-
PDF
- Split View
-
Views
-
Cite
Cite
Pierre Thomas, Luc Valton, Pierre Genton, Absence and myoclonic status epilepticus precipitated by antiepileptic drugs in idiopathic generalized epilepsy, Brain, Volume 129, Issue 5, May 2006, Pages 1281–1292, https://doi.org/10.1093/brain/awl047
Close - Share Icon Share
Abstract
Aggravation of idiopathic generalized epilepsy (IGE) syndromes by inappropriate antiepileptic drugs (AEDs) is increasingly recognized as a serious and common problem. Precipitation of status epilepticus (SE) by inappropriate medication has rarely been reported. We retrospectively studied all adult patients with IGE taking at least one potentially aggravating AED, who developed video-EEG documented SE over 8 years, and whose long-term outcome was favourable after adjustment of medication. We identified 14 patients (seven male patients) aged 15–46 years with a mean duration of epilepsy of 16.4 years. Video-EEG demonstrated typical absence SE (ASE) in five, atypical ASE in five, atypical myoclonic SE (MSE) in three and typical MSE in one. Epilepsy had been misclassified as cryptogenic partial in eight cases and cryptogenic generalized in four. The correct diagnosis proved to be juvenile absence epilepsy (JAE) in six patients, juvenile myoclonic epilepsy (JME) in four, epilepsy with grand mal on awakening (EGMA) in two and childhood absence epilepsy (CAE) in two. All patients had been treated with carbamazepine (CBZ) and had experienced seizure aggravation or new seizure types before referral. Seven patients had polytherapy with phenytoin (PHT), vigabatrin (VGB) or gabapentin (GBP). Potential precipitating factors included dose increase of CBZ or of CBZ and PHT; initiation of CBZ, VGB or GBP; and decrease of phenobarbital. Withdrawal of the aggravating agents and adjustment of medication resulted in full seizure control. This series shows that severe pharmacodynamic aggravation of seizures in IGE may result in ASE or MSE, often with atypical features.
Introduction
Treatment with antiepileptic drugs (AEDs) may provoke a paradoxical aggravation of epilepsy, both in adults (Lerman, 1986; Bauer, 1996; Perucca et al., 1998) and in children (Guerrini et al., 1998; Usui et al., 2005). Various mechanisms may be responsible, but the most puzzling is a truly inverse pharmacodynamic effect, with increased seizure activity sometimes associated with the appearance of new seizure types, which occurs without high drug level, drug tolerance or encephalopathy (Berkovic, 1998). Idiopathic generalized epilepsies (IGE), a subgroup of epilepsies that are genetically determined and have no structural or anatomic cause, are often involved in this type of deterioration (Sazgar and Bourgeois, 2003). In IGE, the use of ill-advised AEDs, especially carbamazepine (CBZ) and phenytoin (PHT) (Genton et al., 2000; Osorio et al., 2000), either in monotherapy or in combination, is a common problem, encountered in up to 69% of patients in a recent series (Benbadis et al., 2003). Paradoxical aggravation in IGE usually results in subtle or overt increased seizures with or without new seizure types, associated with worsening of EEG abnormalities. Status epilepticus (SE) may occur but is believed to be unusual (Callahan and Noezel, 1992; So et al., 1994; Osorio et al., 2000). We report 14 such patients with video-EEG documentation of absence SE (ASE) or myoclonic SE (MSE), and atypical clinical and EEG features at referral.
Patients and methods
Among 3874 adults referred for epilepsy to the Nice University Hospital between 1996 and 2004, we retrospectively identified those with an adequately documented episode of SE and who were finally diagnosed with IGE. In this group of 22 patients, we found 14 with paradoxical worsening of epilepsy by AED leading to SE, diagnosed by the following criteria:
Documentation of SE with continuous video-EEG monitoring under clinical observation, showing either a prolonged seizure that lasted at least 15 min, or a minimum of three seizures without return to the interictal clinical or EEG baseline. Patients with very frequent serial seizures appearing as ‘a fixed and lasting epileptic condition’ (Gastaut, 1983) were also considered to have SE and were included.
Absence of EEG or laboratory evidence (haemogram, electrolytes, blood glucose, ammonia, proteins and liver function studies) of metabolic or AED-induced encephalopathy and AED levels at referral showing no evidence of AED withdrawal, non-compliance or overdosage.
A history of clear worsening of epilepsy over at least 3 months before admission with documentation of increased seizures and/or onset of new seizure types, following the introduction or increase of drugs reported to aggravate IGE.
A final diagnosis of IGE based on (a) onset of initial seizure type during the first two decades of life; (b) normal development and interictal neurological examination; (c) bilateral, symmetrical and synchronous 3–5 Hz spike-and-wave (SW) and/or polyspike-and-wave (PSW) discharges and normal interictal background activity; (d) normal MRI. These criteria were fulfilled either before seizure aggravation or after the SE, when patients where fully investigated, including video-taped seizures and EEGs recorded after change in AED regimen, either during wakefulness or sleep, or both. Patients with slight diffuse brain atrophy or non-specific white matter changes were not excluded if all other criteria were present.
Favourable outcome after discontinuation of the potentially aggravating AEDs and their replacement by appropriate treatment, with a minimum follow-up of 3 years.
Informed consent for video-EEG was obtained from the patients either before or after the episode of SE, and this protocol was approved by the local Ethics Committee.
Ictal EEGs were obtained with an Alvar™ 20-channel analog polygraph or a Deltamed™ Coherence II or Coherence III 36-channel digitized video-EEG workstation. Scalp electrodes were placed according to the International 10–20 system.
We reviewed all video-EEG recordings of SE. ASE was classified into two groups (Kaplan, 2002). Typical ASE was characterized by isolated impairment of consciousness, at times with subtle jerks of the eyelids and/or simple gestural automatisms, which correlated with continuous or repetitive symmetric and bilaterally synchronous SW or PSW at 2.5 Hz or faster. Atypical ASE was characterized by additional symptoms, such as complex automatisms or more prominent myoclonic and/or lateralized ictal manifestations, with a continuous or intermittent EEG pattern characterized by diffuse, irregular SW or PSW at less than 2.5 Hz. Myoclonic status epilepticus (MSE) was considered typical when restricted to bilateral, positive myoclonic jerks synchronous with generalized SW or PSW discharges. Any other symptoms led to classification as atypical MSE.
Clinical and EEG data were analysed from diaries and medical records in an effort to determine why potentially aggravating AEDs were prescribed. We contacted the patient and the referring neurologist by telephone when available clinical data were ambiguous. Interictal EEGs were retrospectively collected or obtained during follow-up evaluation after SE. Epileptic syndromes were classified according to the International Classification of Epilepsies and Related Syndromes (Commission on Classification and Terminology of the International League Against Epilepsy, 1989).
Results
The clinical characteristics of the 14 patients (7 male patients) who fulfilled the inclusion criteria are summarized in Table 1, and the characteristics of seizure aggravation and episodes of SE are summarized in Table 2. Seven patients had a family history of epilepsy, and six a personal history of febrile seizures. Seizures began at a mean age of 13.2 years (range 5–20), with onset between age 10 and 20 in 11. At recording of SE, the mean age was 29.4 years (range 15–46), with a mean duration of epilepsy of 16.4 years (range 2–41).
Clinical characteristics of patients at SE
| Case (sex, age) . | Personal and family history . | Type of first seizure age (years) . | Syndromic classification at SE . | AED treatment at SE (mg/day) Blood levels [mg/l] . | Duration of CBZ therapy (years) . | Treatment after SE (mg/day) . | Evolution Follow up (years) . | Final epileptic syndrome . |
|---|---|---|---|---|---|---|---|---|
| 1 (M, 26) | Febrile Sz | GTCS (14) | CPE (R frontal) | CBZ (1600) [9] VGB (3000) [NA] VPA (2000) [44] | 3 | LTG (200) VPA (1000) | No seizure (4.3) | EGMA |
| 2 (F, 15) | Epilepsy in sister | Abs (11) | CPE (L frontal) | CBZ (800) [8] PB 100 [18] | 0.5 | LTG (150) VPA (750) | Rare Abs (non-compliance) (5.8) | JAE |
| 3 (F, 39) | None | Abs (13) | CPE (R frontal) | CBZ (800) [7.6] PB (50) [9] PHT (350) [8.5] | 4 | LTG (200) VPA (1000) | No seizure (5.5) | JAE |
| 4 (M, 28) | Febrile Sz | MJ (16) | SGE | CBZ (1200) [7.3] PB (150) [14] VGB (2000) [NA] | 4.5 | PB (100) VPA (1000) | No seizure (5) | JME |
| 5 (F, 45) | Epilepsy in brother | Abs (17) | CPE (R temporal) | CBZ (500) [5.2] | 5 | VPA (1000) | No seizure (4) | JAE |
| 6 (F, 25) | Febrile Sz Epilepsy in brother | Abs (12) | CPE (temporal) | CBZ (1400) [8.7] PB (150) [21.7] | 4.5 | VPA (1250) | No seizure (4.75) | JAE |
| 7 (M, 21) | Epilepsy in mother Behavioural problems | CGTC (16) | CGE | CBZ (1200) [10] GBP (800) [NA] | 3 | LTG (200) VPA (1000) | No seizure (5.2) | EGMA |
| 8 (M, 46) | Febrile Sz | Abs (5) | CPE (temporal) | CBZ (1200) [7.5] PB (150) [22] | 7 | LTG (200) VPA (750) | No seizure (7) | CAE |
| 9 (F, 26) | Epilepsy in maternal grandmother | MJ (7) | SGE | CBZ (1600) [11] PB (100) [18] PHT (200) [5] | 5.5 | CLB (30) VPA (1500) PB (75) | No seizure (3.5) | JME |
| 10 (M, 18) | Febrile Sz | MJ (16) | Undetermined GE | CBZ (800) [6.3] | 0.5 | VPA (1000) | No seizure (3.1) | JME |
| 11 (M, 17) | Behavioural problems | Abs (5) | CGE | CBZ (800) [6.5] PHT (300) [8] VPA (1000) [55] | 0.25 | VPA (1250) | No seizure (7) | CAE |
| 12 (F, 38) | Febrile Sz | Abs (17) | CPE (L frontal) | CBZ (800) [7.2] | 0.5 | VPA (500) TPR (100) | No seizure (3) | JAE |
| 13 (F, 25) | Abs in cousin | MJ (16) | Undetermined GE | CBZ (600) [7.4] | 0.5 | VPA (1000) | No seizure (6) | JME |
| 14 (M, 43) | Neonatal Sz in paternal uncle | GTCS (20) | CPE (frontal) | CBZ (1000) [6.7] CLB (30) [NA] VGB (2000) [NA] | 1 | ESM (750) VPA (1000) | No seizure (7.5) | JAE |
| Case (sex, age) . | Personal and family history . | Type of first seizure age (years) . | Syndromic classification at SE . | AED treatment at SE (mg/day) Blood levels [mg/l] . | Duration of CBZ therapy (years) . | Treatment after SE (mg/day) . | Evolution Follow up (years) . | Final epileptic syndrome . |
|---|---|---|---|---|---|---|---|---|
| 1 (M, 26) | Febrile Sz | GTCS (14) | CPE (R frontal) | CBZ (1600) [9] VGB (3000) [NA] VPA (2000) [44] | 3 | LTG (200) VPA (1000) | No seizure (4.3) | EGMA |
| 2 (F, 15) | Epilepsy in sister | Abs (11) | CPE (L frontal) | CBZ (800) [8] PB 100 [18] | 0.5 | LTG (150) VPA (750) | Rare Abs (non-compliance) (5.8) | JAE |
| 3 (F, 39) | None | Abs (13) | CPE (R frontal) | CBZ (800) [7.6] PB (50) [9] PHT (350) [8.5] | 4 | LTG (200) VPA (1000) | No seizure (5.5) | JAE |
| 4 (M, 28) | Febrile Sz | MJ (16) | SGE | CBZ (1200) [7.3] PB (150) [14] VGB (2000) [NA] | 4.5 | PB (100) VPA (1000) | No seizure (5) | JME |
| 5 (F, 45) | Epilepsy in brother | Abs (17) | CPE (R temporal) | CBZ (500) [5.2] | 5 | VPA (1000) | No seizure (4) | JAE |
| 6 (F, 25) | Febrile Sz Epilepsy in brother | Abs (12) | CPE (temporal) | CBZ (1400) [8.7] PB (150) [21.7] | 4.5 | VPA (1250) | No seizure (4.75) | JAE |
| 7 (M, 21) | Epilepsy in mother Behavioural problems | CGTC (16) | CGE | CBZ (1200) [10] GBP (800) [NA] | 3 | LTG (200) VPA (1000) | No seizure (5.2) | EGMA |
| 8 (M, 46) | Febrile Sz | Abs (5) | CPE (temporal) | CBZ (1200) [7.5] PB (150) [22] | 7 | LTG (200) VPA (750) | No seizure (7) | CAE |
| 9 (F, 26) | Epilepsy in maternal grandmother | MJ (7) | SGE | CBZ (1600) [11] PB (100) [18] PHT (200) [5] | 5.5 | CLB (30) VPA (1500) PB (75) | No seizure (3.5) | JME |
| 10 (M, 18) | Febrile Sz | MJ (16) | Undetermined GE | CBZ (800) [6.3] | 0.5 | VPA (1000) | No seizure (3.1) | JME |
| 11 (M, 17) | Behavioural problems | Abs (5) | CGE | CBZ (800) [6.5] PHT (300) [8] VPA (1000) [55] | 0.25 | VPA (1250) | No seizure (7) | CAE |
| 12 (F, 38) | Febrile Sz | Abs (17) | CPE (L frontal) | CBZ (800) [7.2] | 0.5 | VPA (500) TPR (100) | No seizure (3) | JAE |
| 13 (F, 25) | Abs in cousin | MJ (16) | Undetermined GE | CBZ (600) [7.4] | 0.5 | VPA (1000) | No seizure (6) | JME |
| 14 (M, 43) | Neonatal Sz in paternal uncle | GTCS (20) | CPE (frontal) | CBZ (1000) [6.7] CLB (30) [NA] VGB (2000) [NA] | 1 | ESM (750) VPA (1000) | No seizure (7.5) | JAE |
M: male; F: female; R: right: L: left; SE: status epilepticus; Abs: absences; GTCS: generalized tonic-clonic seizure; MJ: myoclonic jerks; CGE: cryptogenic generalized epilepsy; CPE: cryptogenic partial epilepsy; SGE: symptomatic partial epilepsy; NA: not available; CBZ: carbamazepine; CLB: clobazam; CNZ: clonazepam; ESM: ethosuximide; GBP: gabapentin; LTG: lamotrigine; PB: phenobarbital; PHT: phenytoin; TPR: topiramate; VPA: valproate; VGB: vigabatrin; JME: juvenile myoclonic epilepsy; JAE: juvenile absence epilepsy; CAE: childhood absence epilepsy; EGMA: epilepsy with grand mal on awakening.
Clinical characteristics of patients at SE
| Case (sex, age) . | Personal and family history . | Type of first seizure age (years) . | Syndromic classification at SE . | AED treatment at SE (mg/day) Blood levels [mg/l] . | Duration of CBZ therapy (years) . | Treatment after SE (mg/day) . | Evolution Follow up (years) . | Final epileptic syndrome . |
|---|---|---|---|---|---|---|---|---|
| 1 (M, 26) | Febrile Sz | GTCS (14) | CPE (R frontal) | CBZ (1600) [9] VGB (3000) [NA] VPA (2000) [44] | 3 | LTG (200) VPA (1000) | No seizure (4.3) | EGMA |
| 2 (F, 15) | Epilepsy in sister | Abs (11) | CPE (L frontal) | CBZ (800) [8] PB 100 [18] | 0.5 | LTG (150) VPA (750) | Rare Abs (non-compliance) (5.8) | JAE |
| 3 (F, 39) | None | Abs (13) | CPE (R frontal) | CBZ (800) [7.6] PB (50) [9] PHT (350) [8.5] | 4 | LTG (200) VPA (1000) | No seizure (5.5) | JAE |
| 4 (M, 28) | Febrile Sz | MJ (16) | SGE | CBZ (1200) [7.3] PB (150) [14] VGB (2000) [NA] | 4.5 | PB (100) VPA (1000) | No seizure (5) | JME |
| 5 (F, 45) | Epilepsy in brother | Abs (17) | CPE (R temporal) | CBZ (500) [5.2] | 5 | VPA (1000) | No seizure (4) | JAE |
| 6 (F, 25) | Febrile Sz Epilepsy in brother | Abs (12) | CPE (temporal) | CBZ (1400) [8.7] PB (150) [21.7] | 4.5 | VPA (1250) | No seizure (4.75) | JAE |
| 7 (M, 21) | Epilepsy in mother Behavioural problems | CGTC (16) | CGE | CBZ (1200) [10] GBP (800) [NA] | 3 | LTG (200) VPA (1000) | No seizure (5.2) | EGMA |
| 8 (M, 46) | Febrile Sz | Abs (5) | CPE (temporal) | CBZ (1200) [7.5] PB (150) [22] | 7 | LTG (200) VPA (750) | No seizure (7) | CAE |
| 9 (F, 26) | Epilepsy in maternal grandmother | MJ (7) | SGE | CBZ (1600) [11] PB (100) [18] PHT (200) [5] | 5.5 | CLB (30) VPA (1500) PB (75) | No seizure (3.5) | JME |
| 10 (M, 18) | Febrile Sz | MJ (16) | Undetermined GE | CBZ (800) [6.3] | 0.5 | VPA (1000) | No seizure (3.1) | JME |
| 11 (M, 17) | Behavioural problems | Abs (5) | CGE | CBZ (800) [6.5] PHT (300) [8] VPA (1000) [55] | 0.25 | VPA (1250) | No seizure (7) | CAE |
| 12 (F, 38) | Febrile Sz | Abs (17) | CPE (L frontal) | CBZ (800) [7.2] | 0.5 | VPA (500) TPR (100) | No seizure (3) | JAE |
| 13 (F, 25) | Abs in cousin | MJ (16) | Undetermined GE | CBZ (600) [7.4] | 0.5 | VPA (1000) | No seizure (6) | JME |
| 14 (M, 43) | Neonatal Sz in paternal uncle | GTCS (20) | CPE (frontal) | CBZ (1000) [6.7] CLB (30) [NA] VGB (2000) [NA] | 1 | ESM (750) VPA (1000) | No seizure (7.5) | JAE |
| Case (sex, age) . | Personal and family history . | Type of first seizure age (years) . | Syndromic classification at SE . | AED treatment at SE (mg/day) Blood levels [mg/l] . | Duration of CBZ therapy (years) . | Treatment after SE (mg/day) . | Evolution Follow up (years) . | Final epileptic syndrome . |
|---|---|---|---|---|---|---|---|---|
| 1 (M, 26) | Febrile Sz | GTCS (14) | CPE (R frontal) | CBZ (1600) [9] VGB (3000) [NA] VPA (2000) [44] | 3 | LTG (200) VPA (1000) | No seizure (4.3) | EGMA |
| 2 (F, 15) | Epilepsy in sister | Abs (11) | CPE (L frontal) | CBZ (800) [8] PB 100 [18] | 0.5 | LTG (150) VPA (750) | Rare Abs (non-compliance) (5.8) | JAE |
| 3 (F, 39) | None | Abs (13) | CPE (R frontal) | CBZ (800) [7.6] PB (50) [9] PHT (350) [8.5] | 4 | LTG (200) VPA (1000) | No seizure (5.5) | JAE |
| 4 (M, 28) | Febrile Sz | MJ (16) | SGE | CBZ (1200) [7.3] PB (150) [14] VGB (2000) [NA] | 4.5 | PB (100) VPA (1000) | No seizure (5) | JME |
| 5 (F, 45) | Epilepsy in brother | Abs (17) | CPE (R temporal) | CBZ (500) [5.2] | 5 | VPA (1000) | No seizure (4) | JAE |
| 6 (F, 25) | Febrile Sz Epilepsy in brother | Abs (12) | CPE (temporal) | CBZ (1400) [8.7] PB (150) [21.7] | 4.5 | VPA (1250) | No seizure (4.75) | JAE |
| 7 (M, 21) | Epilepsy in mother Behavioural problems | CGTC (16) | CGE | CBZ (1200) [10] GBP (800) [NA] | 3 | LTG (200) VPA (1000) | No seizure (5.2) | EGMA |
| 8 (M, 46) | Febrile Sz | Abs (5) | CPE (temporal) | CBZ (1200) [7.5] PB (150) [22] | 7 | LTG (200) VPA (750) | No seizure (7) | CAE |
| 9 (F, 26) | Epilepsy in maternal grandmother | MJ (7) | SGE | CBZ (1600) [11] PB (100) [18] PHT (200) [5] | 5.5 | CLB (30) VPA (1500) PB (75) | No seizure (3.5) | JME |
| 10 (M, 18) | Febrile Sz | MJ (16) | Undetermined GE | CBZ (800) [6.3] | 0.5 | VPA (1000) | No seizure (3.1) | JME |
| 11 (M, 17) | Behavioural problems | Abs (5) | CGE | CBZ (800) [6.5] PHT (300) [8] VPA (1000) [55] | 0.25 | VPA (1250) | No seizure (7) | CAE |
| 12 (F, 38) | Febrile Sz | Abs (17) | CPE (L frontal) | CBZ (800) [7.2] | 0.5 | VPA (500) TPR (100) | No seizure (3) | JAE |
| 13 (F, 25) | Abs in cousin | MJ (16) | Undetermined GE | CBZ (600) [7.4] | 0.5 | VPA (1000) | No seizure (6) | JME |
| 14 (M, 43) | Neonatal Sz in paternal uncle | GTCS (20) | CPE (frontal) | CBZ (1000) [6.7] CLB (30) [NA] VGB (2000) [NA] | 1 | ESM (750) VPA (1000) | No seizure (7.5) | JAE |
M: male; F: female; R: right: L: left; SE: status epilepticus; Abs: absences; GTCS: generalized tonic-clonic seizure; MJ: myoclonic jerks; CGE: cryptogenic generalized epilepsy; CPE: cryptogenic partial epilepsy; SGE: symptomatic partial epilepsy; NA: not available; CBZ: carbamazepine; CLB: clobazam; CNZ: clonazepam; ESM: ethosuximide; GBP: gabapentin; LTG: lamotrigine; PB: phenobarbital; PHT: phenytoin; TPR: topiramate; VPA: valproate; VGB: vigabatrin; JME: juvenile myoclonic epilepsy; JAE: juvenile absence epilepsy; CAE: childhood absence epilepsy; EGMA: epilepsy with grand mal on awakening.
Characteristics of seizure aggravation and episodes of status epilepticus
| Case (sex, age) . | Seizure characteristics during aggravation (frequency) . | Possible cause of SE . | Clinical presentation of SE (duration) . | Ictal EEG pattern . |
|---|---|---|---|---|
| 1 (M, 26) | Loss of contact with immediate head and eye deviation to the L, then GTCS (1 per month) Loss of contact with delayed slight head and eye deviation to the L, followed by simple gestural automatisms, duration 10–20 s (10 per month) | Decr. VPA Incr. CBZ | Atypical ASE with moderate confusion, episodes of eye rolling, version of head and eyes to the L Resolved after IV BZ and IV PHT (90 min) | Subcontinuous diffuse 2–2.5 Hz SW and PSW discharges |
| 2 (F, 15) | GTCS (1 per month) Loss of contact, either isolated or followed by slight head and eye deviation to the R, duration 10–20 s (20–30 per month) | Incr. CBZ | Typical ASE with mild impairment of consciousness and eyelid myoclonias, in relation to serial absences in quick succession Resolved after IV BZ (65 min) | Rhythmic, generalized 3 Hz PSW discharges of 5–10 s duration repeated at 5–10 s intervals |
| 3 (F, 39) | Head and eye deviation to the L, then GTCS (1 per month) Episodes of impaired consciousness of varying intensity at awakening, lasting 0.5–4 h, (5–7 per month) | Incr. CBZ Incr. PHT | Atypical ASE with severe confusion, disinhibition and stereotyped rhythmic movements of the right arm Resolved spontaneously (180 min) | Generalized 1 Hz PSW and SW discharges with a R predominance, alternating with rhythmic bursts of slow waves |
| 4 (M, 28) | Clonic-tonic-clonic Sz (3 per month) Awakening episodes of MJ of varying amplitude, more marked on the R, increased by eye closure, lasting 30–45 min (6 per month) | Onset of VGB | Severe, atypical MSE. Eye closure induced rhythmic bursts of eyelid myoclonias with eyelid-opening apraxia. Resolved after IV BZ (45 min) | Pseudo-rhythmic, continuous diffuse 4–6 Hz SW Major increase of ictal activity when eyes were closed |
| 5 (F, 45) | GTCS (1 per year) Serial, daily episodes of mild intellectual impairment with diffuse anxiety lasting 5–15 s (30 per month) | Sleep deprivation | Atypical ASE with subjective cognitive impairment, and unpleasant feeling during discharges. Resolved after IV BZ (210 min) | Serial bursts of rapid, generalized Sp, followed by slow PSW lasting 7–12 s, repeated at 20–40 s intervals |
| 6 (F, 25) | GTCS (2 per year) Isolated loss of contact (10 per month) Long-lasting episode of subjective cognitive impairment with MJ ending in a GTCS after 10 h (three episodes) | Incr. CBZ | Typical ASE with subjective cognitive impairment, feeling of drunkenness, rare MJ, and unsteady gait. Resolved after a single GTCS (140 min) | Continuous, diffuse 4 Hz irregular SW |
| 7 (M, 21) | GTCS (6 per year) Long-lasting episodes of confusion with deambulation and amnesia, lasting 10–24 h (3 per year) | Incr. CBZ. Onset of GBP | Atypical ASE with severe confusion, eyelid myoclonias, perseverations, urination. Resolved after IV BZ (120 min) | Continuous 1–2.5 Hz PSW with a L predominance |
| 8 (M, 46) | GTCS (1 per year) Episodes of subjective cognitive impairment with eyelid myoclonias and anxiety, lasting 12–18 h (3 per year) | Incr. CBZ. Decr. PB | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ (60 min) | Bursts of generalized 3 Hz SW lasting 3–15 s, repeated at 10–15 s intervals |
| 9 (F, 26) | Bilateral MJ at awakening, lasting 30–45 min (5–10 per month) GTCS ending series of MJ (5–7 per month) | Sleep deprivation | Severe, typical MSE at awakening, interspersed with 3 GTCS. Resolved after IV BZ (40 min) | Bursts of generalized 0.5–1.5 Hz PSW lasting 10–15 s, repeated at 10 s intervals. |
| 10 9M, 18) | GTCS (two episodes) At awakening, rhythmic 3 Hz bilateral MJ in separate 10–15 s clusters, during 20–30 min (5–10 per month) | Onset of CBZ | Atypical MSE with short (10-20 s) bursts of bilateral, rhythmic 3 Hz MJ repeated at 20–30 s intervals. Resolved after IV BZ (35 min) | Bursts of generalized rhythmic PSW at 3 Hz synchronous with MJ. |
| 11 (M, 17) | GTCS (4 per month). Isolated, brief (5–10 s) loss of contact (10–20 per month). Long-lasting episodes of clumsiness with unsteady gait (2 per month) | Onset of CBZ | Atypical MSE with bilateral, asynchronous, negative myoclonus and marked postural instability. Resolved after IV BZ (120 min) | Continuous diffuse PSW at 1–2 Hz, time-locked with atonias |
| 12 (F, 38) | GTCS (1 per month). Isolated, brief (5–10 s) loss of contact (20–30 per month) | Incr. CBZ | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ and IV VPA (80 min) | Bursts of rhythmic generalized 2.5–3 Hz SW and PSW with a L predominance, repeated at 5 s intervals |
| 13 (F, 25) | GTCS preceded by MJ (8 per year). Long-lasting episodes of obtundation at awakening with bilateral MJ (4 per month) | Unknown | Typical ASE with mild confusion, interspersed with asynchronous, bilateral MJ of the limbs. Resolved spontaneously (60 min) | Bursts of rhythmic generalized PSW at 3 Hz, sometimes synchronous with MJ, repeated at 10–20 s intervals |
| 14 (M, 43) | GTCS (1 per month) Isolated loss of contact lasting 5–10 s (60 per month). Long-lasting episodes of cognitive disturbances with amnesia, ambulatory automatisms and fugue, sometimes with intercalated GTCS (2 per month) | Unknown | Atypical ASE with mild confusion, interspersed with a single GTCS. Resolved spontaneously (120 min) | Subcontinuous pseudo-rhythmic diffuse 4–6 Hz slow waves and Sp with a L predominance |
| Case (sex, age) . | Seizure characteristics during aggravation (frequency) . | Possible cause of SE . | Clinical presentation of SE (duration) . | Ictal EEG pattern . |
|---|---|---|---|---|
| 1 (M, 26) | Loss of contact with immediate head and eye deviation to the L, then GTCS (1 per month) Loss of contact with delayed slight head and eye deviation to the L, followed by simple gestural automatisms, duration 10–20 s (10 per month) | Decr. VPA Incr. CBZ | Atypical ASE with moderate confusion, episodes of eye rolling, version of head and eyes to the L Resolved after IV BZ and IV PHT (90 min) | Subcontinuous diffuse 2–2.5 Hz SW and PSW discharges |
| 2 (F, 15) | GTCS (1 per month) Loss of contact, either isolated or followed by slight head and eye deviation to the R, duration 10–20 s (20–30 per month) | Incr. CBZ | Typical ASE with mild impairment of consciousness and eyelid myoclonias, in relation to serial absences in quick succession Resolved after IV BZ (65 min) | Rhythmic, generalized 3 Hz PSW discharges of 5–10 s duration repeated at 5–10 s intervals |
| 3 (F, 39) | Head and eye deviation to the L, then GTCS (1 per month) Episodes of impaired consciousness of varying intensity at awakening, lasting 0.5–4 h, (5–7 per month) | Incr. CBZ Incr. PHT | Atypical ASE with severe confusion, disinhibition and stereotyped rhythmic movements of the right arm Resolved spontaneously (180 min) | Generalized 1 Hz PSW and SW discharges with a R predominance, alternating with rhythmic bursts of slow waves |
| 4 (M, 28) | Clonic-tonic-clonic Sz (3 per month) Awakening episodes of MJ of varying amplitude, more marked on the R, increased by eye closure, lasting 30–45 min (6 per month) | Onset of VGB | Severe, atypical MSE. Eye closure induced rhythmic bursts of eyelid myoclonias with eyelid-opening apraxia. Resolved after IV BZ (45 min) | Pseudo-rhythmic, continuous diffuse 4–6 Hz SW Major increase of ictal activity when eyes were closed |
| 5 (F, 45) | GTCS (1 per year) Serial, daily episodes of mild intellectual impairment with diffuse anxiety lasting 5–15 s (30 per month) | Sleep deprivation | Atypical ASE with subjective cognitive impairment, and unpleasant feeling during discharges. Resolved after IV BZ (210 min) | Serial bursts of rapid, generalized Sp, followed by slow PSW lasting 7–12 s, repeated at 20–40 s intervals |
| 6 (F, 25) | GTCS (2 per year) Isolated loss of contact (10 per month) Long-lasting episode of subjective cognitive impairment with MJ ending in a GTCS after 10 h (three episodes) | Incr. CBZ | Typical ASE with subjective cognitive impairment, feeling of drunkenness, rare MJ, and unsteady gait. Resolved after a single GTCS (140 min) | Continuous, diffuse 4 Hz irregular SW |
| 7 (M, 21) | GTCS (6 per year) Long-lasting episodes of confusion with deambulation and amnesia, lasting 10–24 h (3 per year) | Incr. CBZ. Onset of GBP | Atypical ASE with severe confusion, eyelid myoclonias, perseverations, urination. Resolved after IV BZ (120 min) | Continuous 1–2.5 Hz PSW with a L predominance |
| 8 (M, 46) | GTCS (1 per year) Episodes of subjective cognitive impairment with eyelid myoclonias and anxiety, lasting 12–18 h (3 per year) | Incr. CBZ. Decr. PB | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ (60 min) | Bursts of generalized 3 Hz SW lasting 3–15 s, repeated at 10–15 s intervals |
| 9 (F, 26) | Bilateral MJ at awakening, lasting 30–45 min (5–10 per month) GTCS ending series of MJ (5–7 per month) | Sleep deprivation | Severe, typical MSE at awakening, interspersed with 3 GTCS. Resolved after IV BZ (40 min) | Bursts of generalized 0.5–1.5 Hz PSW lasting 10–15 s, repeated at 10 s intervals. |
| 10 9M, 18) | GTCS (two episodes) At awakening, rhythmic 3 Hz bilateral MJ in separate 10–15 s clusters, during 20–30 min (5–10 per month) | Onset of CBZ | Atypical MSE with short (10-20 s) bursts of bilateral, rhythmic 3 Hz MJ repeated at 20–30 s intervals. Resolved after IV BZ (35 min) | Bursts of generalized rhythmic PSW at 3 Hz synchronous with MJ. |
| 11 (M, 17) | GTCS (4 per month). Isolated, brief (5–10 s) loss of contact (10–20 per month). Long-lasting episodes of clumsiness with unsteady gait (2 per month) | Onset of CBZ | Atypical MSE with bilateral, asynchronous, negative myoclonus and marked postural instability. Resolved after IV BZ (120 min) | Continuous diffuse PSW at 1–2 Hz, time-locked with atonias |
| 12 (F, 38) | GTCS (1 per month). Isolated, brief (5–10 s) loss of contact (20–30 per month) | Incr. CBZ | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ and IV VPA (80 min) | Bursts of rhythmic generalized 2.5–3 Hz SW and PSW with a L predominance, repeated at 5 s intervals |
| 13 (F, 25) | GTCS preceded by MJ (8 per year). Long-lasting episodes of obtundation at awakening with bilateral MJ (4 per month) | Unknown | Typical ASE with mild confusion, interspersed with asynchronous, bilateral MJ of the limbs. Resolved spontaneously (60 min) | Bursts of rhythmic generalized PSW at 3 Hz, sometimes synchronous with MJ, repeated at 10–20 s intervals |
| 14 (M, 43) | GTCS (1 per month) Isolated loss of contact lasting 5–10 s (60 per month). Long-lasting episodes of cognitive disturbances with amnesia, ambulatory automatisms and fugue, sometimes with intercalated GTCS (2 per month) | Unknown | Atypical ASE with mild confusion, interspersed with a single GTCS. Resolved spontaneously (120 min) | Subcontinuous pseudo-rhythmic diffuse 4–6 Hz slow waves and Sp with a L predominance |
M: male; F: female; R: right: L: left; min: minutes; s: seconds; ASE: absence status epilepticus; MSE: myoclonic status epilepticus; GTCS: generalized tonic-clonic seizure; MJ: myoclonic jerks; Sp: spikes; SW: spike-and-waves: PSW: polyspike-and-waves; incr.: increase in dosage of; decr.: decrease in dosage of; BZ: benzodiazepine; CBZ: carbamazepine; GBP: gabapentin; PB: phenobarbital; PHT: phenytoin; VPA: valproate; VGB: vigabatrin.
Characteristics of seizure aggravation and episodes of status epilepticus
| Case (sex, age) . | Seizure characteristics during aggravation (frequency) . | Possible cause of SE . | Clinical presentation of SE (duration) . | Ictal EEG pattern . |
|---|---|---|---|---|
| 1 (M, 26) | Loss of contact with immediate head and eye deviation to the L, then GTCS (1 per month) Loss of contact with delayed slight head and eye deviation to the L, followed by simple gestural automatisms, duration 10–20 s (10 per month) | Decr. VPA Incr. CBZ | Atypical ASE with moderate confusion, episodes of eye rolling, version of head and eyes to the L Resolved after IV BZ and IV PHT (90 min) | Subcontinuous diffuse 2–2.5 Hz SW and PSW discharges |
| 2 (F, 15) | GTCS (1 per month) Loss of contact, either isolated or followed by slight head and eye deviation to the R, duration 10–20 s (20–30 per month) | Incr. CBZ | Typical ASE with mild impairment of consciousness and eyelid myoclonias, in relation to serial absences in quick succession Resolved after IV BZ (65 min) | Rhythmic, generalized 3 Hz PSW discharges of 5–10 s duration repeated at 5–10 s intervals |
| 3 (F, 39) | Head and eye deviation to the L, then GTCS (1 per month) Episodes of impaired consciousness of varying intensity at awakening, lasting 0.5–4 h, (5–7 per month) | Incr. CBZ Incr. PHT | Atypical ASE with severe confusion, disinhibition and stereotyped rhythmic movements of the right arm Resolved spontaneously (180 min) | Generalized 1 Hz PSW and SW discharges with a R predominance, alternating with rhythmic bursts of slow waves |
| 4 (M, 28) | Clonic-tonic-clonic Sz (3 per month) Awakening episodes of MJ of varying amplitude, more marked on the R, increased by eye closure, lasting 30–45 min (6 per month) | Onset of VGB | Severe, atypical MSE. Eye closure induced rhythmic bursts of eyelid myoclonias with eyelid-opening apraxia. Resolved after IV BZ (45 min) | Pseudo-rhythmic, continuous diffuse 4–6 Hz SW Major increase of ictal activity when eyes were closed |
| 5 (F, 45) | GTCS (1 per year) Serial, daily episodes of mild intellectual impairment with diffuse anxiety lasting 5–15 s (30 per month) | Sleep deprivation | Atypical ASE with subjective cognitive impairment, and unpleasant feeling during discharges. Resolved after IV BZ (210 min) | Serial bursts of rapid, generalized Sp, followed by slow PSW lasting 7–12 s, repeated at 20–40 s intervals |
| 6 (F, 25) | GTCS (2 per year) Isolated loss of contact (10 per month) Long-lasting episode of subjective cognitive impairment with MJ ending in a GTCS after 10 h (three episodes) | Incr. CBZ | Typical ASE with subjective cognitive impairment, feeling of drunkenness, rare MJ, and unsteady gait. Resolved after a single GTCS (140 min) | Continuous, diffuse 4 Hz irregular SW |
| 7 (M, 21) | GTCS (6 per year) Long-lasting episodes of confusion with deambulation and amnesia, lasting 10–24 h (3 per year) | Incr. CBZ. Onset of GBP | Atypical ASE with severe confusion, eyelid myoclonias, perseverations, urination. Resolved after IV BZ (120 min) | Continuous 1–2.5 Hz PSW with a L predominance |
| 8 (M, 46) | GTCS (1 per year) Episodes of subjective cognitive impairment with eyelid myoclonias and anxiety, lasting 12–18 h (3 per year) | Incr. CBZ. Decr. PB | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ (60 min) | Bursts of generalized 3 Hz SW lasting 3–15 s, repeated at 10–15 s intervals |
| 9 (F, 26) | Bilateral MJ at awakening, lasting 30–45 min (5–10 per month) GTCS ending series of MJ (5–7 per month) | Sleep deprivation | Severe, typical MSE at awakening, interspersed with 3 GTCS. Resolved after IV BZ (40 min) | Bursts of generalized 0.5–1.5 Hz PSW lasting 10–15 s, repeated at 10 s intervals. |
| 10 9M, 18) | GTCS (two episodes) At awakening, rhythmic 3 Hz bilateral MJ in separate 10–15 s clusters, during 20–30 min (5–10 per month) | Onset of CBZ | Atypical MSE with short (10-20 s) bursts of bilateral, rhythmic 3 Hz MJ repeated at 20–30 s intervals. Resolved after IV BZ (35 min) | Bursts of generalized rhythmic PSW at 3 Hz synchronous with MJ. |
| 11 (M, 17) | GTCS (4 per month). Isolated, brief (5–10 s) loss of contact (10–20 per month). Long-lasting episodes of clumsiness with unsteady gait (2 per month) | Onset of CBZ | Atypical MSE with bilateral, asynchronous, negative myoclonus and marked postural instability. Resolved after IV BZ (120 min) | Continuous diffuse PSW at 1–2 Hz, time-locked with atonias |
| 12 (F, 38) | GTCS (1 per month). Isolated, brief (5–10 s) loss of contact (20–30 per month) | Incr. CBZ | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ and IV VPA (80 min) | Bursts of rhythmic generalized 2.5–3 Hz SW and PSW with a L predominance, repeated at 5 s intervals |
| 13 (F, 25) | GTCS preceded by MJ (8 per year). Long-lasting episodes of obtundation at awakening with bilateral MJ (4 per month) | Unknown | Typical ASE with mild confusion, interspersed with asynchronous, bilateral MJ of the limbs. Resolved spontaneously (60 min) | Bursts of rhythmic generalized PSW at 3 Hz, sometimes synchronous with MJ, repeated at 10–20 s intervals |
| 14 (M, 43) | GTCS (1 per month) Isolated loss of contact lasting 5–10 s (60 per month). Long-lasting episodes of cognitive disturbances with amnesia, ambulatory automatisms and fugue, sometimes with intercalated GTCS (2 per month) | Unknown | Atypical ASE with mild confusion, interspersed with a single GTCS. Resolved spontaneously (120 min) | Subcontinuous pseudo-rhythmic diffuse 4–6 Hz slow waves and Sp with a L predominance |
| Case (sex, age) . | Seizure characteristics during aggravation (frequency) . | Possible cause of SE . | Clinical presentation of SE (duration) . | Ictal EEG pattern . |
|---|---|---|---|---|
| 1 (M, 26) | Loss of contact with immediate head and eye deviation to the L, then GTCS (1 per month) Loss of contact with delayed slight head and eye deviation to the L, followed by simple gestural automatisms, duration 10–20 s (10 per month) | Decr. VPA Incr. CBZ | Atypical ASE with moderate confusion, episodes of eye rolling, version of head and eyes to the L Resolved after IV BZ and IV PHT (90 min) | Subcontinuous diffuse 2–2.5 Hz SW and PSW discharges |
| 2 (F, 15) | GTCS (1 per month) Loss of contact, either isolated or followed by slight head and eye deviation to the R, duration 10–20 s (20–30 per month) | Incr. CBZ | Typical ASE with mild impairment of consciousness and eyelid myoclonias, in relation to serial absences in quick succession Resolved after IV BZ (65 min) | Rhythmic, generalized 3 Hz PSW discharges of 5–10 s duration repeated at 5–10 s intervals |
| 3 (F, 39) | Head and eye deviation to the L, then GTCS (1 per month) Episodes of impaired consciousness of varying intensity at awakening, lasting 0.5–4 h, (5–7 per month) | Incr. CBZ Incr. PHT | Atypical ASE with severe confusion, disinhibition and stereotyped rhythmic movements of the right arm Resolved spontaneously (180 min) | Generalized 1 Hz PSW and SW discharges with a R predominance, alternating with rhythmic bursts of slow waves |
| 4 (M, 28) | Clonic-tonic-clonic Sz (3 per month) Awakening episodes of MJ of varying amplitude, more marked on the R, increased by eye closure, lasting 30–45 min (6 per month) | Onset of VGB | Severe, atypical MSE. Eye closure induced rhythmic bursts of eyelid myoclonias with eyelid-opening apraxia. Resolved after IV BZ (45 min) | Pseudo-rhythmic, continuous diffuse 4–6 Hz SW Major increase of ictal activity when eyes were closed |
| 5 (F, 45) | GTCS (1 per year) Serial, daily episodes of mild intellectual impairment with diffuse anxiety lasting 5–15 s (30 per month) | Sleep deprivation | Atypical ASE with subjective cognitive impairment, and unpleasant feeling during discharges. Resolved after IV BZ (210 min) | Serial bursts of rapid, generalized Sp, followed by slow PSW lasting 7–12 s, repeated at 20–40 s intervals |
| 6 (F, 25) | GTCS (2 per year) Isolated loss of contact (10 per month) Long-lasting episode of subjective cognitive impairment with MJ ending in a GTCS after 10 h (three episodes) | Incr. CBZ | Typical ASE with subjective cognitive impairment, feeling of drunkenness, rare MJ, and unsteady gait. Resolved after a single GTCS (140 min) | Continuous, diffuse 4 Hz irregular SW |
| 7 (M, 21) | GTCS (6 per year) Long-lasting episodes of confusion with deambulation and amnesia, lasting 10–24 h (3 per year) | Incr. CBZ. Onset of GBP | Atypical ASE with severe confusion, eyelid myoclonias, perseverations, urination. Resolved after IV BZ (120 min) | Continuous 1–2.5 Hz PSW with a L predominance |
| 8 (M, 46) | GTCS (1 per year) Episodes of subjective cognitive impairment with eyelid myoclonias and anxiety, lasting 12–18 h (3 per year) | Incr. CBZ. Decr. PB | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ (60 min) | Bursts of generalized 3 Hz SW lasting 3–15 s, repeated at 10–15 s intervals |
| 9 (F, 26) | Bilateral MJ at awakening, lasting 30–45 min (5–10 per month) GTCS ending series of MJ (5–7 per month) | Sleep deprivation | Severe, typical MSE at awakening, interspersed with 3 GTCS. Resolved after IV BZ (40 min) | Bursts of generalized 0.5–1.5 Hz PSW lasting 10–15 s, repeated at 10 s intervals. |
| 10 9M, 18) | GTCS (two episodes) At awakening, rhythmic 3 Hz bilateral MJ in separate 10–15 s clusters, during 20–30 min (5–10 per month) | Onset of CBZ | Atypical MSE with short (10-20 s) bursts of bilateral, rhythmic 3 Hz MJ repeated at 20–30 s intervals. Resolved after IV BZ (35 min) | Bursts of generalized rhythmic PSW at 3 Hz synchronous with MJ. |
| 11 (M, 17) | GTCS (4 per month). Isolated, brief (5–10 s) loss of contact (10–20 per month). Long-lasting episodes of clumsiness with unsteady gait (2 per month) | Onset of CBZ | Atypical MSE with bilateral, asynchronous, negative myoclonus and marked postural instability. Resolved after IV BZ (120 min) | Continuous diffuse PSW at 1–2 Hz, time-locked with atonias |
| 12 (F, 38) | GTCS (1 per month). Isolated, brief (5–10 s) loss of contact (20–30 per month) | Incr. CBZ | Typical ASE with mild cognitive impairment and occasional eyelid myoclonias. Resolved after IV BZ and IV VPA (80 min) | Bursts of rhythmic generalized 2.5–3 Hz SW and PSW with a L predominance, repeated at 5 s intervals |
| 13 (F, 25) | GTCS preceded by MJ (8 per year). Long-lasting episodes of obtundation at awakening with bilateral MJ (4 per month) | Unknown | Typical ASE with mild confusion, interspersed with asynchronous, bilateral MJ of the limbs. Resolved spontaneously (60 min) | Bursts of rhythmic generalized PSW at 3 Hz, sometimes synchronous with MJ, repeated at 10–20 s intervals |
| 14 (M, 43) | GTCS (1 per month) Isolated loss of contact lasting 5–10 s (60 per month). Long-lasting episodes of cognitive disturbances with amnesia, ambulatory automatisms and fugue, sometimes with intercalated GTCS (2 per month) | Unknown | Atypical ASE with mild confusion, interspersed with a single GTCS. Resolved spontaneously (120 min) | Subcontinuous pseudo-rhythmic diffuse 4–6 Hz slow waves and Sp with a L predominance |
M: male; F: female; R: right: L: left; min: minutes; s: seconds; ASE: absence status epilepticus; MSE: myoclonic status epilepticus; GTCS: generalized tonic-clonic seizure; MJ: myoclonic jerks; Sp: spikes; SW: spike-and-waves: PSW: polyspike-and-waves; incr.: increase in dosage of; decr.: decrease in dosage of; BZ: benzodiazepine; CBZ: carbamazepine; GBP: gabapentin; PB: phenobarbital; PHT: phenytoin; VPA: valproate; VGB: vigabatrin.
Syndromic classification at referral
In eight patients, initial syndromic classification prior to the occurrence of SE was cryptogenic partial epilepsy (CPE) of frontal origin in five (Cases 1, 2, 3, 12 and 14) and of temporal origin in three (Cases 5, 6 and 8). This initial diagnosis had been based either on the presence of simple gestural automatisms during absence seizures that were considered as suggestive of complex partial seizures (Cases 1, 2, 3, 5, 6, 8, 12 and 14) or on that of slight versive movements at the onset of tonic-clonic seizures, misdiagnosed as frontal onset secondarily generalized tonic-clonic seizure (GTCS) (Cases 1, 2 and 3). In six of these patients, interictal EEG recordings prior to referral had been considered as incompatible with the diagnosis of IGE because they showed bilateral bursts of SW or PSW that were either asymmetric (Cases 1, 3, 8, 12 and 14) or of varying frequency (Case 5). Examination of pre-aggravation EEGs in the two remaining patients (Cases 2 and 5) could not show why the diagnosis of IGE had been discarded. MRI showed mild abnormalities in two patients (right ventricular enlargement in Patient 4 and isolated right temporal subcortical T2 hypersignal in Patient 5).
Four patients (Cases 4, 7, 9 and 11) had been diagnosed with non-idiopathic generalized epilepsy (IGE). Aggravation was so severe in Patients 4 and 9 that a diagnosis of Unverricht-Lundborg disease had been considered. School difficulties and behavioural problems had been interpreted as mild mental retardation in Patients 7 and 11, with doubtful brainstem atrophy on MRI in the latter. Interictal EEG in these patients showed either slow SW or rhythmic slow waves on a poorly organized, slow background activity.
Patient 10 had been classified as having undetermined epilepsy following two GTCS prior to the introduction of CBZ, which produced severe aggravation with onset of myoclonic jerks. The last patient (Case 13), despite an initial diagnosis of generalized epilepsy and a good seizure control with valproate (VPA), was given CBZ because of weight gain considered unacceptable.
Seizure aggravation
All patients had experienced seizure aggravation several months before referral. Absence or myoclonic seizures were markedly increased in frequency, duration and/or severity in five patients (Cases 1, 2, 5, 9 and 12), while the others developed, in addition to their usual seizures, new seizure types: prolonged episodes of confusion and/or cognitive disturbances (Cases 3, 6, 7, 8, 13 and 14) later documented as episodes of ASE; development of scotosensitivity with eyelid myoclonia and myoclonic jerks associated with eye closure (Case 4); appearance of clusters of rhythmic 3 Hz myoclonic jerks on awakening (Case 10); and episodes of unsteady gait and clumsiness (Case 11). In contrast, except in Patients 4 and 11, GTCS frequency was less obviously increased than other seizure types. In some patients (Cases 2, 7, 10, 11 and 13), clinical deterioration occurred soon after the introduction of the drug later identified as responsible for the increased seizure frequency. In the others (Cases 1, 3, 4, 5, 6, 8 and 9) aggravation had been more progressive and insidious, leading to the sequential addition of various AEDs by successive doctors over months.
Status epilepticus
Patients were either referred for assessment of intractable epilepsy with elective video-EEG or were admitted to the emergency ward for an acute epileptic event and assessed immediately. Five patients (Cases 2, 6, 8, 12 and 13) had typical ASE (Fig. 1A). Uncommon clinical features were prominent in five others with atypical ASE (Cases 1, 3, 5, 7 and 14): repeated versive movements; a peculiar compulsive motor perseveration; an unpleasant, recurrent, stereotyped feeling during discharges; repeated urination and a versive-onset GTCS that did not interrupt the ASE. In these patients, ictal EEG showed continuous or intermittent discharges of slow PSW, SW or slow waves at less than 2.5 Hz (Fig. 1B). Among the four patients with MSE (Cases 4, 9, 10 and 11), three had additional unusual features. In Case 4, paroxysmal activity and eyelid myoclonia were considerably increased at eyes-closure. In Patient 10, bursts of 3 Hz rhythmic massive myoclonic jerks were time-locked to the PSW. Patient 11 had epileptic negative myoclonus organized in SE (Tassinari et al., 1995) with interruption of tonic muscular activity time-locked to the PSW discharges without an antecedent positive myoclonic jerk (Fig. 2B).
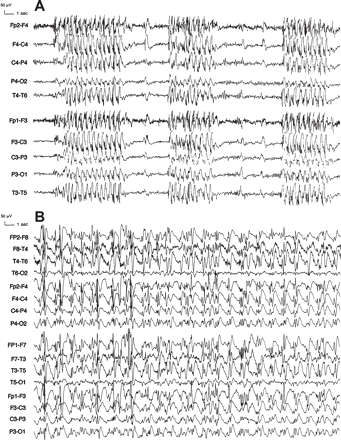
Typical and atypical ASE in JAE. (A) This 15-year-old girl (Patient 2) with JAE was treated with PB, 100 mg/day, and VPA, 1000 mg/day. Weight gain and an erroneous syndromic classification of CPE of frontal origin led to the replacement of VPA by CBZ, 600 mg/day. When CBZ was raised to 800 mg/day, typical ASE developed, characterized by serial bursts of 3 Hz generalized polyspike-and-wave complexes, recurring every 5–10 s. Mild impairment of consciousness and eyelid myoclonia were prominent during discharges. (B) This 39-year-old woman (Patient 3) with JAE had long-standing aggravation on CBZ, 800 mg/day, PHT, 350 mg/day, and PB, 50 mg/day. Shortly after awakening, she very often had episodes of prolonged cognitive impairment of varying intensity, one of which led to a car accident. During a 5-day video-EEG monitoring, a 180 min atypical ASE was recorded, with severe confusion, inappropriate familiarity, sexual disinhibition and a peculiar compulsive rhythmic stereotyped movement of flexion-extension of the right arm. EEG shows continuous, diffuse, irregular, high-amplitude, 1 Hz polyspike-and-slow wave and spike–and-wave activity with slight right-sided predominance.
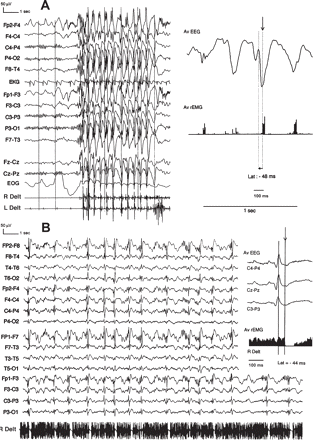
Positive and negative MSE as an effect of CBZ. (A). This 18-year-old man (Patient 10) with JME received CBZ, 800 mg/day, from the onset and had since experienced numerous episodes of MSE lasting up to 30 min after awakening. EEG shows very frequent 10–20 s bursts of generalized, rhythmic polyspike-and-slow wave complexes (PSW) at 3 Hz, synchronous with the myoclonia. Jerk-locked back-averaging of 30 traces shows that the last spike component precedes the onset of the myoclonic jerk with a 48 ms delay. (B) This 17-year-old man (Patient 11) with CAE was poorly controlled on VPA, 1000 mg/day, and PHT, 300 mg/day. Prolonged episodes of unsteady gait and clumsiness, related to negative MSE, occurred shortly after CBZ was added. EEG shows continuous 1–2 Hz PSW with a frontal predominance synchronous with the atonia. The EMG shows that interruption of tonic muscular activity occurs without evidence of an antecedent positive myoclonic jerk. Silent period-locked back-averaging of 50 consecutive traces shows a 44 ms delay between the peak of the last spike and the onset of atonia. R Delt: right deltoid; L Delt: left deltoid; Av EEG: averaged EEG; AV rEMG: averaged rectified EMG; Lat: latency.
Intravenous (IV) benzodiazepines (BZD) alone (diazepam, 10–20 mg, or clonazepam, 1–2 mg) successfully stopped SE in eight patients. Two patients with ASE required combination therapy with either IV PHT (Case 1) or IV VPA (Case 12). In three other patients (Cases 3, 13 and 14), SE ended spontaneously and ASE ended with a GTCS in Patient 6.
Antiepileptic drug regimen
All patients received CBZ, with a mean exposure of 2.8 years (range, 0.25–5.5 years), a mean dose of 1020 mg/day (range, 500–1600) and a mean blood level of 7.74 mg/l (range, 5.2–11). Four patients (Cases 5, 10, 12 and 13) received CBZ monotherapy, with a mean dose of 625 mg/day (range, 500–800) and a mean blood level of 6.52 mg/l. Ten patients took one or several other AEDs: phenobarbital (PB) in six (50–150 mg/day); PHT in three (200–350 mg/day); vigabatrin (VGB) in three (2000–3000 mg/day); VPA in two (1000 and 2000 mg/day); gabapentin (GBP) in one (800 mg/day) and clobazam (30 mg/day) in one. In three patients (Cases 2, 12 and 13), VPA had been replaced with CBZ owing to side-effects (weight gain in two and fatigue in the last). Among the 12 patients in whom SE was presumably precipitated by modification of the drug regimen, an increase of CBZ dosage was implicated in 7.
Blood test results were normal in all. Blood levels of CBZ, PB, PHT and VPA were within the usual therapeutic ranges; levels of CBZ 10,11-epoxide, VGB, GBP and clobazam were not assessed. None of the patients had acute AED withdrawal. Patient 8 had reduced PB by 50 mg/day two weeks before onset of SE, but was still receiving 150 mg/day (blood level: 22 mg/l).
Final diagnosis, course and prognosis
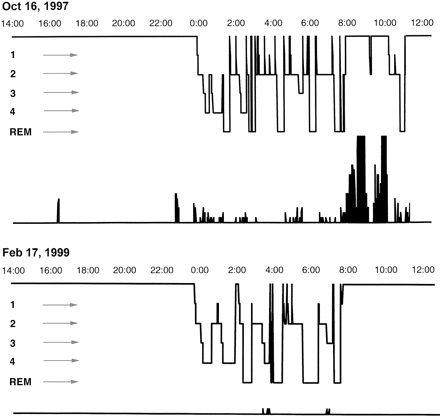
Final syndrome diagnoses were as follows: juvenile absence epilepsy (JAE) in six patients, juvenile myoclonic epilepsy (JME) in four, epilepsy with grand mal on awakening (EGMA) in two and childhood absence epilepsy (CAE) in two. All patients but one became seizure-free during follow-up (mean duration: 5.1 years, range 3–7.5 years), including five on VPA monotherapy and eight with a combination therapy that included VPA. Patient 2 had persisting rare absences due to poor compliance. In all patients, interictal EEGs after discontinuation of aggravating drugs and prescription of adequate treatment showed either complete normalization or rare bursts of rapid SW or PSW on a normal background. A 24 h ambulatory EEG was recorded before and after change of treatment in Patient 13 and demonstrated the occurrence of MSE at awakening on CBZ and the near-complete disappearance of interictal changes on VPA (Fig. 3).
This 25-year-old woman (Patient 13) with JME since age 16 had been on VPA, 1000 mg/day, which was changed at age 23 to CBZ, 600 mg/day, by her general practitioner because of weight gain. She was referred at age 25 for severe generalized epilepsy with long-lasting episodes of obtundation with asynchronous jerks of the limbs that occurred shortly after awakening and lasted up to 2 h. Top: 24 h ambulatory EEG recording with quantification of the duration of paroxysmal activity (PA), performed on CBZ, 600 mg/day (blood level: 7.4 mg/l), showing the presence of nearly continuous discharges of SW or PSW after awakening. Total duration of PA: 45 min 30 s. Bottom: Same procedure 16 months later on VPA, 1000 mg/day (blood level: 88 mg/l). Total duration of PA: 15 s.
Discussion
Paradoxical seizure worsening by AED in IGE is not always easy to diagnose. Although presenting with unusual ASE or MSE, all of our patients fulfilled criteria for this type of aggravation (Genton and McMenamin, 1998). None had toxic AED levels, poor compliance, irregular lifestyles, acute drug withdrawal or other metabolic encephalopathies. Before developing SE, all experienced a clear increase in seizures and nine had new seizure types. Although we considered the possibility of refractory frontal-lobe epilepsy in cases with atypical ASE or unusual clinical seizure patterns, the appearance of new seizure types was clearly restricted to the aggravating period.
This paradoxical aggravation of seizures should not be confused with spontaneous fluctuations in seizure activity, because all patients had an immediate and satisfactory seizure control and EEG improvement or normalization when initial AED treatments were discontinued and replaced by an adequate medication. To confirm fully that narrow-spectrum AEDs were the cause of aggravation, we would have had to stop them first to observe spontaneous improvement before reintroducing the potential aggravating drug. However, all patients required immediate treatment following a long-standing aggravation that terminated into SE. Furthermore, this reintroduction procedure is obviously ethically impossible just to acquire scientific evidence (Gelisse et al., 2004).
Our patients had various syndromes of IGE, a subgroup of epilepsies that are more likely to be aggravated by narrow-spectrum AEDs (Benbadis et al., 2003). This study shows that there are many clinical and EEG pitfalls in IGE diagnosis that may result in erroneous classification and treatment choices. Clinical manifestations may be asymmetric, with lateralized myoclonic jerks or versive onset of GTCS, particularly in patients with JME (Montalenti et al., 2001; Usui et al., 2005). In this syndrome, EEG abnormalities may be asymmetric or even focal (Lancman et al., 1994; Genton et al., 1995) and this EEG feature may even be exacerbated by inadequate treatment (Talwar et al., 1994). In CAE and JAE, absences may be misdiagnosed as complex focal seizures, particularly if ictal automatisms occur (Snead and Hosey, 1985). Such confusing features were responsible for the choice of inappropriate treatment in most of our patients. Abnormal neuroimaging may also cause misdiagnosis in IGE, and such coincidental findings have been demonstrated to have no impact on the clinical course of JME (Gelisse et al., 2000).
In the present series, syndromic diagnosis was changed during hospitalization, and the occurrence of SE was the major reason. The phenomenology of SE helped somewhat in rectifying the syndromic diagnosis, as all patients with JAE presented with ASE, and most patients with JME had MSE. However, ASE was atypical in half the cases, and MSE was atypical in three-quarters of them. Also, no patients presented with generalized tonic-clonic SE, even those with a final diagnosis of EGMA.
MSE is a rare spontaneous complication of JME (Thomas et al., 2005) and was found in three of our four JME patients. In two of these, the intensity of myoclonus, the duration of the episodes and their daily occurrence mimicked the symptoms of a progressive myoclonus epilepsy. A major increase in eyelid myoclonia and paroxysmal activity on eye closure produced a clinical picture similar to apraxia of eyelid opening (Defazio et al., 1998) in Patient 4. In Patient 10, rhythmic 3 Hz myoclonic jerks with abrupt on- and offset, but without impairment of consciousness resembled the EEG ictal pattern of epilepsy with myoclonic absences (Bureau and Tassinari, 2005). Generalized MSE with only negative myoclonus was prominent in Patient 11, with a final diagnosis of CAE. Although never documented in IGE in the form of SE, epileptic (Nanba and Maegaki, 1999; Shirasaka and Mitsuyoshi, 1999) and non-epileptic (Aguglia et al., 1987) negative myoclonus have been reported as an adverse effect of CBZ in children with benign partial epilepsies.
Similarly, in absence epilepsies, the occurrence of typical ASE has been repeatedly reported as a rare spontaneous complication (Kaplan, 2002), although prevalence may be higher in patients with persistence of typical absences in adult life (Michelucci et al., 1996), or in some recently identified syndromes such as perioral myoclonias with absences (Agathonikou et al., 1998) or phantom absences with GTCS (Panayiotopoulos et al. 1997b). In these idiopathic forms, ASE is typically restricted to a prolonged variable confusional state associated with symmetric and bilaterally synchronous fast SW or PSW discharges (Shorvon, 1994). Clinical and EEG features were clearly atypical in half of our ASE patients, which could lead to a misdiagnosis of a cryptogenic or symptomatic epilepsy syndrome (Kaplan, 2002). Osorio et al. (2000) recorded atypical ASE in eight patients receiving both CBZ and PHT, four with CAE and four with JME. Interestingly, ASE was much more resistant to IV BZD or IV VPA, compared with 10 ASE patients with IGE who either were on no medication or were not being treated with this combination of AEDs. In our series, IV BZD or IV VPA easily controlled SE in all patients, save for one who required IV PHT.
CBZ appears to be the major cause of seizure aggravation in our series: it was being taken by all patients when SE developed, was the only drug in four cases and probably masked or reduced the benefit of effective AEDs (PB and VPA) in eight others. Although associated with other potentially aggravating agents (PHT, VGB, GBP) in seven patients, SE was clearly triggered in two of them by starting CBZ and in three others by an increased CBZ dose. Other patients had a longer exposure to CBZ, leading to an insidious worsening over months, which explained the successive addition of other drugs.
Exacerbation of seizures in patients treated with CBZ has sometimes been reported as a toxic effect with elevated CBZ blood levels (Bauer, 1996), or with elevated 10,11 epoxy CBZ levels, even without elevated CBZ levels (Dhuna et al., 1991; So et al., 1994). However, in our patients, blood levels of CBZ were well below the toxic range, and none had clinical signs of intoxication. Several seizure types can be aggravated by therapeutic levels of CBZ, including typical and atypical absences (Lerman, 1986; Talwar et al., 1994; Parker et al., 1998), tonic (Snead and Hosey, 1985), atonic (Shields and Saslow, 1983) and myoclonic seizures (Parmeggiani et al., 1998; Genton et al., 2000). This paradoxical aggravation has been most commonly reported in children with generalized cryptogenic or symptomatic epilepsies (Guerrini et al., 1998) and is less well documented in adults (Liporace et al., 1994). However, in a series of 59 children with various focal epileptic syndromes and CBZ-associated EEG changes (Talwar et al., 1994), the appearance of generalized paroxysmal discharges after starting CBZ was highly correlated with seizure exacerbation.
In idiopathic epilepsies, CBZ can increase seizures in absence epilepsies (Parker et al., 1998), benign epilepsy with centrotemporal spikes (Corda et al., 2001) and JME: in a large retrospective series (Genton et al., 2000), 68% of cases exposed to CBZ experienced seizure aggravation, mostly in the form of increased myoclonus. De novo appearance of absences (Yang et al., 2003) and myoclonic jerks (Parmeggiani et al., 1998) has also been reported in patients with GTCS only. Oxcarbazepine, a new drug chemically related to CBZ can also exacerbate myoclonus and absence seizures in JME and JAE (Gelisse et al., 2004)
Seizure exacerbation by PHT is often associated in IGE with toxic drug levels (Bauer, 1996). PHT may increase absences and tonic-clonic seizures in generalized epilepsies (Levy and Fenichel, 1968) and has also been reported to precipitate ASE in JME and in CAE (Osorio et al., 2000). PHT can also aggravate myoclonic jerks in patients with JME, but to a lesser extent than CBZ (Genton et al., 2000). Increased PHT dose was a co-factor in MSE in only one of our patients.
VGB has also been reported to aggravate absence, tonic and myoclonic seizures, especially in children. However, except in absence epilepsies, clear aggravation of IGE is relatively uncommon (Michelucci and Tassinari, 1989). In two patients with either CAE or JAE, adding VGB to a previously inappropriate treatment dramatically increased both frequency and severity of ASE (Panayiotopoulos et al., 1997a). Similarly, in our series, starting VGB may have triggered ASE in a JME patient (Case 4), and was a co-factor in two other cases.
Lamotrigine (LTG) has been shown to be useful for absences, but may worsen myoclonic seizures in various clinical settings. Aggravation of IGE has been reported, including six patients with JME that were clearly worsened (Biraben et al., 2000), three other patients who developed ASE with mild myoclonic components when switched from VPA (Trinka et al., 2002) and five patients with exacerbation or de novo appearance of myoclonic jerks (Crespel et al., 2005). However, five of our non-JME patients were finally controlled on a combination of AEDs, including LTG.
There is some evidence for precipitating absences or myoclonic jerks with GBP. However, if GBP-associated myoclonus is relatively frequent, it may easily remain unnoticed (Asconape et al., 2000). One of our patients experienced ASE following the introduction of GBP, but CBZ dosage had been increased at the same time.
Similarly, high-dose PB has been reported to increase absences (Bauer, 1996; Berkovic, 1998) but today most clinicians in developed countries use barbiturates sparingly in IGE. Six of our patients initially received PB, but its role in the provocation of SE was unclear: although dosage had been decreased in a single patient with ASE, it had remained stable in five, and all were receiving other potentially aggravating AEDs.
This series also confirms the major efficacy of VPA in IGE patients, a property that may have been masked by the co-prescription of potentially aggravating AEDs in two cases. VPA may also be withdrawn owing to side-effects, and the control of epilepsy typically worsens thereafter: this happened in three patients who were switched to CBZ for weight gain or asthenia. Syndromic worsening with VPA is very unusual: eight children with absence seizures associated with generalized 3 Hz SW activity developed paradoxical increase in absences within days of starting VPA (Lerman-Sagie et al., 2001). These puzzling but well-documented cases show that no AED can be excluded as a cause of paradoxical aggravation of seizures.
Among cellular mechanisms by which AEDs may precipitate seizures, the gamma aminobutyric acid (GABA) system is the most extensively studied. In a genetic absence rat model, a reverberating thalamocortical circuit generates abnormal oscillatory rhythms, resulting in non-convulsive seizures resembling human absences (Marescaux et al., 1992; McCormick, 2002). GABA-B-induced hyperpolarization of thalamic relay neurons enhances oscillatory thalamocortical activity, leading to more prominent and sustained SW discharges (Liu et al., 1992). In this model, GABAergic drugs such as VGB, tiagabine and GBP, as well as CBZ and PHT, increase SW discharges and clinical seizures. In addition, if one superimposes a GABA-B receptor agonist, baclofen, on the gamma-hydroxybutyrate model of absence seizures, ASE results (Snead, 1996). CBZ and PHT act mainly as voltage-dependent sodium channel blockers, enhancing membrane stabilization, a property that may indirectly increase hypersynchronization of neuronal discharges in a thalamocortical loop already showing intensified oscillatory activity (McLean and Macdonald, 1986).
This series shows that severe pharmacodynamic AED-induced seizure aggravation in IGE may express itself as ASE or MSE, often with atypical clinical and EEG features. Careful syndrome diagnosis is needed to avoid such complications. Although the risk of aggravation has probably increased with the number of available narrow-spectrum anticonvulsants, CBZ appears to be the drug most often implicated and should be contraindicated in absence epilepsies and JME, and used with great care when a diagnosis of IGE is considered.
The authors thank F. Andermann, F. Mauguière and B. G. Zifkin for their expert reviewing of the manuscript and helpful comments.
References
Agathonikou A, Panayiotopoulos CP, Giannakodimos S, Koutroumanidis M. Typical absence status in adults: diagnostic and syndromic considerations.
Aguglia U, Zappia M, Quattrone A. Carbamazepine-induced nonepileptic myoclonus in a child with benign epilepsy.
Asconape J, Diedrich A, DellaBadia J. Myoclonus associated with the use of gabapentin.
Bauer J. Seizure-inducing effects of antiepileptic drugs: a review. [Review].
Benbadis SR, Tatum WO, Gieron M. Idiopathic generalized epilepsy and choice of antiepileptic drugs.
Berkovic SF. Aggravation of generalized epilepsies. [Review].
Biraben A, Allain H, Scarabin JM, Schuck S, Edan G. Exacerbation of juvenile myoclonic epilepsy with lamotrigine.
Bureau M, Tassinari CA. The syndrome of myoclonic absences. In: Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P, editors. Epileptic syndromes in infancy, childhood and adolescence. 4th edn. Montrouge: John Libbey Eurotext;
Callahan DJ, Noetzel MJ. Prolonged absence status epilepticus associated with carbamazepine therapy, increased intracranial pressure and transient MRI abnormalities.
Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes.
Corda D, Gelisse P, Genton P, Dravet C, Baldy-Moulinier M. Incidence of drug-induced aggravation in benign epilepsy with centrotemporal spikes.
Crespel A, Genton P, Berramdane M, Coubes P, Monicard C, Baldy-Moulinier M, et al. Lamotrigine associated with exacerbation or de novo myoclonus in idiopathic generalized epilepsies.
Defazio G, Livrea P, Lamberti P, De Salvia R, Laddomada G, Giorelli M, et al. Isolated so-called apraxia of eyelid opening: report of 10 cases and a review of the literature.
Dhuna A, Pascual-Leone A, Talwar D. Exacerbation of partial seizures and onset of nonepileptic myoclonus with carbamazepine.
Gastaut H. Classification of status epilepticus. In: Delgado-Escueta AV, Wasterlain CG, Treiman DM, Porter RJ, editors. Status epilepticus. Advances in neurology, Volume 34. New York: Raven Press;
Gelisse P, Genton P, Raybaud C, Thomas P, Dravet C. Structural brain lesions do not influence the prognosis of juvenile myoclonic epilepsy.
Gelisse P, Genton P, Kuate C, Pesenti A, Baldy-Moulinier M, Crespel A. Worsening of seizures by oxcarbazepine in juvenile idiopathic generalized epilepsies.
Genton P, McMenamin J. Aggravation of seizures by antiepileptic drugs: what to do in clinical practice. [Review].
Genton P, Gonzalez-Sanchez MSE, Saltarelli A, Bureau M, Dravet C, Roger J. Misleading aspects of the standard electroencephalogram in juvenile myoclonic epilepsy: a retrospective study of 56 consecutive cases.
Genton P, Gelisse P, Thomas P, Dravet C. Do carbamazepine and phenytoin aggravate juvenile myoclonic epilepsy?
Guerrini R, Belmonte A, Genton P. Antiepileptic drug-induced worsening of seizures in children.
Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus.
Lancman ME, Asconape JJ, Penry JK. Clinical and EEG asymmetries in juvenile myoclonic epilepsy.
Lerman-Sagie T, Watemberg N, Kramer U, Shahar E, Lerman P. Absence seizures aggravated by valproic acid.
Liporace JD, Sperling MR, Dichter MA. Absence seizures and carbamazepine in adults.
Liu Z, Vergnes M, Depaulis A, Marescaux C. Involvement of intrathalamic GABA-B neurotransmission in the control of absence seizures in the rat.
Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg—a review. [Review].
McCormick DA. Cortical and subcortical generators of normal and abnormal rhythmicity. [Review].
McLean MJ, Macdonald RL. Carbamazepine and 10,11–epoxycarbamazepine produce use- and voltage-dependent limitation of rapidly firing action potentials of mouse central neurons in cell culture.
Michelucci R, Tassinari CA. Response to vigabatrin in relation to seizure type.
Michelucci R, Rubboli G, Passarelli D, Riguzzi P, Volpi L, Parmeggiani L, et al. Electroclinical features of idiopathic generalised epilepsy with persisting absences in adult life.
Montalenti E, Imperiale D, Rovera A, Bergamasco B, Benna P. Clinical features, EEG findings and diagnostic pitfalls in juvenile myoclonic epilepsy: a series of 63 patients.
Nanba Y, Maegaki Y. Epileptic negative myoclonus induced by carbamazepine in a child with BECTS.
Osorio I, Reed RC, Peltzer JN. Refractory idiopathic absence status epilepticus: a probable paradoxical effect of phenytoin and carbamazepine.
Panayiotopoulos CP, Agathonikou A, Ahmed-Sharoqi I, Parker APJ. Vigabatrin aggravates absences and absence status.
Panayiotopoulos CP, Koutroumanidis M, Giannakodimos S, Agathonikou A. Idiopathic generalised epilepsy in adults manifested by phantom absences, generalised tonic-clonic seizures, and frequent absence status.
Parker AP, Agathonikou A, Robinson RO, Panayiotopoulos CP. Inappropriate use of carbamazepine and vigabatrin in typical absence seizures.
Parmeggiani A, Fraticelli E, Rossi PG. Exacerbation of epileptic seizures by carbamazepine: report of 10 cases.
Perucca E, Gram L, Avanzini G, Dulac O. Antiepileptic drugs as a cause of worsening seizures. [Review].
Sazgar M, Bourgeois BF. Aggravation of epilepsy by antiepileptic drugs. [Review].
Shields WD, Saslow E. Myoclonic, atonic and absence seizures following institution of carbamazepine therapy in children.
Shirasaka Y, Mitsuyoshi I. A case of epileptic negative myoclonus: therapeutic considerations.
Shorvon S. Status epilepticus, its clinical features and treatment in children and adults. Cambridge: Cambridge University Press; 1994.
Snead OC. Antiabsence activity of specific GABA-B and γ-hydroxybutyric acid antagonists.
Snead OC, Hosey LC. Exacerbation of seizures in children by carbamazepine.
So E, Ruggles K, Cascino G, Ahmann P, Weatherford K. Seizure exacerbation and status epilepticus related to carbamazepine-10,11–epoxide.
Talwar D, Arora MSE, Sher PK. EEG changes and seizures exacerbation in young children treated with carbamazepine.
Thomas P, Genton P, Gelisse P, Wolf P. Juvenile myoclonic epilepsy. In: Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P, editors. Epileptic syndromes in infancy, childhood and adolescence. 4th edn. Montrouge: John Libbey Eurotext;
Trinka E, Dilitz E, Unterberger I, Luef G, Deisenhammer F, Niedermuller U, et al. Non convulsive status epilepticus after replacement of valproate with lamotrigine.
Usui N, Kotagal P, Matsumoto R, Kellinghaus C, Luders HO. Focal semiologic and electroencephalographic features in patients with juvenile myoclonic epilepsy.
Author notes
1Unité Fonctionnelle EEG-Epileptologie, Service de Neurologie, Hôpital Pasteur, Nice, 2Unité d'Epileptologie, Service de Neurologie, Hôpital Rangueil, Toulouse and 3Centre Saint Paul-Hôpital Henri Gastaut, Marseilles, France
- anticonvulsants
- phenytoin
- patient referral
- epilepsy
- seizures
- carbamazepine
- defibrillator, automatic external
- electroencephalography
- gabapentin
- adult
- absence epilepsy
- myoclonic epilepsy, juvenile
- precipitating factors
- precipitation
- status epilepticus
- vigabatrin
- diagnosis
- immunoglobulin e
- phenobarbital
- valproic acid
- pharmacodynamics
- idiopathic generalized epilepsy
- tonic - clonic seizures
- awakening
- video eeg
- cyclophosphamide/doxorubicin/etoposide
- symptom aggravating factors