-
PDF
- Split View
-
Views
-
Cite
Cite
Nicholas D Schiff, Uncovering hidden integrative cerebral function in the intensive care unit, Brain, Volume 140, Issue 9, September 2017, Pages 2259–2262, https://doi.org/10.1093/brain/awx209
Close - Share Icon Share
This scientific commentary refers to ‘Early detection of consciousness in patients with acute severe traumatic brain injury’, by Edlow et al. (doi:10.1093/brain/awx176).
Over the past two decades a wide range of hidden integrative cerebral function has been identified in some behaviourally unresponsive or minimally responsive patients (see Laureys and Schiff, 2012 for a review). Modern neuroimaging tools and sophisticated electrophysiological methods have provided an increasingly clear picture of brain function following severe injuries. To date, however, the investigation of graded cerebral function has largely focused on chronic recovery long after the period of acute injury (with exceptions, e.g. Claassen et al., 2016). In this context, a study by Edlow et al. in this issue of Brain breaks considerable new ground (Edlow et al., 2017). The investigators provide the first prospective study combining neuroimaging and electrophysiological assessment of the level of consciousness in severely brain-injured patients in the first 2 weeks of an intensive care period.
Edlow and colleagues enrolled 16 patients with acute severe traumatic brain injuries and carried out a series of structured experimental paradigms using functional MRI and quantitative EEG combined with sophisticated multivariate pattern recognition algorithms. Their work was based on two a priori hypotheses: (i) that direct brain measurements with functional MRI or quantitative EEG would reveal evidence of language comprehension or cortical processing in patients without behavioural evidence of language function; and (ii) if present, such evidence would provide predictive information about 6-month outcomes. The major success of this study is the identification of cognitive motor dissociation (CMD) in half of the subjects without behavioural evidence of language function. CMD has been proposed as a term to categorize patients with no, or only very limited, behavioural evidence of awareness who nonetheless demonstrate unequivocal empirical evidence of command-following via functional MRI, quantitative EEG or similar indirect measurements of brain response to spoken language (Schiff, 2015) (Fig. 1). In the present study, four of eight patients without behavioural evidence of language function were identified as CMD patients by the functional MRI method; this observation, though made in a small sample, suggests that such dissociations are likely to be common in the ICU.
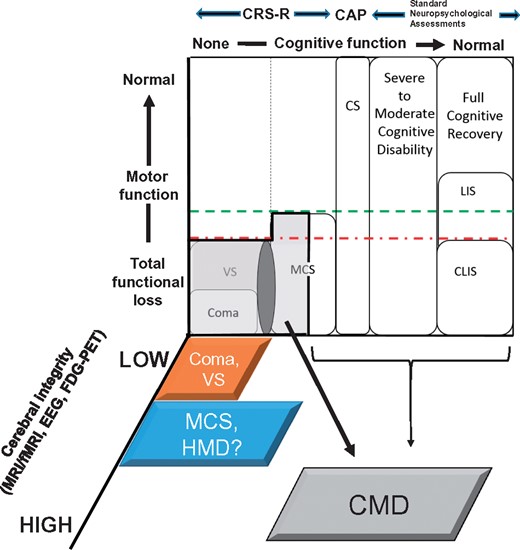
Dissociations of behavioural and physiological measures in recovery patterns following severe brain injuries. Patterns of recovery of consciousness and cognition following severe brain injuries are represented on a 3D coordinate system. Dimensions illustrate the dissociations of behavioural and physiological measurements observed in the assessment of patients with disorders of consciousness, as seen in both the chronic recovery phase and the acute intensive care unit setting examined by Edlow et al. in this issue of Brain. A light grey zone encompassing coma, vegetative state (VS) and the left half of the minimally conscious state (MCS) identifies patients with cognitive motor dissociation (CMD). The dark grey oval between coma and vegetative state and minimally conscious state indicates a transition zone in which behavioural fragments may be present in patients otherwise fulfilling the criteria for vegetative state; an interrupted vertical line indicates the boundary of evidence for awareness as judged behaviourally (none to the left of the line). CMD is a clinical syndrome operationally defined as having a bedside examination consistent with coma, vegetative state or the limited non-reflexive behaviours seen in MCS patients who are unable to follow commands and the concurrent demonstration of command-following using functional MRI (fMRI), EEG or similar technologies alone. Uncertainty exists regarding the ultimate underlying cognitive capacity in CMD as patients may span the range from command-following to higher integrative function (as indicated by the brackets to the right of the light grey region). Many studies now show that CMD is associated with highly preserved cerebral integrity (Forgacs et al., 2014; Stender et al., 2014; Schiff, 2015) suggesting that CMD patients are likely to be closer in preservation of cerebral function to patients in locked-in state (LIS) or the complete locked-in state (CLIS), but distinct from such patients because of multiple injuries across the corticothalamic systems. As defined by Edlow and colleagues, ‘higher-order cortex motor dissociation’, HMD, denotes a determination of higher-order cortical responses to structured stimuli in patients without behavioural evidence of language. Quantitative measurements of behavioural function smoothly span recovery of cognitive function when expressed through observable behaviours using the Coma Recovery Scale-Revised (CRS-R) to measure recovery from coma through emergence from MCS; the Confusion Assessment Protocol (CAP) to measure function in the confusional state (CS), and a wide array of standardized measures to capture the recovery of normative neuropsychological function. Because motor impairments following severe brain injuries may mask even full cognitive recovery, the search for reliable correlative physiological measures to identify levels of cognitive function is critical. Ongoing work demonstrates that functional neuroimaging with MRI and PET tools (functional MRI, FDG-PET) and the EEG suggest high degrees of preservation of cerebral functional integrity in CMD.
The investigators also examined evidence for higher-order cortical processing of language and musical stimuli at the acute stage using functional MRI and quantitative EEG. They introduce a new term, higher-order cortex motor dissociation (HMD), to label the presence of contingent brain responses to these stimuli in patients without evidence of language function. Patients with statistical evidence of a contingent functional MRI or quantitative EEG response to either language or musical stimuli when compared to control or rest periods were identified as having HMD. Earlier studies have assessed similar isolated evidence of higher-order responses from association cortices (e.g. Menon et al., 1998 demonstrated isolated and selective processing within visual association cortex) but, as Edlow et al. note, whether such responses are evidence of awareness is more ambiguous. Although patient outcomes were not statistically linked to functional MRI or quantitative EEG responses across the group of subjects, the use of HMD and CMD designations to augment the best behavioural assessment of highest level of consciousness improved sensitivity to detect recovery beyond post-traumatic confusion at 6 months after injury.
As shown in Fig. 1, CMD represents a sharp recategorization of patients from measurements available using quantitative behavioural assessment tools (Schiff, 2015). The Edlow et al. study validates the direct translation to the ICU of the functional MRI strategies employed in earlier work with patients in the later stages of recovery. Collectively, both the HMD and CMD findings in the study highlight an often neglected third dimension of recovery that is critical in thinking carefully about the severely injured brain—the functional integrity of the cerebrum or corticothalamic system per se (Fig. 1). Several studies have now provided evidence that CMD is associated with broad preservation of the dynamic structure of wake and sleep cerebral network physiology and metabolism (Forgacs et al., 2014; Stender et al., 2014; Schiff, 2015). Although we lack a predictive physiological model for conscious awareness or cognitive capacities at present, accumulating correlative evidence points toward a coincident high degree of preservation of cerebral function associated with CMD. While similar correlations are not yet known for HMD, HMD alone may be particularly useful in the ICU as the investigators note; the demonstration of higher-order cortical response does provide an unambiguous assay of the relative integrity of corticothalamic systems. Clearly larger studies are required, but these findings show that the degree of preserved integrative cerebral function is a critical unmeasured variable in current ICU practice. Once greater numbers of patients are enrolled in future studies, assessment of the probability of awareness and use of such measures for prognostic predictions are likely to follow.
Thus, the results of the Edlow et al. study also highlight the many challenges ahead for measuring recovery in the ICU. While functional MRI measurements identified CMD in four patients, the quantitative EEG measures did not provide concordant evidence in the three subjects tested. Functional MRI studies of ICU patients are not widely available and many patients may be excluded from undergoing functional MRI during an ICU course, making the lack of quantitative EEG findings in the CMD subjects of considerable practical concern. The analytical approach that Edlow et al. adopted for the quantitative EEG experiment relied upon the use of a multivariate pattern classifier, which had the statistical strength of being applied to single-subject data in each case. However, even with single subject modelling of signals, quantitative EEG classifier approaches are very data hungry and under-perform more classical statistics applied to the power spectrum when using limited datasets (Goldfine et al., 2013). The lack of quantitative EEG evidence for command-following using motor imagery in a quarter of the healthy volunteers tested by Edlow et al. is consistent with this limitation. Aside from constraints imposed by different quantitative EEG analysis methods, the ICU environment is a particularly difficult one for recording of the EEG. While a graded sedation scale did not show an effect on quantitative EEG measurements or interaction, the investigators noted that qualitative assessments of the patients showed a dose-response variance in the apparent level of effective sedation. This finding raises an issue overlapping with current research in anaesthesia and pharmacologically induced coma where electrophysiological data suggest heterogeneity and variable sensitivity to sedatives (Chander et al., 2014). Modelling and accounting for the dynamic features of specific sedatives such as propofol or dexmedetomidine (Akeju et al., 2014) may aid such analyses in all ICU patients with severe brain injuries including cohorts with subarachnoid haemorrhage (Claassen et al., 2016), post-anoxic encephalopathy following cardiac arrest and other aetiologies.
Glossary
Cognitive motor dissociation (CMD): A specific subset of patients who fulfil CRS-R criteria for coma, vegetative state or MCS without behavioural evidence of language function, who verifiably demonstrate command-following response when tested using proxy methods of neuroimaging or electrophysiological assessment.
Confusional state (CS): Recovery following emergence from MCS begins with the confusional state, also known as post-traumatic confusional state when a consequence of traumatic brain injury. Patients in a confusional state cannot be formally tested using standard neuropsychometric measures and remain disoriented; their cognitive capacities can be formally assessed using the Confusion Assessment Protocol (CAP).
Higher-order cortex motor dissociation (HMD): A term to indicate the presence of contingent responses of association cortices to complex auditory stimuli (linguistic or musical) in patients without behavioural evidence of consciousness (i.e. coma, vegetative state, or the subset of MCS patients without language function).
Locked-in state (LIS): Normal conscious awareness but severe motor impairment, limiting communication channels typically to eye movements, while complete locked-in state (CLIS) indicates the same level of function in a patient without any motor function to allow verification of this degree of cognitive recovery.
Minimally conscious state (MCS): A state in which unequivocal but often only intermittent behavioural evidence of consciousness is present, such as visual tracking or turning of the head toward a sound. The definition allows for a wide range of higher level behaviours to be present that indicate evidence of language function, including intermittent command-following, verbalization, or inaccurate communication. The Coma Recovery Scale-Revised (CRS-R) is a quantitative behavioural assessment tool that measures behavioural features of coma, vegetative state and MCS.
Vegetative state (VS): An unconscious brain state in which no behavioural evidence of consciousness is present and both cognitive and motor functions are absent. Vegetative state differs from coma only by the presence of intermittent eyes-open periods that are not accompanied by normal sleep-wake physiology.
It is likely that the present study from Edlow et al. will represent an historical watershed for revealing this critical vein of new discovery regarding the presence of hidden integrative cerebral function in the ICU. As noted above, the findings of this study set several challenges for future work and suggest refinements of measurement strategies tailored to the ICU patient. Our colleagues in anaesthesiology make considerable efforts to ensure that awareness is not present in apparently comatose patients; as Edlow et al. demonstrate, the reverse consideration is equally critical and ethically mandated in the ICU. There will undoubtedly be a steep learning curve to establish the rigorous assessment of sustained cerebral capacity of the severely brain-injured ICU patient. The present study shows that we will need increasing precision as stratification of level of brain function is related over time to patient outcomes. Moreover, our judgements of capacity will have to be clearly separated from the effects of initial injuries, course of ICU treatments, and the instrumental sedation required for ICU management. Taking this emerging future into view, the most general and likely enduring contribution of the Edlow et al. study is to show that such a rigorous approach is feasible and to underscore the urgent need to further its development.