| F 25–34 years | 15 | 29 000 | 51.6 | |||||
| F 35–44 years | 25 | 29 000 | 85.9 | |||||
| F 45–54 years | 9 | 22 000 | 41.6 | |||||
| F 55–64 years | 3 | 15 000 | 19.2 | |||||
| F >65 years | 4 | 13 000 | 31.0 | |||||
| M 15–24 years | 1 | 23 000 | 2.6 | |||||
| M 25–34 years | 4 | 25 000 | 17 | |||||
| M 35–44 years | 5 | 26 000 | 19.8 | |||||
| M 45–54 years | 2 | 18 000 | 10.9 | |||||
| M 55–64 years | 2 | 14 000 | 14.6 | |||||
| Guadeloupe according to sex and age | ||||||||
| F 15–24 years | 2 | 24 000 | 8.2 | |||||
| F 25–34 years | 10 | 29 000 | 34.2 | |||||
| F 35–44 years | 6 | 27 000 | 21.4 | |||||
| F 45–54 years | 4 | 20 000 | 19.4 | |||||
| F 55–64 years | 3 | 13 000 | 21.6 | |||||
| M 15–24 years | 1 | 24 000 | 4 | |||||
| M 25–34 years | 1 | 25 000 | 3.9 | |||||
| M 35–44 years | 1 | 25 000 | 3.9 | |||||
| M 45–54 years | 1 | 18 000 | 5.4 | |||||
| M 55–64 years | 0 | 12 000 | – | |||||
| Clinical form | ||||||||
| Martinique: RNMO | 8 | 343 000 | 2.3 (0.6–3.9) | |||||
| Guadeloupe: RNMO | 10 | 340 000 | 2.9 (1.1–4.7) | |||||
| Habitat | ||||||||
| Urban | 51 | 263 000 | 19.4 (14.2–24.6) | |||||
| Rural | 50 | 420 000 | 11.1 (8.6–15.2) | |||||
| Socioeconomic class | ||||||||
| High | 56 | 200 000 | 28 (20.8–35.2) | |||||
| Low | 42 | 483 000 | 8.7 (6.0–11.4) | |||||
| French West Indies according to migrationc | ||||||||
| Migrants | 52 | 144 000 | 36.1 (26.3–45.9) | 32.1 (22.7–41.5) | ||||
| Non-migrants | 45 | 297 000 | 15.1 (10.8–19.4) | 14.8 (10.5–19.1) | ||||
| Migrants M1 | 23 | 24 000 | 95.8 (56.6–135) | 107.2 (52.7–161.7) | ||||
| Migrants M2 | 29 | 120 000 | 24.2 (15.4–33) | 20.3 (12.7–27.9) | ||||
| Martinique according to migrationc | ||||||||
| Migrants | 37 | 83 000 | 44.6 (30.3–58.6) | |||||
| Non-migrants | 31 | 141 000 | 22.0 (14.4–29.6) | |||||
| Migrants M1 | 19 | 16 000 | 118.7 (65.4–172) | |||||
| Migrants M2 | 18 | 67 000 | 26.9 (14.6–39.2) | |||||
| Guadeloupe according to migrationc | ||||||||
| Migrants | 15 | 61 000 | 24.6 (12.3–36.9) | |||||
| Non-migrants | 14 | 156 000 | 9.0 (4.3–13.7) | |||||
| Migrants M1 | 4 | 8000 | 50.0 (1.0–99) | |||||
| Migrants M2 | 11 | 53 000 | 20.7 (8.4–33) | |||||
| . | No. . | Population at risk . | As on December 31, 1999 . | . | ||||
|---|---|---|---|---|---|---|---|---|
. | . | . | Prevalencea (95% CI) . | Prevalenceb (95% CI) . | ||||
| French West Indies | 101 | 683 000 | 14.8 (11.9–17.7) | 14.1 (11.4–16.8) | ||||
| Martinique | 72 | 343 000 | 21 (16.1–25.9) | 19.6 (14.9–24.3) | ||||
| Guadeloupe | 29 | 340 000 | 8.5 (5.4–11.6) | 8.8 (5.7–11.9) | ||||
| Martinique according to sex and age | ||||||||
| F 15–24 years | 2 | 23 000 | 8.7 | |||||
| F 25–34 years | 15 | 29 000 | 51.6 | |||||
| F 35–44 years | 25 | 29 000 | 85.9 | |||||
| F 45–54 years | 9 | 22 000 | 41.6 | |||||
| F 55–64 years | 3 | 15 000 | 19.2 | |||||
| F >65 years | 4 | 13 000 | 31.0 | |||||
| M 15–24 years | 1 | 23 000 | 2.6 | |||||
| M 25–34 years | 4 | 25 000 | 17 | |||||
| M 35–44 years | 5 | 26 000 | 19.8 | |||||
| M 45–54 years | 2 | 18 000 | 10.9 | |||||
| M 55–64 years | 2 | 14 000 | 14.6 | |||||
| Guadeloupe according to sex and age | ||||||||
| F 15–24 years | 2 | 24 000 | 8.2 | |||||
| F 25–34 years | 10 | 29 000 | 34.2 | |||||
| F 35–44 years | 6 | 27 000 | 21.4 | |||||
| F 45–54 years | 4 | 20 000 | 19.4 | |||||
| F 55–64 years | 3 | 13 000 | 21.6 | |||||
| M 15–24 years | 1 | 24 000 | 4 | |||||
| M 25–34 years | 1 | 25 000 | 3.9 | |||||
| M 35–44 years | 1 | 25 000 | 3.9 | |||||
| M 45–54 years | 1 | 18 000 | 5.4 | |||||
| M 55–64 years | 0 | 12 000 | – | |||||
| Clinical form | ||||||||
| Martinique: RNMO | 8 | 343 000 | 2.3 (0.6–3.9) | |||||
| Guadeloupe: RNMO | 10 | 340 000 | 2.9 (1.1–4.7) | |||||
| Habitat | ||||||||
| Urban | 51 | 263 000 | 19.4 (14.2–24.6) | |||||
| Rural | 50 | 420 000 | 11.1 (8.6–15.2) | |||||
| Socioeconomic class | ||||||||
| High | 56 | 200 000 | 28 (20.8–35.2) | |||||
| Low | 42 | 483 000 | 8.7 (6.0–11.4) | |||||
| French West Indies according to migrationc | ||||||||
| Migrants | 52 | 144 000 | 36.1 (26.3–45.9) | 32.1 (22.7–41.5) | ||||
| Non-migrants | 45 | 297 000 | 15.1 (10.8–19.4) | 14.8 (10.5–19.1) | ||||
| Migrants M1 | 23 | 24 000 | 95.8 (56.6–135) | 107.2 (52.7–161.7) | ||||
| Migrants M2 | 29 | 120 000 | 24.2 (15.4–33) | 20.3 (12.7–27.9) | ||||
| Martinique according to migrationc | ||||||||
| Migrants | 37 | 83 000 | 44.6 (30.3–58.6) | |||||
| Non-migrants | 31 | 141 000 | 22.0 (14.4–29.6) | |||||
| Migrants M1 | 19 | 16 000 | 118.7 (65.4–172) | |||||
| Migrants M2 | 18 | 67 000 | 26.9 (14.6–39.2) | |||||
| Guadeloupe according to migrationc | ||||||||
| Migrants | 15 | 61 000 | 24.6 (12.3–36.9) | |||||
| Non-migrants | 14 | 156 000 | 9.0 (4.3–13.7) | |||||
| Migrants M1 | 4 | 8000 | 50.0 (1.0–99) | |||||
| Migrants M2 | 11 | 53 000 | 20.7 (8.4–33) | |||||
M1: migrants to a temperate region before the age of 15 years, M2: migrants to a temperate region after the age of 15 years;
Uncorrected prevalence of MS;
prevalence of MS adjusted to the European population RNMO;
in the population aged from 15 to 64 years on December 31, 1999.
Prevalence of MS in the French West Indies
| . | No. . | Population at risk . | As on December 31, 1999 . | . | ||||
|---|---|---|---|---|---|---|---|---|
. | . | . | Prevalencea (95% CI) . | Prevalenceb (95% CI) . | ||||
| French West Indies | 101 | 683 000 | 14.8 (11.9–17.7) | 14.1 (11.4–16.8) | ||||
| Martinique | 72 | 343 000 | 21 (16.1–25.9) | 19.6 (14.9–24.3) | ||||
| Guadeloupe | 29 | 340 000 | 8.5 (5.4–11.6) | 8.8 (5.7–11.9) | ||||
| Martinique according to sex and age | ||||||||
| F 15–24 years | 2 | 23 000 | 8.7 | |||||
| F 25–34 years | 15 | 29 000 | 51.6 | |||||
| F 35–44 years | 25 | 29 000 | 85.9 | |||||
| F 45–54 years | 9 | 22 000 | 41.6 | |||||
| F 55–64 years | 3 | 15 000 | 19.2 | |||||
| F >65 years | 4 | 13 000 | 31.0 | |||||
| M 15–24 years | 1 | 23 000 | 2.6 | |||||
| M 25–34 years | 4 | 25 000 | 17 | |||||
| M 35–44 years | 5 | 26 000 | 19.8 | |||||
| M 45–54 years | 2 | 18 000 | 10.9 | |||||
| M 55–64 years | 2 | 14 000 | 14.6 | |||||
| Guadeloupe according to sex and age | ||||||||
| F 15–24 years | 2 | 24 000 | 8.2 | |||||
| F 25–34 years | 10 | 29 000 | 34.2 | |||||
| F 35–44 years | 6 | 27 000 | 21.4 | |||||
| F 45–54 years | 4 | 20 000 | 19.4 | |||||
| F 55–64 years | 3 | 13 000 | 21.6 | |||||
| M 15–24 years | 1 | 24 000 | 4 | |||||
| M 25–34 years | 1 | 25 000 | 3.9 | |||||
| M 35–44 years | 1 | 25 000 | 3.9 | |||||
| M 45–54 years | 1 | 18 000 | 5.4 | |||||
| M 55–64 years | 0 | 12 000 | – | |||||
| Clinical form | ||||||||
| Martinique: RNMO | 8 | 343 000 | 2.3 (0.6–3.9) | |||||
| Guadeloupe: RNMO | 10 | 340 000 | 2.9 (1.1–4.7) | |||||
| Habitat | ||||||||
| Urban | 51 | 263 000 | 19.4 (14.2–24.6) | |||||
| Rural | 50 | 420 000 | 11.1 (8.6–15.2) | |||||
| Socioeconomic class | ||||||||
| High | 56 | 200 000 | 28 (20.8–35.2) | |||||
| Low | 42 | 483 000 | 8.7 (6.0–11.4) | |||||
| French West Indies according to migrationc | ||||||||
| Migrants | 52 | 144 000 | 36.1 (26.3–45.9) | 32.1 (22.7–41.5) | ||||
| Non-migrants | 45 | 297 000 | 15.1 (10.8–19.4) | 14.8 (10.5–19.1) | ||||
| Migrants M1 | 23 | 24 000 | 95.8 (56.6–135) | 107.2 (52.7–161.7) | ||||
| Migrants M2 | 29 | 120 000 | 24.2 (15.4–33) | 20.3 (12.7–27.9) | ||||
| Martinique according to migrationc | ||||||||
| Migrants | 37 | 83 000 | 44.6 (30.3–58.6) | |||||
| Non-migrants | 31 | 141 000 | 22.0 (14.4–29.6) | |||||
| Migrants M1 | 19 | 16 000 | 118.7 (65.4–172) | |||||
| Migrants M2 | 18 | 67 000 | 26.9 (14.6–39.2) | |||||
| Guadeloupe according to migrationc | ||||||||
| Migrants | 15 | 61 000 | 24.6 (12.3–36.9) | |||||
| Non-migrants | 14 | 156 000 | 9.0 (4.3–13.7) | |||||
| Migrants M1 | 4 | 8000 | 50.0 (1.0–99) | |||||
| Migrants M2 | 11 | 53 000 | 20.7 (8.4–33) | |||||
| . | No. . | Population at risk . | As on December 31, 1999 . | . | ||||
|---|---|---|---|---|---|---|---|---|
. | . | . | Prevalencea (95% CI) . | Prevalenceb (95% CI) . | ||||
| French West Indies | 101 | 683 000 | 14.8 (11.9–17.7) | 14.1 (11.4–16.8) | ||||
| Martinique | 72 | 343 000 | 21 (16.1–25.9) | 19.6 (14.9–24.3) | ||||
| Guadeloupe | 29 | 340 000 | 8.5 (5.4–11.6) | 8.8 (5.7–11.9) | ||||
| Martinique according to sex and age | ||||||||
| F 15–24 years | 2 | 23 000 | 8.7 | |||||
| F 25–34 years | 15 | 29 000 | 51.6 | |||||
| F 35–44 years | 25 | 29 000 | 85.9 | |||||
| F 45–54 years | 9 | 22 000 | 41.6 | |||||
| F 55–64 years | 3 | 15 000 | 19.2 | |||||
| F >65 years | 4 | 13 000 | 31.0 | |||||
| M 15–24 years | 1 | 23 000 | 2.6 | |||||
| M 25–34 years | 4 | 25 000 | 17 | |||||
| M 35–44 years | 5 | 26 000 | 19.8 | |||||
| M 45–54 years | 2 | 18 000 | 10.9 | |||||
| M 55–64 years | 2 | 14 000 | 14.6 | |||||
| Guadeloupe according to sex and age | ||||||||
| F 15–24 years | 2 | 24 000 | 8.2 | |||||
| F 25–34 years | 10 | 29 000 | 34.2 | |||||
| F 35–44 years | 6 | 27 000 | 21.4 | |||||
| F 45–54 years | 4 | 20 000 | 19.4 | |||||
| F 55–64 years | 3 | 13 000 | 21.6 | |||||
| M 15–24 years | 1 | 24 000 | 4 | |||||
| M 25–34 years | 1 | 25 000 | 3.9 | |||||
| M 35–44 years | 1 | 25 000 | 3.9 | |||||
| M 45–54 years | 1 | 18 000 | 5.4 | |||||
| M 55–64 years | 0 | 12 000 | – | |||||
| Clinical form | ||||||||
| Martinique: RNMO | 8 | 343 000 | 2.3 (0.6–3.9) | |||||
| Guadeloupe: RNMO | 10 | 340 000 | 2.9 (1.1–4.7) | |||||
| Habitat | ||||||||
| Urban | 51 | 263 000 | 19.4 (14.2–24.6) | |||||
| Rural | 50 | 420 000 | 11.1 (8.6–15.2) | |||||
| Socioeconomic class | ||||||||
| High | 56 | 200 000 | 28 (20.8–35.2) | |||||
| Low | 42 | 483 000 | 8.7 (6.0–11.4) | |||||
| French West Indies according to migrationc | ||||||||
| Migrants | 52 | 144 000 | 36.1 (26.3–45.9) | 32.1 (22.7–41.5) | ||||
| Non-migrants | 45 | 297 000 | 15.1 (10.8–19.4) | 14.8 (10.5–19.1) | ||||
| Migrants M1 | 23 | 24 000 | 95.8 (56.6–135) | 107.2 (52.7–161.7) | ||||
| Migrants M2 | 29 | 120 000 | 24.2 (15.4–33) | 20.3 (12.7–27.9) | ||||
| Martinique according to migrationc | ||||||||
| Migrants | 37 | 83 000 | 44.6 (30.3–58.6) | |||||
| Non-migrants | 31 | 141 000 | 22.0 (14.4–29.6) | |||||
| Migrants M1 | 19 | 16 000 | 118.7 (65.4–172) | |||||
| Migrants M2 | 18 | 67 000 | 26.9 (14.6–39.2) | |||||
| Guadeloupe according to migrationc | ||||||||
| Migrants | 15 | 61 000 | 24.6 (12.3–36.9) | |||||
| Non-migrants | 14 | 156 000 | 9.0 (4.3–13.7) | |||||
| Migrants M1 | 4 | 8000 | 50.0 (1.0–99) | |||||
| Migrants M2 | 11 | 53 000 | 20.7 (8.4–33) | |||||
M1: migrants to a temperate region before the age of 15 years, M2: migrants to a temperate region after the age of 15 years;
Uncorrected prevalence of MS;
prevalence of MS adjusted to the European population RNMO;
in the population aged from 15 to 64 years on December 31, 1999.
Mean annual incidencea of MS in the French West Indies
. | No. . | Population at risk . | Rateb (95% CI) . | Ratec (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| French West Indies | 47 | 683 000 | 1.4 (1.0–1.8) | 1.3 (0.9–1.7) | ||||
| Martinique | 35 | 343 000 | 2.0 (1.4–2.6) | 1.9 (1.2–2.6) | ||||
| Guadeloupe | 12 | 340 000 | 0.7 (0.3–1.0) | 0.6 (0.3–0.9) | ||||
| French West Indies according to migrationd | ||||||||
| Migrants | 21 | 144 000 | 2.9 (1.7–4.1) | 2.5 (1.3–3.7) | ||||
| Non-migrants | 25 | 297 000 | 1.7 (1.1–2.3) | 1.6 (0.9–2.3) | ||||
| Migrants M1 | 9 | 24 000 | 7.5 (2.6–11.9) | 6.9 (1.5–12.3) | ||||
| Migrants M2 | 12 | 120 000 | 2.0 (0.8–3.2) | 1.4 (0.6–2.2) | ||||
. | No. . | Population at risk . | Rateb (95% CI) . | Ratec (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| French West Indies | 47 | 683 000 | 1.4 (1.0–1.8) | 1.3 (0.9–1.7) | ||||
| Martinique | 35 | 343 000 | 2.0 (1.4–2.6) | 1.9 (1.2–2.6) | ||||
| Guadeloupe | 12 | 340 000 | 0.7 (0.3–1.0) | 0.6 (0.3–0.9) | ||||
| French West Indies according to migrationd | ||||||||
| Migrants | 21 | 144 000 | 2.9 (1.7–4.1) | 2.5 (1.3–3.7) | ||||
| Non-migrants | 25 | 297 000 | 1.7 (1.1–2.3) | 1.6 (0.9–2.3) | ||||
| Migrants M1 | 9 | 24 000 | 7.5 (2.6–11.9) | 6.9 (1.5–12.3) | ||||
| Migrants M2 | 12 | 120 000 | 2.0 (0.8–3.2) | 1.4 (0.6–2.2) | ||||
M1: migrants to a temperate region before the age of 15 years; M2: migrants to a temperate region after the age of 15 years;
Annual incidence between July 1, 1997 and June 30, 2002;
uncorrected prevalence and incidence rates;
prevalence and incidence rates adjusted to the European population;
In the population aged from 15 to 64 years on December 31, 1999.
Mean annual incidencea of MS in the French West Indies
. | No. . | Population at risk . | Rateb (95% CI) . | Ratec (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| French West Indies | 47 | 683 000 | 1.4 (1.0–1.8) | 1.3 (0.9–1.7) | ||||
| Martinique | 35 | 343 000 | 2.0 (1.4–2.6) | 1.9 (1.2–2.6) | ||||
| Guadeloupe | 12 | 340 000 | 0.7 (0.3–1.0) | 0.6 (0.3–0.9) | ||||
| French West Indies according to migrationd | ||||||||
| Migrants | 21 | 144 000 | 2.9 (1.7–4.1) | 2.5 (1.3–3.7) | ||||
| Non-migrants | 25 | 297 000 | 1.7 (1.1–2.3) | 1.6 (0.9–2.3) | ||||
| Migrants M1 | 9 | 24 000 | 7.5 (2.6–11.9) | 6.9 (1.5–12.3) | ||||
| Migrants M2 | 12 | 120 000 | 2.0 (0.8–3.2) | 1.4 (0.6–2.2) | ||||
. | No. . | Population at risk . | Rateb (95% CI) . | Ratec (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| French West Indies | 47 | 683 000 | 1.4 (1.0–1.8) | 1.3 (0.9–1.7) | ||||
| Martinique | 35 | 343 000 | 2.0 (1.4–2.6) | 1.9 (1.2–2.6) | ||||
| Guadeloupe | 12 | 340 000 | 0.7 (0.3–1.0) | 0.6 (0.3–0.9) | ||||
| French West Indies according to migrationd | ||||||||
| Migrants | 21 | 144 000 | 2.9 (1.7–4.1) | 2.5 (1.3–3.7) | ||||
| Non-migrants | 25 | 297 000 | 1.7 (1.1–2.3) | 1.6 (0.9–2.3) | ||||
| Migrants M1 | 9 | 24 000 | 7.5 (2.6–11.9) | 6.9 (1.5–12.3) | ||||
| Migrants M2 | 12 | 120 000 | 2.0 (0.8–3.2) | 1.4 (0.6–2.2) | ||||
M1: migrants to a temperate region before the age of 15 years; M2: migrants to a temperate region after the age of 15 years;
Annual incidence between July 1, 1997 and June 30, 2002;
uncorrected prevalence and incidence rates;
prevalence and incidence rates adjusted to the European population;
In the population aged from 15 to 64 years on December 31, 1999.
SPRs for MS based on migration and duration of residence
. | Population at risk . | MS-O . | MS-E . | SPR . | χ2-test . | 95% CI . | P-value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Migration | ||||||||||||||
| Migrants | 144 000 | 52 | 26 | 1.98 | 24.26 | 1.48–2.60 | <0.0001 | |||||||
| Migrants M1 | 24 000 | 23 | 3.84 | 5.99 | 42.77 | 3.79–8.98 | <0.0001 | |||||||
| Migrants M2 | 120 000 | 29 | 22.42 | 1.29 | 1.65 | 0.87–1.86 | 0.20 | |||||||
| Duration of residence (years) | ||||||||||||||
| <5 years | 57 000 | 11 | 9.89 | 1.11 | 0.04 | 0.55–1.99 | 0.95 | |||||||
| 5–10 years | 39 000 | 14 | 7.67 | 1.83 | 3.65 | 0.99–3.04 | 0.06 | |||||||
| >10 years | 48 000 | 27 | 8.71 | 3.10 | 23.5 | 2.01–4.47 | <0.0001 | |||||||
. | Population at risk . | MS-O . | MS-E . | SPR . | χ2-test . | 95% CI . | P-value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Migration | ||||||||||||||
| Migrants | 144 000 | 52 | 26 | 1.98 | 24.26 | 1.48–2.60 | <0.0001 | |||||||
| Migrants M1 | 24 000 | 23 | 3.84 | 5.99 | 42.77 | 3.79–8.98 | <0.0001 | |||||||
| Migrants M2 | 120 000 | 29 | 22.42 | 1.29 | 1.65 | 0.87–1.86 | 0.20 | |||||||
| Duration of residence (years) | ||||||||||||||
| <5 years | 57 000 | 11 | 9.89 | 1.11 | 0.04 | 0.55–1.99 | 0.95 | |||||||
| 5–10 years | 39 000 | 14 | 7.67 | 1.83 | 3.65 | 0.99–3.04 | 0.06 | |||||||
| >10 years | 48 000 | 27 | 8.71 | 3.10 | 23.5 | 2.01–4.47 | <0.0001 | |||||||
SPRs calculated based on migration and duration of residence in a temperate region prior to onset of disease in the French West Indian population aged from 15 to 64 years on December 31, 1999. M1 = migrants to a temperate region before the age of 15 years; M2 = migrants to a temperate region after the age of 15 years; MS = multiple sclerosis; MS-O = MS cases observed; MS-E = MS cases expected deriving from age-specific prevalence of MS in non-migrants.
SPRs for MS based on migration and duration of residence
. | Population at risk . | MS-O . | MS-E . | SPR . | χ2-test . | 95% CI . | P-value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Migration | ||||||||||||||
| Migrants | 144 000 | 52 | 26 | 1.98 | 24.26 | 1.48–2.60 | <0.0001 | |||||||
| Migrants M1 | 24 000 | 23 | 3.84 | 5.99 | 42.77 | 3.79–8.98 | <0.0001 | |||||||
| Migrants M2 | 120 000 | 29 | 22.42 | 1.29 | 1.65 | 0.87–1.86 | 0.20 | |||||||
| Duration of residence (years) | ||||||||||||||
| <5 years | 57 000 | 11 | 9.89 | 1.11 | 0.04 | 0.55–1.99 | 0.95 | |||||||
| 5–10 years | 39 000 | 14 | 7.67 | 1.83 | 3.65 | 0.99–3.04 | 0.06 | |||||||
| >10 years | 48 000 | 27 | 8.71 | 3.10 | 23.5 | 2.01–4.47 | <0.0001 | |||||||
. | Population at risk . | MS-O . | MS-E . | SPR . | χ2-test . | 95% CI . | P-value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Migration | ||||||||||||||
| Migrants | 144 000 | 52 | 26 | 1.98 | 24.26 | 1.48–2.60 | <0.0001 | |||||||
| Migrants M1 | 24 000 | 23 | 3.84 | 5.99 | 42.77 | 3.79–8.98 | <0.0001 | |||||||
| Migrants M2 | 120 000 | 29 | 22.42 | 1.29 | 1.65 | 0.87–1.86 | 0.20 | |||||||
| Duration of residence (years) | ||||||||||||||
| <5 years | 57 000 | 11 | 9.89 | 1.11 | 0.04 | 0.55–1.99 | 0.95 | |||||||
| 5–10 years | 39 000 | 14 | 7.67 | 1.83 | 3.65 | 0.99–3.04 | 0.06 | |||||||
| >10 years | 48 000 | 27 | 8.71 | 3.10 | 23.5 | 2.01–4.47 | <0.0001 | |||||||
SPRs calculated based on migration and duration of residence in a temperate region prior to onset of disease in the French West Indian population aged from 15 to 64 years on December 31, 1999. M1 = migrants to a temperate region before the age of 15 years; M2 = migrants to a temperate region after the age of 15 years; MS = multiple sclerosis; MS-O = MS cases observed; MS-E = MS cases expected deriving from age-specific prevalence of MS in non-migrants.
Evaluation of the risk of multiple sclerosis according to migration
Seventy-four incident cases of multiple sclerosis were observed between January 1, 1995 and December 31, 2004, including 56 in Martinique and 18 in Guadeloupe. Thirty-five cases were incident between January 1, 1995 and December 31, 1999 and 39 cases were incident between December 31, 1999 and December 31, 2004. The mean delay in the diagnosis of these incident cases was 1.45 years (SD: 1.9 years) ranging between 0 and 9 years. The mean delay in diagnosis of the incidental cases before December 31, 1999 of 2.3 years (SD: 2.3 years) fell to 0.7 years (SD: 0.8 years) for incident cases after December 31, 1999 (P < 0.001). During the 1 440 000 person-years of follow-up, 33 incident multiple sclerosis cases were identified in migrants. Since the number of expected cases was 19.3, the overall SIR was 1.71 (95% CI: 1.19–2.38; P < 0.01) among migrants (Table 4). The SIR was very significantly increased for West Indians who migrated before the age of 15 (4.05, 95% CI: 2.17–6.83; P < 0.0001) but not for those who migrated after that age (Table 4). There was no significant difference between the mean delay in the diagnosis of incident cases in migrants of 1.6 years (SD: 1.9 years) and that observed in non-migrants of 1.3 years (SD: 1.8 years) (P = 0.43).
SIRs for MS based on age at migration to a temperate region
. | Population at risk . | MS-O . | MS-E . | SIR . | χ2-test . | 95% CI . | P-value . |
|---|---|---|---|---|---|---|---|
| Any age | 144 000 | 33 | 19.3 | 1.71 | 9.02 | 1.19–2.38 | <0.01 |
| Before age of 15 | 24 000 | 14 | 3.46 | 4.05 | 16.33 | 2.17–6.83 | <0.0001 |
| Between age of 15 and 20 | 48 000 | 10 | 6.85 | 1.46 | 1.09 | 0.52–2.71 | 0.28 |
| After age of 20 | 72 000 | 9.0 | 9.0 | 1.0 | 0.01 | 0.45–1.87 | 0.95 |
. | Population at risk . | MS-O . | MS-E . | SIR . | χ2-test . | 95% CI . | P-value . |
|---|---|---|---|---|---|---|---|
| Any age | 144 000 | 33 | 19.3 | 1.71 | 9.02 | 1.19–2.38 | <0.01 |
| Before age of 15 | 24 000 | 14 | 3.46 | 4.05 | 16.33 | 2.17–6.83 | <0.0001 |
| Between age of 15 and 20 | 48 000 | 10 | 6.85 | 1.46 | 1.09 | 0.52–2.71 | 0.28 |
| After age of 20 | 72 000 | 9.0 | 9.0 | 1.0 | 0.01 | 0.45–1.87 | 0.95 |
SIRs calculated based on age at migration to a temperate region prior to onset of disease in the French West Indian population aged from 15 to 64 years on December 31, 1999. MS = multiple sclerosis; MS-O = MS cases observed; MS-E = MS cases expected deriving from age-specific mean annual incidence of MS in non-migrants for the period January 1, 1995 to December 31, 2004.
SIRs for MS based on age at migration to a temperate region
. | Population at risk . | MS-O . | MS-E . | SIR . | χ2-test . | 95% CI . | P-value . |
|---|---|---|---|---|---|---|---|
| Any age | 144 000 | 33 | 19.3 | 1.71 | 9.02 | 1.19–2.38 | <0.01 |
| Before age of 15 | 24 000 | 14 | 3.46 | 4.05 | 16.33 | 2.17–6.83 | <0.0001 |
| Between age of 15 and 20 | 48 000 | 10 | 6.85 | 1.46 | 1.09 | 0.52–2.71 | 0.28 |
| After age of 20 | 72 000 | 9.0 | 9.0 | 1.0 | 0.01 | 0.45–1.87 | 0.95 |
. | Population at risk . | MS-O . | MS-E . | SIR . | χ2-test . | 95% CI . | P-value . |
|---|---|---|---|---|---|---|---|
| Any age | 144 000 | 33 | 19.3 | 1.71 | 9.02 | 1.19–2.38 | <0.01 |
| Before age of 15 | 24 000 | 14 | 3.46 | 4.05 | 16.33 | 2.17–6.83 | <0.0001 |
| Between age of 15 and 20 | 48 000 | 10 | 6.85 | 1.46 | 1.09 | 0.52–2.71 | 0.28 |
| After age of 20 | 72 000 | 9.0 | 9.0 | 1.0 | 0.01 | 0.45–1.87 | 0.95 |
SIRs calculated based on age at migration to a temperate region prior to onset of disease in the French West Indian population aged from 15 to 64 years on December 31, 1999. MS = multiple sclerosis; MS-O = MS cases observed; MS-E = MS cases expected deriving from age-specific mean annual incidence of MS in non-migrants for the period January 1, 1995 to December 31, 2004.
Sociodemographic and genetic characteristics of the migrants
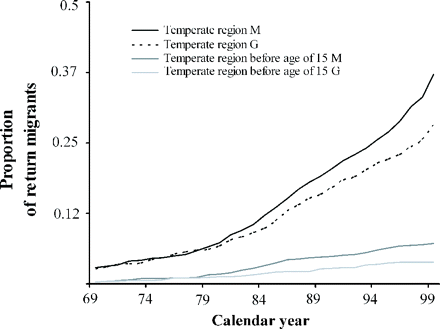
On prevalence day December 31, 1999, for the 15–64-year age range, return migration involved 36.6% of the Martinican population and 28.1% of the Guadeloupan population (P = 0.0002). There were more migrants who had lived in a temperate region under the age of 15 years in Martinique than in Guadeloupe: 7.1% versus 3.8% (P = 0.004). The total duration of the stay outside the West Indies was 9.8 years (SD: 9) in Martinicans—greater than the value of 7.2 years (SD: 5.7) in Guadeloupans (P < 0.0001). The proportion of Martinicans who lived in metropolitan France north of the 45th parallel (82%) was greater than that for Guadeloupans (76%) (P < 0.0001). Analysis of the return migration curve showed that this was negligible until the end of the 1970s. There was then a continuous acceleration in return migration which was more marked for Martinique than for Guadeloupe (Fig. 3). From the demographic point of view, there was no difference in the male/female sex ratio between migrants and non-migrants. On the contrary, migrants were significantly older: 40 years (SD: 12.4) in comparison with non-migrants: 37.1 years (SD: 13.5) (P < 0.0001). In migrants, the proportion of subjects of high socioeconomic class was 58.3% compared with 39% in non-migrants (P < 0.0001). Analysis of the distribution of ABO rhesus blood groups and in particular the O+ group failed to show any difference between migrants and non-migrants (Table 5). Finally, the analysis of the frequencies of HLA-I alleles at the A and B loci was conducted to evaluate the genetic drift of the migrant (0.246) and non-migrant (0.229) West Indian populations in comparison with that of the population of Equatorial Guinea from which the West Indian population derives by interbreeding. The difference of 0.017 in the penetrance of Caucasian genes between the two migrating and non-migrating populations was very low (Table 6).
Cumulative frequency of West Indians who stayed for at least 1 year in a temperate region as a function of age at time of residence in the population aged from 15 to 64 years on 12/31/1999. M: Martinique, G: Guadeloupe.
Sociodemographic and genetic characteristics of the West Indian population (population aged from 15 to 64 years on December 31, 1999 according to migration status)
. | Migrants (N = 518) . | Non-migrants (N = 1082) . | P-value . | |||
|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||
| Sex | ||||||
| F/M (ratio) | 264/254 (1.04) | 572/510 (1.1) | 0.43 | |||
| Age, years (mean ± SD) | 40 (12.4) | 37.1 (13.5) | <0.0001 | |||
| Socioeconomic class (%) | ||||||
| High | 302/518 (58.3) | 422/1082 (39) | <0.0001 | |||
| Low | 216/518 (41.7) | 660/1082 (61) | ||||
| Genetic characteristics | ||||||
| ABO group (%) | ||||||
| O+ | 204/409 (49.9) | 315/672 (46.9) | 0.8 | |||
| A+ | 116/409 (28.4) | 181/672 (26.9) | 0.19 | |||
| B+ | 57/409 (13.9) | 106/672 (15.8) | 0.53 | |||
| O− | 12/409 (2.9) | 18/672 (2.7) | 0.95 | |||
| AB+ | 11/409 (2.7) | 31/672 (4.6) | 0.15 | |||
| B− | 5/409 (1.2) | 12/672 (1.8) | 0.62 | |||
| A− | 4/409 (1.0) | 9/672 (1.3) | 0.73 | |||
. | Migrants (N = 518) . | Non-migrants (N = 1082) . | P-value . | |||
|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||
| Sex | ||||||
| F/M (ratio) | 264/254 (1.04) | 572/510 (1.1) | 0.43 | |||
| Age, years (mean ± SD) | 40 (12.4) | 37.1 (13.5) | <0.0001 | |||
| Socioeconomic class (%) | ||||||
| High | 302/518 (58.3) | 422/1082 (39) | <0.0001 | |||
| Low | 216/518 (41.7) | 660/1082 (61) | ||||
| Genetic characteristics | ||||||
| ABO group (%) | ||||||
| O+ | 204/409 (49.9) | 315/672 (46.9) | 0.8 | |||
| A+ | 116/409 (28.4) | 181/672 (26.9) | 0.19 | |||
| B+ | 57/409 (13.9) | 106/672 (15.8) | 0.53 | |||
| O− | 12/409 (2.9) | 18/672 (2.7) | 0.95 | |||
| AB+ | 11/409 (2.7) | 31/672 (4.6) | 0.15 | |||
| B− | 5/409 (1.2) | 12/672 (1.8) | 0.62 | |||
| A− | 4/409 (1.0) | 9/672 (1.3) | 0.73 | |||
Sociodemographic and genetic characteristics of the West Indian population (population aged from 15 to 64 years on December 31, 1999 according to migration status)
. | Migrants (N = 518) . | Non-migrants (N = 1082) . | P-value . | |||
|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||
| Sex | ||||||
| F/M (ratio) | 264/254 (1.04) | 572/510 (1.1) | 0.43 | |||
| Age, years (mean ± SD) | 40 (12.4) | 37.1 (13.5) | <0.0001 | |||
| Socioeconomic class (%) | ||||||
| High | 302/518 (58.3) | 422/1082 (39) | <0.0001 | |||
| Low | 216/518 (41.7) | 660/1082 (61) | ||||
| Genetic characteristics | ||||||
| ABO group (%) | ||||||
| O+ | 204/409 (49.9) | 315/672 (46.9) | 0.8 | |||
| A+ | 116/409 (28.4) | 181/672 (26.9) | 0.19 | |||
| B+ | 57/409 (13.9) | 106/672 (15.8) | 0.53 | |||
| O− | 12/409 (2.9) | 18/672 (2.7) | 0.95 | |||
| AB+ | 11/409 (2.7) | 31/672 (4.6) | 0.15 | |||
| B− | 5/409 (1.2) | 12/672 (1.8) | 0.62 | |||
| A− | 4/409 (1.0) | 9/672 (1.3) | 0.73 | |||
. | Migrants (N = 518) . | Non-migrants (N = 1082) . | P-value . | |||
|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||
| Sex | ||||||
| F/M (ratio) | 264/254 (1.04) | 572/510 (1.1) | 0.43 | |||
| Age, years (mean ± SD) | 40 (12.4) | 37.1 (13.5) | <0.0001 | |||
| Socioeconomic class (%) | ||||||
| High | 302/518 (58.3) | 422/1082 (39) | <0.0001 | |||
| Low | 216/518 (41.7) | 660/1082 (61) | ||||
| Genetic characteristics | ||||||
| ABO group (%) | ||||||
| O+ | 204/409 (49.9) | 315/672 (46.9) | 0.8 | |||
| A+ | 116/409 (28.4) | 181/672 (26.9) | 0.19 | |||
| B+ | 57/409 (13.9) | 106/672 (15.8) | 0.53 | |||
| O− | 12/409 (2.9) | 18/672 (2.7) | 0.95 | |||
| AB+ | 11/409 (2.7) | 31/672 (4.6) | 0.15 | |||
| B− | 5/409 (1.2) | 12/672 (1.8) | 0.62 | |||
| A− | 4/409 (1.0) | 9/672 (1.3) | 0.73 | |||
Distribution of HLA-I allelic frequencies at the A and B loci in the migrant West Indian population, non-migrant West Indian population and the population of Equatorial Guinea
. | Migrants (N = 42) . | Non-migrants (N = 58) . | Equatorial Guinea (N = 101) . | |||
|---|---|---|---|---|---|---|
| HLA-A locus | ||||||
| A*01 | 0.11 | 0.05 | 0.014 | |||
| A*02 | 0.17 | 0.16 | 0.19 | |||
| A*03 | 0.10 | 0.06 | 0.03 | |||
| A*11 | 0.03 | 0 | 0.01 | |||
| A*2301 | 0.11 | 0.10 | 0.09 | |||
| A*24 | 0.06 | 0.03 | 0 | |||
| A*25 | 0 | 0.03 | 0 | |||
| A*26 | 0.03 | 0.03 | 0.01 | |||
| A*29 | 0 | 0.04 | 0.02 | |||
| A*30 | 0.15 | 0.12 | 0.25 | |||
| A*32 | 0.01 | 0.03 | 0.09 | |||
| A*33 | 0.06 | 0.08 | 0.04 | |||
| A*3402 | 0.01 | 0.03 | 0.04 | |||
| A*36 | 0.04 | 0.06 | 0.004 | |||
| A*6601 | 0.03 | 0.01 | 0 | |||
| A*68 | 0.07 | 0.10 | 0.10 | |||
| A*74 | 0.03 | 0.06 | 0.06 | |||
| A*8001 | 0 | 0.01 | 0.004 | |||
| HLA-B locus | ||||||
| B*07 | 0.05 | 0.14 | 0.07 | |||
| B*08 | 0.09 | 0.01 | 0.015 | |||
| B*13 | 0.01 | 0 | 0.10 | |||
| B*14 | 0.07 | 0.04 | 0.015 | |||
| B*15 | 0.11 | 0.10 | 0.20 | |||
| B*18 | 0.03 | 0.06 | 0.014 | |||
| B*27 | 0.03 | 0.01 | 0 | |||
| B*35 | 0.09 | 0.15 | 0.01 | |||
| B*39 | 0.01 | 0.03 | 0 | |||
| B*41 | 0.01 | 0 | 0 | |||
| B*42 | 0.07 | 0.03 | 0.11 | |||
| B*44 | 0.03 | 0.08 | 0.14 | |||
| B*45 | 0.07 | 0.05 | 0.08 | |||
| B*49 | 0.04 | 0.04 | 0.01 | |||
| B*50 | 0.01 | 0 | 0 | |||
| B*51 | 0.04 | 0.01 | 0.02 | |||
| B*52 | 0.03 | 0.01 | 0 | |||
| B*53 | 0.08 | 0.14 | 0.13 | |||
| B*55 | 0.01 | 0.01 | 0 | |||
| B*57 | 0.07 | 0.05 | 0.04 | |||
| B*58 | 0.05 | 0.04 | 0.03 | |||
| Genetic drift* | 0.246 | 0.229 | ||||
. | Migrants (N = 42) . | Non-migrants (N = 58) . | Equatorial Guinea (N = 101) . | |||
|---|---|---|---|---|---|---|
| HLA-A locus | ||||||
| A*01 | 0.11 | 0.05 | 0.014 | |||
| A*02 | 0.17 | 0.16 | 0.19 | |||
| A*03 | 0.10 | 0.06 | 0.03 | |||
| A*11 | 0.03 | 0 | 0.01 | |||
| A*2301 | 0.11 | 0.10 | 0.09 | |||
| A*24 | 0.06 | 0.03 | 0 | |||
| A*25 | 0 | 0.03 | 0 | |||
| A*26 | 0.03 | 0.03 | 0.01 | |||
| A*29 | 0 | 0.04 | 0.02 | |||
| A*30 | 0.15 | 0.12 | 0.25 | |||
| A*32 | 0.01 | 0.03 | 0.09 | |||
| A*33 | 0.06 | 0.08 | 0.04 | |||
| A*3402 | 0.01 | 0.03 | 0.04 | |||
| A*36 | 0.04 | 0.06 | 0.004 | |||
| A*6601 | 0.03 | 0.01 | 0 | |||
| A*68 | 0.07 | 0.10 | 0.10 | |||
| A*74 | 0.03 | 0.06 | 0.06 | |||
| A*8001 | 0 | 0.01 | 0.004 | |||
| HLA-B locus | ||||||
| B*07 | 0.05 | 0.14 | 0.07 | |||
| B*08 | 0.09 | 0.01 | 0.015 | |||
| B*13 | 0.01 | 0 | 0.10 | |||
| B*14 | 0.07 | 0.04 | 0.015 | |||
| B*15 | 0.11 | 0.10 | 0.20 | |||
| B*18 | 0.03 | 0.06 | 0.014 | |||
| B*27 | 0.03 | 0.01 | 0 | |||
| B*35 | 0.09 | 0.15 | 0.01 | |||
| B*39 | 0.01 | 0.03 | 0 | |||
| B*41 | 0.01 | 0 | 0 | |||
| B*42 | 0.07 | 0.03 | 0.11 | |||
| B*44 | 0.03 | 0.08 | 0.14 | |||
| B*45 | 0.07 | 0.05 | 0.08 | |||
| B*49 | 0.04 | 0.04 | 0.01 | |||
| B*50 | 0.01 | 0 | 0 | |||
| B*51 | 0.04 | 0.01 | 0.02 | |||
| B*52 | 0.03 | 0.01 | 0 | |||
| B*53 | 0.08 | 0.14 | 0.13 | |||
| B*55 | 0.01 | 0.01 | 0 | |||
| B*57 | 0.07 | 0.05 | 0.04 | |||
| B*58 | 0.05 | 0.04 | 0.03 | |||
| Genetic drift* | 0.246 | 0.229 | ||||
Genetic drift in comparison with the population of Equatorial Guinea calculated using Nei's law.
Distribution of HLA-I allelic frequencies at the A and B loci in the migrant West Indian population, non-migrant West Indian population and the population of Equatorial Guinea
. | Migrants (N = 42) . | Non-migrants (N = 58) . | Equatorial Guinea (N = 101) . | |||
|---|---|---|---|---|---|---|
| HLA-A locus | ||||||
| A*01 | 0.11 | 0.05 | 0.014 | |||
| A*02 | 0.17 | 0.16 | 0.19 | |||
| A*03 | 0.10 | 0.06 | 0.03 | |||
| A*11 | 0.03 | 0 | 0.01 | |||
| A*2301 | 0.11 | 0.10 | 0.09 | |||
| A*24 | 0.06 | 0.03 | 0 | |||
| A*25 | 0 | 0.03 | 0 | |||
| A*26 | 0.03 | 0.03 | 0.01 | |||
| A*29 | 0 | 0.04 | 0.02 | |||
| A*30 | 0.15 | 0.12 | 0.25 | |||
| A*32 | 0.01 | 0.03 | 0.09 | |||
| A*33 | 0.06 | 0.08 | 0.04 | |||
| A*3402 | 0.01 | 0.03 | 0.04 | |||
| A*36 | 0.04 | 0.06 | 0.004 | |||
| A*6601 | 0.03 | 0.01 | 0 | |||
| A*68 | 0.07 | 0.10 | 0.10 | |||
| A*74 | 0.03 | 0.06 | 0.06 | |||
| A*8001 | 0 | 0.01 | 0.004 | |||
| HLA-B locus | ||||||
| B*07 | 0.05 | 0.14 | 0.07 | |||
| B*08 | 0.09 | 0.01 | 0.015 | |||
| B*13 | 0.01 | 0 | 0.10 | |||
| B*14 | 0.07 | 0.04 | 0.015 | |||
| B*15 | 0.11 | 0.10 | 0.20 | |||
| B*18 | 0.03 | 0.06 | 0.014 | |||
| B*27 | 0.03 | 0.01 | 0 | |||
| B*35 | 0.09 | 0.15 | 0.01 | |||
| B*39 | 0.01 | 0.03 | 0 | |||
| B*41 | 0.01 | 0 | 0 | |||
| B*42 | 0.07 | 0.03 | 0.11 | |||
| B*44 | 0.03 | 0.08 | 0.14 | |||
| B*45 | 0.07 | 0.05 | 0.08 | |||
| B*49 | 0.04 | 0.04 | 0.01 | |||
| B*50 | 0.01 | 0 | 0 | |||
| B*51 | 0.04 | 0.01 | 0.02 | |||
| B*52 | 0.03 | 0.01 | 0 | |||
| B*53 | 0.08 | 0.14 | 0.13 | |||
| B*55 | 0.01 | 0.01 | 0 | |||
| B*57 | 0.07 | 0.05 | 0.04 | |||
| B*58 | 0.05 | 0.04 | 0.03 | |||
| Genetic drift* | 0.246 | 0.229 | ||||
. | Migrants (N = 42) . | Non-migrants (N = 58) . | Equatorial Guinea (N = 101) . | |||
|---|---|---|---|---|---|---|
| HLA-A locus | ||||||
| A*01 | 0.11 | 0.05 | 0.014 | |||
| A*02 | 0.17 | 0.16 | 0.19 | |||
| A*03 | 0.10 | 0.06 | 0.03 | |||
| A*11 | 0.03 | 0 | 0.01 | |||
| A*2301 | 0.11 | 0.10 | 0.09 | |||
| A*24 | 0.06 | 0.03 | 0 | |||
| A*25 | 0 | 0.03 | 0 | |||
| A*26 | 0.03 | 0.03 | 0.01 | |||
| A*29 | 0 | 0.04 | 0.02 | |||
| A*30 | 0.15 | 0.12 | 0.25 | |||
| A*32 | 0.01 | 0.03 | 0.09 | |||
| A*33 | 0.06 | 0.08 | 0.04 | |||
| A*3402 | 0.01 | 0.03 | 0.04 | |||
| A*36 | 0.04 | 0.06 | 0.004 | |||
| A*6601 | 0.03 | 0.01 | 0 | |||
| A*68 | 0.07 | 0.10 | 0.10 | |||
| A*74 | 0.03 | 0.06 | 0.06 | |||
| A*8001 | 0 | 0.01 | 0.004 | |||
| HLA-B locus | ||||||
| B*07 | 0.05 | 0.14 | 0.07 | |||
| B*08 | 0.09 | 0.01 | 0.015 | |||
| B*13 | 0.01 | 0 | 0.10 | |||
| B*14 | 0.07 | 0.04 | 0.015 | |||
| B*15 | 0.11 | 0.10 | 0.20 | |||
| B*18 | 0.03 | 0.06 | 0.014 | |||
| B*27 | 0.03 | 0.01 | 0 | |||
| B*35 | 0.09 | 0.15 | 0.01 | |||
| B*39 | 0.01 | 0.03 | 0 | |||
| B*41 | 0.01 | 0 | 0 | |||
| B*42 | 0.07 | 0.03 | 0.11 | |||
| B*44 | 0.03 | 0.08 | 0.14 | |||
| B*45 | 0.07 | 0.05 | 0.08 | |||
| B*49 | 0.04 | 0.04 | 0.01 | |||
| B*50 | 0.01 | 0 | 0 | |||
| B*51 | 0.04 | 0.01 | 0.02 | |||
| B*52 | 0.03 | 0.01 | 0 | |||
| B*53 | 0.08 | 0.14 | 0.13 | |||
| B*55 | 0.01 | 0.01 | 0 | |||
| B*57 | 0.07 | 0.05 | 0.04 | |||
| B*58 | 0.05 | 0.04 | 0.03 | |||
| Genetic drift* | 0.246 | 0.229 | ||||
Genetic drift in comparison with the population of Equatorial Guinea calculated using Nei's law.
Discussion
Emergence of multiple sclerosis in the French West Indies
Our study demonstrated that prevalence of multiple sclerosis in the French West Indies on December 31, 1999 was 14.8/105 (95% CI: 11.9–17.7), an average figure for the world as a whole. The prevalence was higher in Martinique than Guadeloupe. These data contrast with the generally held opinion that this disease is rare in populations descended from Africans and living in a tropical region because of their supposed increased genetic resistance to the disease and to an unfavourable environment for its development. Despite the absence of previous formal epidemiological studies, there is circumstantial evidence that multiple sclerosis has emerged recently in the French West Indies. The rarity of the illness in the 1980s was evident to neurologists practising in the French West Indies, as it was included in the differential diagnosis of HTLV-1-associated myelopathy, together with nearly the whole spectrum of inflammatory central nervous system diseases. In particular, analysis of age and sex-specific prevalence of multiple sclerosis currently shows the classical prevalence peak in men and women in the age range 35–44 years followed by an unusual fall for the 45–54-year decade. These data suggest that it emerged recently in Martinique. Likewise, the unusually early prevalence peak in the Guadeloupan population in the 25–34-year age range suggests an even more recent emergence of the disease, consistent with its lower global prevalence than in Martinique.
Analysis of the different clinical forms shows that in Martinique the prevalence of the typical form of multiple sclerosis is currently much greater than that of RNMO. This phenomenon of reversal of the clinical spectrum of the illness in the French West Indies is still apparent, though much less clear in Guadeloupe. In the two islands, the increased prevalence of demyelinating disease is due to the emergence of the typical form and not to an increase in RNMO, the prevalence of which remains low, even though its frequency in the French West Indies is slightly greater than that observed in South-east Asia (Kira, 2003) where it oscillates between 0.27/105 and 0.74/105. The female/male sex ratio of multiple sclerosis in Martinique remains high, though lower than in Guadeloupe where the prevalence in the male population remains low whatever the age range. Although the nosological status of RNMO is open to debate (Weinshenker, 2003), these data support the conclusion that RNMO may be an emerging primordial ancestor of multiple sclerosis (Compston, 2004). When multiple sclerosis is rare, its clinical expression is limited to its borderline form, RNMO, which is more or less an exclusively female disease. When multiple sclerosis emerges in a population, RNMO becomes of secondary importance, with the occurrence of typical cases in females but also in males leading to a progressive fall in the female/male sex ratio. Our study also demonstrated a prevalence of multiple sclerosis in urban areas, where it is twice as frequent as in rural areas, confirming the epidemiological data observed in Israel (Martyn, 1991). Finally, the emergence of multiple sclerosis in the French West Indies is also demonstrated by a mean annual incidence of 1.4/105 (95% CI: 1.0–1.8) for the period July 1997 to June 2002. As for the prevalence data, the incidence is lower in Guadeloupe than in Martinique, where the rate of 2.0/105 suggests a more or less epidemic disease as the current incidence rate there is similar to the high rate observed in Western Europe.
Role of return migration
Our study demonstrated that the prevalence of multiple sclerosis was twice as high in migrants than in non-migrants and that the prevalence was very high if this stay in a temperate region occurred before the age of 15 years: 95.8/105 (95% CI : 56.6–135) for the population aged from 15 to 64 years. A clear dose–response relationship between the duration of residence out of the French West Indies and the prevalence of multiple sclerosis was also found. Studies of Caucasian populations migrating from high prevalence areas to low prevalence areas suggest that the risk of acquiring the disease is determined before the age of 15 years for populations from the UK who emigrated to South Africa (Dean and Kurtzke, 1971) and for Jewish populations emigrating to Israel (Alter et al., 1978), whereas migrant populations from Ireland and the UK emigrating to Australia reduced their risk of developing the disease whatever their age on migration (Hammond et al., 2000). Studies evaluating the role of migration on the occurrence of multiple sclerosis in black populations are limited to British West Indian immigrants. These showed that the prevalence in children born of migrants in the UK was similar to that of the white population (Elian and Dean, 1987; Elian et al., 1990), whereas the migrants increased their risk—an effect independent on their age at migration (Dean and Elian, 1997). The importance of geographical factors was also emphasized in a cohort of black American army veterans who developed a risk of multiple sclerosis that was independently correlated with the latitude of their residence on incorporation (Kürtzke and Page, 1997). Prevalence is not, however, the best epidemiological instrument for evaluating the change in risk induced by migration. In our study, it cannot be excluded that migration to temperate regions leads to a less severe progressive form of multiple sclerosis, artificially biasing the epidemiological data in favour of an increased prevalence in migrants. Most importantly, the incidence of the disease was increased in migrants, and those migrants under the age of 15 years carried a larger risk than migrants after that age. The epidemiological data could not have been artificially biased by the demographic characteristics of the migrant population in our study as the population of women was identical in the migrant and non-migrant population, and age-corrected statistics again demonstrated that migration increased risk of the disease. Furthermore, a similar delay in diagnosis of incident cases in migrants and non-migrants is not in favour of less successful detection in our health system in the non-migrant AfroCaribbean population.
We also demonstrated both quantitative and qualitative differences in return migration between the two islands of the French West Indies. There was a greater return of migrants to Martinique. The duration of residence of Martinicans in metropolitan France was greater than that of Guadeloupans, and the percentage of Martinicans who lived in the most northerly parts of metropolitan France was significantly greater than that observed for Guadeloupans. As metropolitan France is located in a high multiple sclerosis prevalence area with a north-south prevalence gradient (Kurtzke and Delasnerie-Laupretre, 1996), the higher incidence in Martinique in comparison with Guadeloupe also strongly supports the hypothesis that return migration has played a role in the emergence of the illness in the French West Indies.
Study limitations
The prevalence of multiple sclerosis in subjects of high socioeconomic class was three times as high as in underprivileged subjects. The correlation between prevalence and socioeconomic class has also been demonstrated in high prevalence areas, such as the USA (Kurtzke and Page, 1997) and Orkney Islands (Poskanzer et al., 1980). In our study, migrants had a significantly higher socioeconomic class than non-migrants following return migration. This may partly explain the increased risk of the disease in migrants, lowering the role of migration in acquiring multiple sclerosis if migrants were also not typical of the general West Indian population in the socioeconomic class at the time they migrated (Gale and Martyn, 1995). Bias attributable to genetic differences was minimized in our study as we showed from the blood group distribution and by calculating the genetic drift from the allelic frequencies at the type 1 HLA complex that crossbreeding for the West Indian migrant population was no greater than for the non-migrant population. However, more powerful tools such as admixture mapping (Smith et al., 2005) would be of great interest, in order to draw a firm conclusion about European ancestry in migrating and non-migrating West Indian populations. Based on incidence cases, a higher-than-expected number of people with multiple sclerosis was found in migrants between 15 and 20 years of age (10 versus 6.8) without reaching statistical significance. Again, more statistical power could have demonstrated that the risk of developing multiple sclerosis, rather than it being largely decided by the age of 15 years, actually spans a much wider age range.
Explanatory hypotheses
Our data suggest that environmental factors are involved in the emergence of multiple sclerosis in the French West Indies, even if genetic factors cannot be totally ignored, as shown by the increased frequency of the DRB1*1503 allele in our patients (Quelvennec et al., 2003). The non-negligible introduction of Caucasian genes into the AfroCaribbean population would not, however, have been sufficient to allow the onset of multiple sclerosis because of a tropical environment unfavourable until the recent past but could begin to operate under a temperate latitude. Return migration may have served as a vector for the introduction in the French West Indies of possible infectious environmental factors operating, in particular, before the age of 15 years. Migrants initially carrying these transmissible factors acquired in metropolitan France would then have secondarily permitted the emergence of multiple sclerosis in the non-migrant population by contamination. This scenario is, therefore, identical to the Faeroe Islands model, where the first epidemic of the illness was observed in an island population previously free from the disease following contact with occupying British troops during World War II (Kurtzke and Hyllested, 1992). Alternatively, local, socioeconomic or sanitary changes to the environment might be involved, as in Sardinia where repeated determination of the incidence of the disease demonstrated that its emergence coincided with rapid post-war westernization (Rosati et al., 1988; Granieri et al., 2000). These environmental changes may either trigger or protect from the disease. Hence the recent gradual disappearance from the French West Indies of factors with a protective effect on children <15 years in age may explain the emergence of multiple sclerosis in the non-migrant population, a fortiori if the population is suddenly isolated from these protective factors by migration and, in particular, if migration to metropolitan France took place at an early age. These local environmental changes may also explain the increased proportion of the typical form of multiple sclerosis in comparison with RNMO. In Japan, a gradual decrease in the NMO/typical multiple sclerosis ratio was demonstrated according to the decade of the birth of Japanese patients in parallel with the economic growth of the country (Kira et al., 1999). In Martinique, a survey of intestinal parasitism conducted in 1978 showed that 70% of the 5–15 age group carried at least one parasite and that one of the most prevalent parasitosis was schistosomiasis (Villon et al., 1983). In contrast, a new survey in 1994 showed a highly significant reduction of the intestinal parasitism rate to 8% following the improved economic and sanitation level of Martinique (Gardien et al., 1997). The recent demonstration that Schistosoma mansoni ova pre-treatment improved experimental autoimmune encephalomyelitis (Sewell et al., 2003) suggests that helminth pathogens are able to modulate CNS autoimmunity by the Th1–Th2 switch in the immune response (Sewell et al., 2002). Alternatively, helminth infections may induce an increase in immunoregulator cells such as Treg cells (CD25+CD4+) leading to a Th2 polarization of the immune response (McKee and Pearce, 2004). Considering that RNMO is predominately mediated by Th2 cells and typical multiple sclerosis by Th1 cells, clearance of helminth pathogens recently observed in the French West Indies might be responsible for the change in the clinical spectrum of multiple sclerosis with the rate of change amplified by the large numbers of return migrants. It is, therefore, of crucial importance in the future, to study the respective incidences of RNMO and typical multiple sclerosis in the French West Indies to determine if the first clinical form will disappear at the expense of the second some time after the clearance of helminth pathogens.
We thank Maryse Levy and Sylvie Merle for their assistance with the statistical analysis on migration in Guadeloupe and Martinique respectively. Participating physicians include hospital neurologists Georges Buisson (Fort de France Regional Hospital Centre), A. Lannuzel (Pointe à Pître Regional Hospital Centre), and Serge Sainte-Foie (Basse-Terre Hospital in Guadeloupe), private neurologists Rémi Bellance, Marie-Anne François, and Josiane Montezume-Barnay from Martinique, and Alex Mozar and Mohamed Beghdadi from Guadeloupe, hospitable and private ophthalmologists from French West Indies under the behalf of Harold Merle (Fort de France Regional Hospital Centre), and radiologists Alex Ridarch, Myriam Raynaud, and Sylvia Cirille (Fort de France Regional Hospital Centre). We thank Remy Hubert-Brierre and Christophe Riocreux for providing data deriving from health insurance files. This project was supported by grants from the Region of Martinique, Faculty of Medicine of French West Indies, and Schering SA. Contributors: P.C. and D.S. designed and coordinated the study. S.O., H.M., and D.C-L. participated in the data collection. A.S. was involved in the migration study. O.B. conducted HLA study. P.C. interpretated the results and wrote the paper, with revisions and contributions from David Warrel (Oxford, UK) and Christian Confavreux (Lyon, France).
Conflict of interest statement. None declared.
References
Alter M, Kahana E, Loewenson R. Migration and risk of multiple sclerosis.
Bera O, Cesaire R, Quelvennec E, Quillivic F, De Chavigny V, Ribal C, et al. HLA class I and class II allele and haplotype diversity in Martinicans.
Bodmer JG, Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Charron D, et al. Nomenclature for factors of the HLA system, 1996.
Breslow NE, Day NE. Statistical methods in cancer research. Volume II: the design and analysis of cohort studies.
Cabre P, Heinzlef O, Merle H, Buisson GG, Bera O, Bellance R, et al. MS and neuromyelitis optica in Martinique (French West Indies).
Compston A. ‘The marvellous harmony of the nervous parts’: the origin of multiple sclerosis.
Cosnet JE. Multiple sclerosis and neuromyelitis optica in tropical and subtropical countries.
Dean G, Elian M. Age at immigration to England of Asian and Carribean immigrants and the risk of developing multiple sclerosis.
Dean G, Kurtzke JF. On the risk of multiple sclerosis according to age at immigration to South Africa.
Elian M, Dean G. Multiple sclerosis among the United Kingdom-born children of immigrants from the West Indies.
Elian M, Nightingale S, Dean G. Multiple sclerosis among United Kingdom-born children of immigrants from the Indian subcontinent, Africa and the West Indies.
Gardien E, Schlegel L, Desbois N, Chout R. Intestinal parasitism prevalence in public laboratories of Martinique: evolution from 1988 to 1995.
Granieri E, Casetta I, Govoni V. The increasing incidence and prevalence of MS in a Sardinian province.
Hammond SR, English DR, McLeod JG. The age-rank of risk of developing multiple sclerosis. Evidence from a migrant population in Australia.
Kira J, Yamasaki K, Horiuchi I, Ohyagi Y, Taniwaki T, Kawano Y. Changes in the clinical phenotypes of multiple sclerosis during the past 50 years in Japan.
Kurtzke JF, Delasnerie-Laupretre N. Reflection on the distribution of multiple sclerosis in France.
Kurtzke JF, Hyllested K. Multiple sclerosis in the Faroe islands and the lack of protection by exposure in infancy.
Kurtzke JF, Page WF. Epidemiology of multiple sclerosis in US veterans VII. Risk factors for MS.
Martyn C. Epidemiology. In: Matthews WB, editors. McAlpine's multiple sclerosis. New York: Churchill Livingstone;
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis.
McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development.
Monplaisir N, Valette I, Lepage V, Dijon V, Lavocat E, Ribal C, et al. Study of HLA antigens of the Martinican population.
Najim Al-Din AS, Khogali M, Poser CM, Al-Nassar KE, Shakir E, Hussain J, et al. Epidemiology of multiple sclerosis in Arabs.
Poser CM, Vernant JC. Multiple sclerosis in black populations.
Poser CM, Paty DW, Scheinberg L, Mc Donald WI. New diagnostic criteria for multiple sclerosis: guidelines for research protocols.
Poskanzer DC, Sheridan JL, Prenney LB, Walker AM. Multiple sclerosis in the Orkney and Shetland islands. II. The search for an exogenous aetiology.
Quelvennec E, Bera O, Cabre P, Alizadeh M, Smadja D, Jugde F, et al. Genetic and functional studies in multiple sclerosis patients from Martinique attest for specific and direct role of the HLA-DR locus in the syndrome.
Rees JH, Thompson RD, Smeeton NC, Hughes RAC. Capture–recapture methods in surveys of diseases of the nervous system.
Rosati G, Aiello I, Mannu L. Incidence of multiple sclerosis in the town of Sassari, Sardinia, 1965 to 1985: evidence for increasing occurrence of the disease.
Samuels SJ, Beaumont JJ, Breslow NE. Power and detectable risk of seven tests for standardized mortality ratios.
Sewell D, Reinke E, Hogan L, Sandor M, Fabry Z. Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections.
Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization.
Smith MW, Patterson N, Lautenberger JA, Truelove AL, McDonald GJ, Waliszewska A, et al. A high-density map for disease gene discovery in African Americans.
Vernant JC, Maurs L, Gessain A, Barin F, Gout O, Delaporte JM, et al. Endemic tropical spastic paraparesis associated with human lymphotropic virus type I: a clinical and seroepidemiological study of 25 cases.
Vernant JC, Cabre P, Smadja D, Merle H, Caubarrère I, Mikol J, et al. Recurrent optic neuromyelitis with endocrinopathies: a new syndrome.
Villon A, Foulon G, Ancelle R, Nguyen NQ, Martin-Bouyer G. Intestinal parasitism prevalence in Martinique.
Wingerchük DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome).
Author notes
Departments of 1Neurology, 2Ophthalmology and 3Viro-Immunology, CHU Fort de France, Martinique and 4Department of Neurology, CHU Pointe à Pître, Guadeloupe, French West Indies, France