-
PDF
- Split View
-
Views
-
Cite
Cite
Chiharu Moriya, Hiroaki Taniguchi, Kanjiro Miyata, Nobuhiro Nishiyama, Kazunori Kataoka, Kohzoh Imai, Inhibition of PRDM14 expression in pancreatic cancer suppresses cancer stem-like properties and liver metastasis in mice, Carcinogenesis, Volume 38, Issue 6, June 2017, Pages 638–648, https://doi.org/10.1093/carcin/bgx040
Close - Share Icon Share
Abstract
Pancreatic cancer is one of the most lethal types of cancer, with aggressive properties characterized by metastasis, recurrence and drug resistance. Cancer stem cells are considered to be responsible for these properties. PRDM14, a transcriptional regulator that maintains pluripotency in embryonic stem cells, is overexpressed in some cancers. Here, we assessed PRDM14 expression and the effects of PRDM14 knockdown on cancer stem-like phenotypes in pancreatic cancer. We observed that PRDM14 protein was overexpressed in pancreatic cancer tissues compared with normal pancreatic tissues. Using lentiviral shRNA-transduced pancreatic cancer cells, we found that PRDM14 knockdown decreased sphere formation, number of side population and cell surface marker-positive cells and subcutaneous xenograft tumors and liver metastasis in mice. This was accompanied by upregulation of some microRNAs (miRNAs), including miR-125a-3p. miR-125a-3p, a tumor suppressor that is down-regulated in pancreatic cancer, has been suggested to regulate the expression of the Src-family kinase, Fyn. In PRDM14-knockdown cells, Fyn was expressed at lower levels and downstream proteins were less activated. These changes were considered to cause suppression of the above cancer phenotypes. In addition, we used small interfering RNA (siRNA)-based therapy targeting PRDM14 in a mouse model of liver metastasis induced using MIA-PaCa2 cells, and this treatment significantly decreased metastasis and in vitro migration. Taken together, these results suggest that targeting the overexpression of PRDM14 suppresses cancer stem-like phenotypes, including liver metastasis, via miRNA regulation and siRNA-based therapy targeting it shows promise as a treatment for patients with pancreatic cancer.
Introduction
Pancreatic cancer is one of the most aggressive and lethal forms of the disease, and remains one of the four leading causes of cancer deaths in the United States (1). Owing to the difficulty of diagnosing the disease during its early stages, most patients show metastases to other organs, which often cannot be treated surgically (2). The cancer is relatively chemoresistant; therefore, the prognoses and survival rates are poor despite the use of high-dose anti-cancer drugs that can have severe side effects (2). In addition, other properties of pancreatic cancer, such as invasion, metastasis, and recurrence, also lead to poor prognosis and survival rates (2).
Recently, cancer stem cells (CSCs) have been identified as being responsible for the aforementioned properties. The CSC theory was proposed based on clinical and experimental observations that tumors arise from a rare cell population, which are capable of self-renewal and differentiation, similar to normal stem cells (3). The theory has been discussed extensively, and supporting data have been reported for various cancers, such as acute myeloid leukemia, breast cancer, brain cancer, colorectal cancer, ovarian cancer and pancreatic cancer (4–9). With their stem-like capacities, CSCs are thought to be responsible for cancer initiation, progression, invasion, metastasis, recurrence and drug resistance (10,11). Moreover, the differentiation capacity of CSCs is considered to cause phenotypic and functional heterogeneity in tumors, which results in difficulty in treating cancers.
Several methods that utilize flow cytometry are currently used to identify CSCs (12). Cell surface markers were first used to distinguish tumor-initiating CSCs from other non-CSCs in acute myeloid leukemia (5), and these markers have been reported in various cancer types (12). Cell surface markers of CSCs vary by organ, and the representative markers of pancreatic CSCs are CD24, CD44 and epithelial-specific antigen (ESA) (8,13) or CD133 (9). Side population (SP) cells have been used to assess cancer stem-like properties by estimating their drug resistance. SP cells, which are detected as Hoechst 33342 dye-expelling cells in flow cytometry, are thought to expel cancer drugs in a similar manner to the dye, resulting in their drug-resistant properties, and they have been reported in a variety of cancer types (14). They have been reported to have higher tumorigenic properties than non-SP cells; therefore, SP cells are considered to include CSCs. Aldehyde dehydrogenase (ALDH) is known to be highly expressed in CSCs, as in normal stem cells, and ALDH activity is therefore used to distinguish CSCs (15,16). The results are assessed using a combination of in vitro sphere formation and in vivo tumor formation properties.
PRDM14, a member of the PR domain-containing family, possesses multiple zinc fingers and a PR domain, which shares high homology with the SET methyltransferase domain (17). PRDM14 is exclusively expressed in mouse and human embryonic stem cells (ESCs) and murine primordial germ cells, and is required for the maintenance of ESC pluripotency and early differentiation in primordial germ cells (18,19). PRDM14 is not reported to be expressed in adult normal tissues such as the brain, heart, spleen, lung, liver and kidney (19), but is over-expressed in some human cancers (20–22). Recent studies have reported that PRDM14 overexpression is correlated with cell proliferation, colony formation and drug resistance in breast cancer, and migration in non-small cell lung cancer (20,21). Although we recently reported overexpression of PRDM14 in several cancers including pancreatic cancer (22), the detailed expression analysis and function of PRDM14 in pancreatic cancer have not been determined.
microRNAs (miRNAs), short non-coding RNAs that post-transcriptionally regulate gene expression, are known to regulate cancer properties, and various miRNAs function as oncogenes or tumor suppressors according to tumor type (23). miR-125a-3p is a tumor suppressor miRNA that is down-regulated in some cancers, including pancreatic cancer (24–27). miR-125a-3p contains a short sequence that is complementary to the 3′-UTR of the Fyn gene, which encodes a member of the Src family of cytoplasmic tyrosine kinases (SFKs), Fyn. Transfection-induced overexpression of miR-125a-3p was reported to suppress Fyn expression and the activities of downstream proteins, including focal adhesion kinase (FAK) and Akt, resulting in inhibition of cell proliferation and migration in a prostate cancer cell line (28,29). Moreover, Fyn expression has been reported to be correlated to liver metastasis in patients with pancreatic cancer, and Fyn knockdown reduced metastasis in mice (30).
In the present study, we assessed PRDM14 expression and its effect on cancer stem-like properties in pancreatic cancer. Using immunohistochemical analysis, we observed that PRDM14 was overexpressed in pancreatic cancer tissues compared with normal pancreatic tissues. To assess the correlation between PRDM14 expression level and cancer stem-like phenotypes in pancreatic cancer, we used lentiviral shRNA-transduced pancreatic cancer cell lines. To determine the mechanism underlying the effects of PRDM14, we analyzed miRNA expression profiles. Moreover, we assessed the effect of small-interfering RNA (siRNA)-based therapy targeting PRDM14 on liver metastasis of pancreatic cancer in mice.
Materials and methods
Cell culture
PK-1 cell line was obtained from the RIKEN BioResource Center (Tsukuba, Ibaraki, Japan). Panc-1, BxPC-3, AsPC-1, Capan-2 and MIA-PaCa2 cells were purchased from the American Type Culture Collection (Manassas, VA). These cells were identified by the cell banks using short tandem repeat analysis. PK-1 and AsPC-1 are cell lines from metastatic liver and ascites sites, respectively, and Panc-1, BxPC-3, Capan-2 and MIA-PaCa2 cells are from pancreatic ductal adenocarcinomas (PDAC). PK-1 and Panc-1 cells were cultured in Dulbecco’s modified eagle medium (DMEM; Thermo Fisher Scientific, San Jose, CA) containing 10% fetal bovine serum (FBS; GE Healthcare, Piscataway, NJ). BxPC-3, AsPC-1, Capan-2 and MIA-PaCa2 cells were cultured in RPMI medium 1640 (Thermo Fisher Scientific) containing 10% FBS. All cell lines were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Establishment of stable transfectants
Stable transfectants were established using the Lenti-pack HIV Expression Packaging Kit (GeneCopoeia, Rockville, MD) according to the manufacturer’s instructions. The plasmid vectors were control shRNA (CSHCTR001-HIVU6, GeneCopoeia), two anti-human PRDM14 shRNAs (HSH016733-1-HIVU6 [sh#1]: 1087 CTACGGAGACAATTCTGTG and HSH016733-3-HIVU6 [sh#3]: 1299 ATGGAGACTGCTATGAGAA, GeneCopoeia), Halo-tag fusion human PRDM14 (Halo-PRDM14; EX-W1089-Lv110, GeneCopoeia) and Halo-tag fusion EGFP (Halo-con; EX-EGFP-Lv110, GeneCopoeia). PRDM14 knockdown in shRNA-transduced cells was confirmed by western blotting (Supplementary Figure S1A and B, available at Carcinogenesis Online).
DNA-chimeric siRNA
DNA-chimeric siRNA is a double-stranded RNA-DNA chimera, in which the seed region of the siRNA guide strand is replaced with DNA. It is better protected from degradation than non-chimeric siRNA, while maintaining silencing activity equal to that of non-chimeric siRNA (31–33). Two DNA-chimeric siRNAs targeting the coding region of PRDM14 (PRDM14_1082-1104 and PRDM14_1504-1526; si#2 and si#3, respectively) and a negative control DNA-chimeric siRNA (siNEGA), not matching any human genes, were purchased from RNAi Inc. (Tokyo, Japan). The sequences of the DNA-chimeric RNA targeting PRDM14 used were as follows (RNA residues are indicated with lowercase “r”):
PRDM14_1082-1104 (si#2):
GrArCrCrUrArCrGrGrArGrArCAATTCTGT
AGAATTrGrUrCrUrCrCrGrUrArGrGrUrCrUrU
PRDM14_1504-1526 (si#3):
rCrUrCrUrCrUrGrCrArArArCrGATCCTTTG
AAGGATrCrGrUrUrUrGrCrArGrArGrArGrArA
For in vitro experiments, cells were transfected with DNA-chimeric siRNAs using Lipofectamine RNAiMAX Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. For animal injection, DNA-chimeric siRNAs were mixed with poly(ethylene glycol)-poly(L-lysine) (PEG-PLL; a gift from Prof. Kataoka) to obtain DNA-chimeric siRNA-loaded polyion complexes (PICs) to deliver the DNA-chimeric siRNA to tumors more efficiently and to protect it from degradation and kidney filtration (34).
Immunohistochemistry
Tissue microarray (TMA) slides containing pancreatic cancer and normal pancreatic tissues were purchased from Biomax (Rockville, MD; PA243, PA483b and PA811) and ISU ABXIS, Co. (Seoul, Korea; AccuMax array A717III and A207V). TMA slides were stained using anti-PRDM14 antibody (1:250 dilution; ab187881, Abcam Inc., Cambridge, MA), anti-rabbit secondary antibody (EnVision+ Systems; Dako, CA), and ImmPACT DAB substrate (Vector Laboratories, Inc., CA), and counterstained in hematoxylin. Mouse tissues were formalin-fixed, paraffin-embedded (FFPE), and sliced. Slides of mouse tissue were stained with hematoxylin and eosin (H&E). Using light microscopy, TMA tissue sections were scored semi-quantitatively for staining. Labeling scores were determined by multiplying the percentage of positive tumor cells per slide (0–100%) by the dominant staining intensity (0 = negative, 1 = trace, 2 = weak, 3 = intermediate, and 4 = strong). The resulting scores ranged from 0 to 400. Specimens with staining scores of 0–200, 201–400 were classified as low-level and high-level expression, respectively. To analyze the correlation of staining scores for PRDM14 in PDAC tissues with pathological features, we used the pathological data on the datasheet of the purchased TMA.
Western blotting, automated capillary electrophoresis and immunodetection
We used the following primary antibodies: PRDM14 (1:500; ab187881) from Abcam, and GAPDH (1:200; #5174), Fyn (1:200; #4023), FAK (1:200; #3285), phospho-FAK (1:200; Tyr397, #8556), Akt (1:200; #4691) and phospho-Akt (1:50; Ser473, #4060) from Cell Signaling Technology Inc. (MA). The primary antibodies were detected with HRP-conjugated goat anti-rabbit or anti-mouse antibodies and enhanced chemiluminescence (GE Healthcare) for western blotting according to the manufacturer’s instructions. We also used an automated capillary electrophoresis and immunoassay system, Wes (ProteinSimple, CA). All procedures were performed using the manufacturer’s reagents according to the manufacturer’s standard protocol. Briefly, cell lysate was mixed with a master mix containing SDS and heated at 95°C for 5 min. The samples, blocking reagent, wash buffer, primary antibodies, secondary antibodies and chemiluminescent substrate were dispensed into the pre-filled microplate provided by the manufacturer. Electrophoresis and immunodetection were performed automatically in Wes using the default settings. The data were analyzed using the built-in Compass software.
Sphere-formation assay
Cells were suspended in sphere formation medium (DMEM/F12 medium supplemented with 50 ng/ml epidermal growth factor, 50 ng/ml basic fibroblast growth factor and 1% B27 supplement; all from Thermo Fisher Scientific), seeded in each well (1 × 103 cells per well) of Ultra-Low Attachment 24-well plates (Corning, NY), and incubated at 37°C in a humidified atmosphere containing 5% CO2. After a 2-week incubation, sphere numbers were counted under a microscope.
Flow cytometry
To isolate SP cells, cells in suspension were incubated with Hoechst 33342 dye (Sigma-Aldrich, St. Louis, MO) for 90 min at 37°C with or without Reserpine (Sigma-Aldrich). The cells were washed with ice-cold PBS and stained with 7-amino actinomycin D (7-AAD) (BD Pharmingen, CA). The cells were analyzed using a FACSAria flow cytometer (BD Biosciences, CA). To analyze cell surface markers, cells were stained with BV421-CD24 (Biolegend, CA), APC-H7-CD44, APC-EpCAM (both from BD Pharmingen), PE-CD133/2 (Miltanyi Biotech, Bergisch Gladbach, Germany) and 7-AAD. The ALDH assay was performed using ALDEFLUOR reagent (STEMCELL Technologies, Vancouver, Canada) according to the manufacturer’s instructions. The ALDH inhibitor N-diethylaminobenzaldehyde (DEAB) was used as a negative control. ALDEFLUOR-treated cells were stained with 7-AAD. These cells were analyzed using a FACS Aria or a FACS Verse flow cytometer (BD Biosciences).
Animals
Six-week-old male BALB/c-nu/nu mice were obtained from CLEA Japan, Inc. (Tokyo, Japan). Liver metastases were induced through splenic injection of pancreatic cancer cells. Before the inoculation, cells were trypsinized, washed and resuspended in HBSS. Approximately 1 × 106 cells in 0.05 ml HBSS were injected into the spleen of the mice and the spleen was removed surgically 4 min later to avoid peritoneal dissemination and tumorigenesis in the spleen. In a treatment model, the mice with experimental liver metastasis were injected with DNA-chimeric siRNA-loaded PICs (25 μg siRNA per mouse) through the tail vein three times per week for 1 month (twelve injections in total) from the day following cell injection. shRNA-transduced PK-1 or MIA-PaCa2 cells were injected into six or eight mice per group, respectively. In the treatment model, 18 mice were injected with wild-type MIA-PaCa2 cells, divided into three groups, and treated with DNA-chimeric siRNA-loaded PICs (siNEGA, si#2 or si#3). Liver metastases were confirmed at 2 months after cell injection. In the subcutaneous xenograft tumor model, plasmid-transduced AsPC-1 cells were suspended in a 1:1 mixture of PBS and matrigel (Corning) and subcutaneously injected into a mouse (1 × 106 cells per mouse). Xenograft tumors were removed 4 weeks after the injection. All animal experiments were performed in accordance with the guidelines for the care and use of laboratory animals of the University of Tokyo and were approved by the Institutional Animal Care and Use Committee of the University of Tokyo.
miRNA array and assay
TaqMan MicroRNA arrays and TaqMan MicroRNA assays (both from Thermo Fisher Scientific) were performed according to the manufacturer’s instructions. Briefly, we prepared total RNA, including miRNA, from the cells using the mirVana miRNA Isolation Kit. Total RNA was transcribed using the TaqMan MicroRNA Reverse Transcription Kit with the Megaplex RT Primers (Pool A v2.1 and Pool B v3.0) for miRNA array or with the RT primers for miRNA assays. The transcribed samples were amplified and quantified by quantitative PCR using TaqMan Universal PCR Master Mix and the TaqMan Array Human MicroRNA A+B Cards Set v3.0 or the TaqMan Small RNA Assay with ViiA7. For the miRNA assay, RNU-48 was used as an endogenous control. All reagents were purchased from Thermo Fisher Scientific. miRNA data were analyzed using the Expression Suite software. Only the results for sh#1-transduced PK-1 and sh#3-tranceduced MIA-PaCa2 cells are shown in Figure 4C–N, and all results for shPRDM14-transduced cells using the miRNA inhibitor are shown in the Supplementary figures.
In vitro cell-based assays
In the wound healing assay, PK-1 and MIA-PaCa2 cells were seeded into 24-well tissue culture plates (1 × 105 cells per well) and transfected using Lipofectamine RNAiMAX Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. shRNA-transduced cells were transfected with a negative control (NC) or an miR-125a-3p inhibitor, and wild-type cells were transfected with DNA-chimeric siRNA. The cells were slowly scratched with a new 200-μl pipette tip more than 24 h after transfection and washed with medium to remove detached cells. Cell migration distance was measured under a microscope at 0 and 24 h post scratch. The proliferation assay using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan), invasion assay using CytoSelect 96-well Cell Invasion (Fluorometric; Cell Biolabs, Inc., MA), adhesion assay using CytoSelect 48-well Cell Adhesion Assay (Fluorometric; Cell Biolabs, Inc.), and apoptosis assay using ApoTox-Glo Triplex Assay kit (Promega, WI) were performed according to the manufacturer’s instructions, respectively.
Statistical analyses
Quantitative data are presented as the mean ± SD in graphs. The P-values for comparisons between groups were determined using a Student’s t-test or a one-way ANOVA. P-values < 0.05 were considered statistically significant.
Results
PRDM14 is expressed in pancreatic cancer tissues and cells
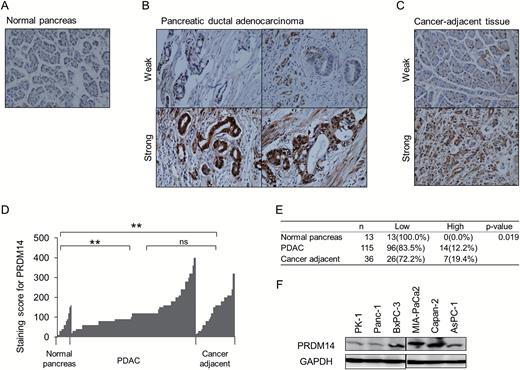
We initially performed immunohistochemistry using the anti-PRDM14 antibody on FFPE TMA slides containing PDAC, normal pancreas and cancer-adjacent tissues, which were histologically non-neoplasmic areas around the tumor (Figure 1A–C). We found that PRDM14 was overexpressed in PDAC compared with pancreatic normal tissues (P < 0.002) (Figure 1D and E). Intense nuclear staining for PRDM14 was observed in the ducts of PDAC, while strong cytoplasmic staining of the duct was often exhibited in strongly stained tissues (Figure 1B). In addition, cancer-adjacent tissues also exhibited strong staining for PRDM14, similar to PDAC (P > 0.05) compared to normal pancreas (P < 0.002) (Figure 1C–1E). Staining scores for PRDM14 in PDAC tissues was not correlated with pathological features such as tumor stage and differentiation score (P > 0.05) (Supplementary Figure S1C, available at Carcinogenesis Online).
Expression of PRDM14 in pancreatic cancer tissues and cell lines. (A–E) Representative immunohistochemistry images for PRDM14 in 13 normal pancreas (A), 115 pancreatic ductal adenocarcinomas (PDAC) (B) and 36 cancer-adjacent (C) tissues. PDAC and cancer-adjacent tissues are shown with low and high staining intensity. Staining scores for PRDM14 in the tissues are shown in (D and E). **P < 0.002, ns: not significant. (F) Western blotting of PRDM14 protein expression in six pancreatic cancer cell lines. GAPDH was used as a loading control.
Next, we tested PRDM14 expression in six pancreatic cancer cell lines: PK-1, AsPC-1, Panc-1, BxPC-3, Capan-2 and MIA-PaCa2 cells. All cell lines expressed PRDM14 at the mRNA (Supplementary Figure S1D, available at Carcinogenesis Online) and protein levels (Figure 1F). We mainly used the PK-1, from metastatic liver, and high metastatic MIA-PaCa2 cell lines, which are appropriate models for liver metastasis, in further experiments because none of the six cell lines exhibited all phenotypes of CSC. We also used other cell lines, for the CSC phenotypes that these two cell lines do not exhibit.
Stable knockdown of PRDM14 suppresses some stem-like properties
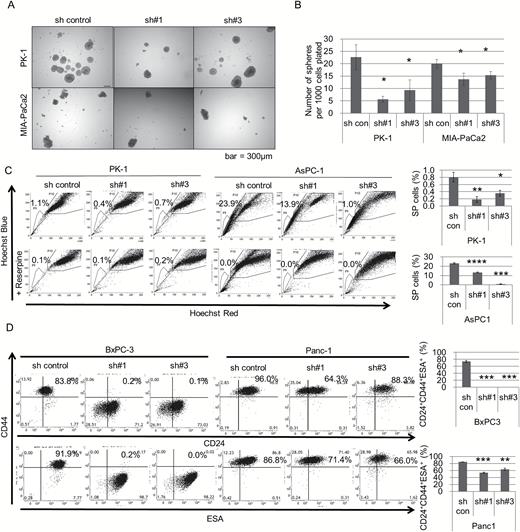
To examine the relationship between PRDM14 expression level and cancer properties, we performed experiments using PRDM14 knockdown pancreatic cancer cells. Five pancreatic cancer cell lines (PK-1, MIA-PaCa2, Panc-1, AsPC-1 and BxPC-3 cell lines) were transduced with shPRDM14 or with control shRNA plasmids using lentiviral vectors. In the sphere-formation assay, we observed that the number of spheres formed by shPRDM14-transduced cells was lower than that of the control shRNA-transduced cells in the PK-1 and MIA-PaCa2 cell lines (P < 0.05) (Figure 2A and B), whereas sphere size did not differ and shRNA did not typically decrease cell proliferation (Supplementary Figure S2A and B, available at Carcinogenesis Online). Subsequently, we analyzed CSC markers. Because MIA-PaCa2 cells include few SP cells, as reported previously (35) (Supplementary Figure S2C, available at Carcinogenesis Online), we used PK-1 and AsPC-1 cells for SP cells. In both cell lines, more SP cells were observed in control cells than in shPRDM14 cells (P < 0.05) (Figure 2C), suggesting that PRDM14 knockdown reduced the quantity of SP cells with a high dye-expelling capacity. ALDH activity of PK-1 and MIA-PaCa2 cells was not decreased by PRDM14 knockdown (Supplementary Figure S2D, available at Carcinogenesis Online), and the other three cell lines, which were reported to have a smaller ALDH-high population (36,37), were not tested. The cell surface marker profiles of PK-1 and MIA-PaCa2 cells were not that of pancreatic CSC, that is CD44+CD24+ESA+ or CD133+, and PRDM14 knockdown did not change the profile (CD44+CD24-ESA+CD133− and CD44+CD24−ESA−CD133−, respectively) (Supplementary Figure S2E, available at Carcinogenesis Online). In Panc-1 and BxPC-3 cells, which include CD44+CD24+ESA+ cells, the positive cells were decreased by PRDM14 knockdown (P < 0.01) (Figure 2D) Interestingly, the markers changed by PRDM14 knockdown differed between the cell lines. However, CD133 expression was negative in both cell lines, and did not change (Supplementary Figure S2F, available at Carcinogenesis Online).
Effect of PRDM14 knockdown on cancer stem-like properties in vitro. (A, B) Sphere formation assay using control shRNA- and shPRDM14-transduced PK-1 and MIA-PaCa2 cells. The spheres were captured (A) and counted (B) after a 2-week incubation. Scale bars in image, 300μm. Error bars represent the mean ± SD of triplicate samples. *P < 0.05. (C) SP cells in shRNA-transduced PK-1 and AsPC-1 cells analyzed by flow cytometry using Hoechst 33342 dye. Reserpine, a multi-drug transporter inhibitor, was used as a control for SP. Error bars in graphs represent the mean ± SD. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. (D) Expression of CD24, CD44 and ESA in shRNA-transfected BxPC-3 and Panc-1 cells as analyzed by flow cytometry. Error bars in graphs represent the mean ± SD. ***P < 0.001, **P < 0.01.
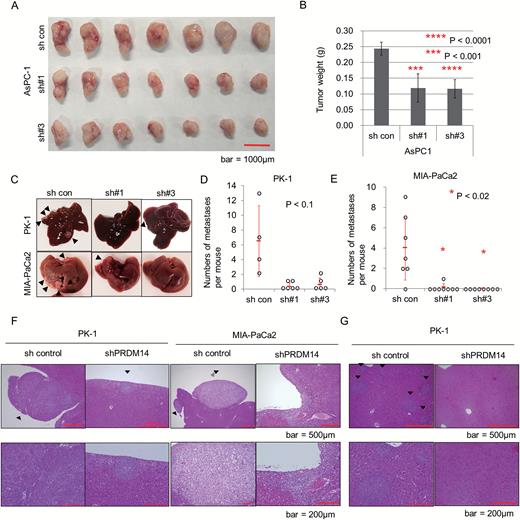
To further assess tumor formation and the metastatic capacity of the PRDM14-knockdown pancreatic cancer cells in vivo, we used mouse models of subcutaneous xenograft and experimental liver metastasis. In the xenograft model of shRNA-transduced AsPC-1 and PK-1 cells, shPRDM14-transduced cells formed smaller tumors than control cells (P < 0.05) (Figure 3A and B, Supplementary Figure S3A and S3B, available at Carcinogenesis Online) (22). PRDM14 overexpression in AsPC-1 and PK-1 cells (Supplementary Figure S3C, available at Carcinogenesis Online) did not significantly increase tumor volume compared to controls (Supplementary Figure S3D and E, available at Carcinogenesis Online). Liver metastases were induced through splenic injection of shRNA-transduced pancreatic cancer cells. Liver metastases were assessed only in those mice that were sacrificed at two months because two of the six mice in the control PK-1 group and one of the eight mice in the control MIA-PaCa2 group died before liver metastases could be fully evaluated. The control PK-1 and MIA-PaCa2 cells developed many metastases on the liver surface, while the shPRDM14-transduced cells developed few or no metastases (Figure 3C–F); the difference between the shPRDM14 and control groups of MIA-PaCa2 cells was particularly significant (P < 0.02), while that of PK-1 barely failed to attain statistical significance (P < 0.10) (Figure 3D and E). In addition, control PK-1 cells developed additional micro-metastases inside the livers, while shPRDM14 cells did not (Figure 3G). Body size did not differ between groups (Supplementary Figure S3F, available at Carcinogenesis Online).
Effect of PRDM14 knockdown on a mouse model of xenograft tumor and liver metastasis. (A, B) Subcutaneous xenograft tumor of shRNA-transduced AsPC-1 cells. Scale bar, 1000μm. Error bars in graphs represent the mean ± SD of tumors (n = 7). ****P < 0.0001, ***P < 0.001. (C–G) Experimental model of liver metastasis using shRNA-transduced cells. shRNA-transduced PK-1 and MIA-PaCa2 cells were introduced through splenic injection into balb/c-nu/nu mice. Two months after the injection, the livers were removed and the number of liver metastases was estimated. Representative images of liver tissue (C, Arrowheads: liver metastases), number of PK-1 and MIA-PaCa2 liver metastases (D and E, respectively), and H&E staining of the metastases (F) and micro-metastases inside the liver in the groups treated with shRNA-transduced PK-1 (G). Error bars in graphs represent the mean ± SD of mice (n = 4–8 per group). *P < 0.02. Scale bars in H&E staining, 500μm (top) and 200μm (bottom).
Stable knockdown of PRDM14 changes the miRNA expression profile of pancreatic cancer cells
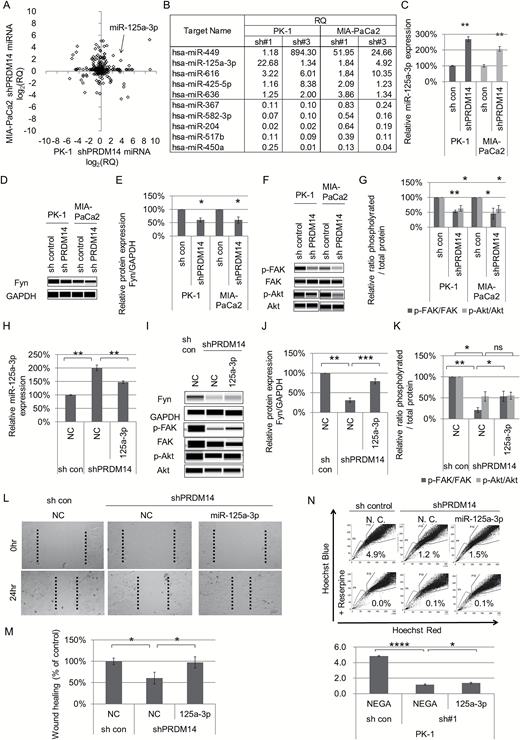
The above experiments using shRNA-transduced pancreatic cancer cells suggested that the stable knockdown of PRDM14 reduced some CSC phenotypes in vitro and in vivo. Therefore, to determine the molecular mechanism underlying the PRDM14-mediated tumor regulation, we analyzed the miRNA profile of shRNA-transduced cells. miRNA array analysis revealed that five miRNAs were increased and five miRNAs were decreased in two shPRDM14-transduced cells compared to control cells, commonly in both cell lines (Figure 4A and B). We then performed an miRNA assay for miR-125a-3p, a tumor suppressor miRNA that is down-regulated in pancreatic cancer, and confirmed its up-regulation in shPRDM14 cells (P < 0.01) (Figure 4C).
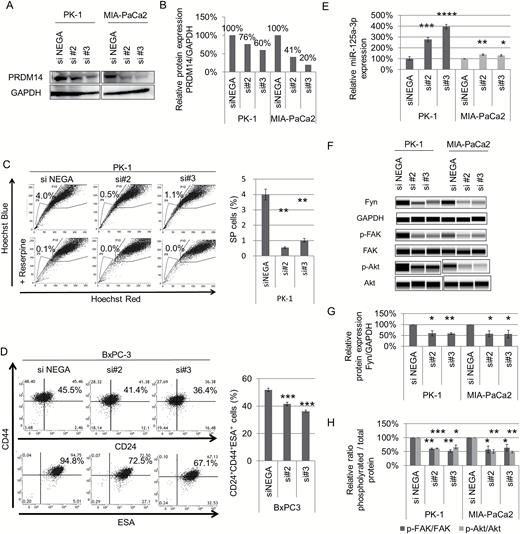
Effect of PRDM14 knockdown on miRNA expression profiles. (A, B) miRNA arrays of shRNA-transduced PK-1 and MIA-PaCa2 cells. miRNA expression levels of shPRDM14 cells were normalized to that of control shRNA cells (relative quantity; RQ). (A) The graph plots the log2 of the RQ (average of two shPRDM14) of PK-1 shPRDM14 (x-axis) versus that of MIA-PaCa2 shPRDM14 (y-axis). (B) List of the RQ of miRNAs in shPRDM14 cells, which are increased or decreased commonly in all shPRDM14 cells (two sequences in PK-1 and MIA-PaCa2). These are listed in descending order of the average of both cells. (C) miRNA assays for miR-125a-3p. Error bars represent the mean ± SD of triplicate samples. **P < 0.01. (D, E) Expression levels of Fyn protein analyzed using Wes, normalized to that of GAPDH, and plotted as fold changes relative to control. A gel image (D) and relative Fyn protein expression (E) are shown. Error bars represent the mean ± SD. *P < 0.05. (F, G) Expression levels of FAK, phospho-FAK (Tyr397), Akt, and phospho-Akt (Ser473) analyzed using Wes. Levels of phosphorylated proteins were normalized to that of total protein and plotted as the fold change relative to controls. A gel image (F) and relative phosphorylated protein expression (G) are shown. Error bars represent the mean ± SD. **P < 0.01, *P < 0.05. (H–N) The treatment effect of the miR-125a-3p inhibitor on the phenotype of shPRDM14 cells. The expression of miR-125a-3p (H), the downstream (I), Fyn expression (J), and activities of FAK and Akt (K), a wound healing assay (L, M) and SP cells (N) are shown. Error bars represent the mean ± SD of triplicate samples. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, ns: not significant.
To examine the participation of miR-125a-3p and Fyn in the tumor-suppression effect of the shPRDM14 pancreatic cells, we examined the expression level and downstream signaling activation of Fyn. The expression level of Fyn protein in shPRDM14 cells was lower than that in control cells (P < 0.05) (Figure 4D and E). The active state ratios of FAK and Akt, which are located downstream of Fyn, were decreased (P < 0.05) (Figure 4F and 4G). In addition, miR-125a-3p inhibitor reduced miR-125a-3p expression (P < 0.01) (Figure 4H and Supplementary Figure S4A, available at Carcinogenesis Online) resulting in increases of Fyn expression and active FAK (P < 0.05) (Figure 4I–K and Supplementary Figure S4B–E, available at Carcinogenesis Online), wound healing ability (P < 0.05) (Figure 4L, M and Supplementary Figure S4F, available at Carcinogenesis Online), and SP cells (P < 0.05) (Figure 4N and Supplementary Figure S4G, available at Carcinogenesis Online) in shPRDM14 cells. However, active Akt was not changed by miR-125a-3p inhibitor (Figure 4K and Supplementary Figure S4E, available at Carcinogenesis Online). These data suggested that up-regulation of miR125a-3p suppresses Fyn expression and activities of its downstream targets related to tumor regulation in shPRDM14-transduced pancreatic cancer cells.
Treatment with DNA-chimeric siRNA targeting PRDM14 suppresses liver metastasis in mice
The above results suggested that the stable knockdown of PRDM14 decreases CSC phenotypes, including in vivo tumor growth. Therefore, we assessed the effects of treatment with siRNA-based therapy targeting PRDM14 on pancreatic cancer cells.
Knockdown efficiency of PRDM14 protein in cells treated with DNA-chimeric siRNA was confirmed by western blotting (Figure 5A and B, and Supplementary Figure S5A, available at Carcinogenesis Online). PRDM14 knockdown using DNA-chimeric siRNA also reduced SP cells in PK-1 and AsPC-1 cells (P < 0.01) (Figure 5C and Supplementary Figure S5B, available at Carcinogenesis Online) and decreased CD44+CD24+ESA+ cells in BxPC-3 and Panc-1 cells (P < 0.05) (Figure 5D and Supplementary Figure S5C, available at Carcinogenesis Online). In miRNA assays, miR-125a-3p was up-regulated by DNA-chimeric siRNA targeting PRDM14 (P < 0.05) (Figure 5E) similar to shRNA-transduced cells. Downstream of miR-125a-3p, Fyn expression and the active state ratios of FAK and Akt were also decreased (P < 0.05) (Figure 5F–H). These data indicated that the treatment with DNA-chimeric siRNA targeting PRDM14 reduced some CSC phenotypes in vitro and regulated miR-125a-3p expression and the downstream as well as stable knockdown in shPRDM14-transduced cells.
Effect of DNA-chimeric siRNA targeting PRDM14 on pancreatic cancer cells. (A, B) PK-1 and MIA-PaCa2 cell lines were treated with DNA-chimeric siRNA targeting PRDM14. The efficiency of PRDM14 knockdown, confirmed by western blotting (A). Expression levels of PRDM14 protein were normalized to that of GAPDH and plotted as fold changes relative to control (B). (C) SP cells in DNA-chimeric siRNA-treated PK-1 cells. Error bars in graphs represent the mean ± SD. **P < 0.01. (D) Expression of CD24, CD44 and ESA in the siRNA-treated BxPC-3 cells, as analyzed by flow cytometry. Error bars in graphs represent the mean ± SD. ***P < 0.001. (E) miRNA assays for miR-125a-3p in DNA-chimeric siRNA-treated cells. Error bars represent the mean ± SD of triplicate samples. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. (F–H) Expression levels of Fyn, FAK, phospho-FAK (Tyr397), Akt, and phospho-Akt (Ser473) analyzed using Wes. Gel image (F), relative Fyn protein expression (G), and relative phosphorylated protein expression (H) are shown. Error bars in graphs represent the mean ± SD. ***P < 0.001, **P < 0.01, *P < 0.05.
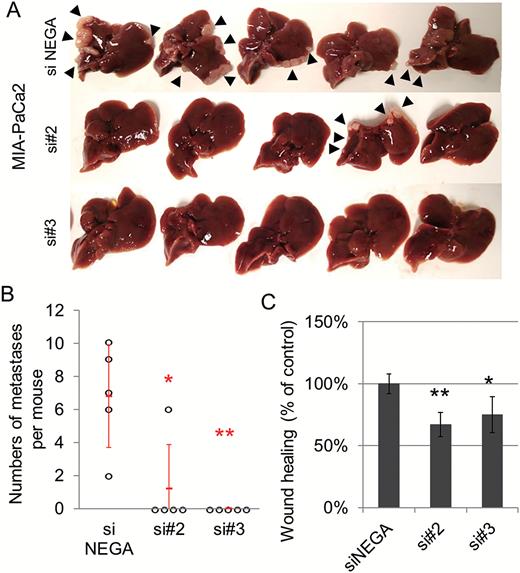
Next, as a cancer treatment model targeting PRDM14, we used an experimental liver metastasis model. Eighteen mice were injected with wild-type MIA-PaCa2 cells, and 15 of the 18 mice injected with MIA-PaCa2 were divided to three groups (five mice per group) and treated with three DNA-chimeric siRNA-loaded PICs. Treatment with two siRNAs targeting PRDM14 significantly decreased the number of metastases on the liver surface compared with siNEGA (P < 0.02) (Figure 6A and B). Treatment with DNA-chimeric siRNAs targeting PRDM14 did not decrease the body size of mice compared with siNEGA (Supplementary Figure S6A, available at Carcinogenesis Online). To identify the mechanism by which PRDM14 knockdown decreases metastasis, we assessed its cellular properties. Migration in the wound healing assay was reduced in DNA-chimeric siRNA transfected cells (P < 0.05) (Figure 6C). However, invasion, proliferation, apoptosis and adhesion (Fibronectin, Collagen I and Collagen IV) capacity remained unchanged (Supplementary Figure S6B–E, available at Carcinogenesis Online). These data suggested that treatment with siRNA-based therapy targeting PRDM14 decreased metastasis of pancreatic cancer cells, regulating the migration process of metastasis.
(A, B) Experimental liver metastasis model with DNA-chimeric siRNA treatment. The mice were given splenic injections of MIA-PaCa2 cells and treated with DNA-chimeric siRNA-loaded PIC 3 times per week for 1 month. After 2 months of cell injection, the livers were removed and the number of liver metastases was estimated. All livers (A)and the number of liver metastases (B) are shown. Error bars in the graph represent the mean ± SD of the mice (n = 5 per group). **P < 0.01, *P < 0.02. (C) Wound healing assay using DNA-chimeric siRNA–treated cells. **P < 0.01, *P < 0.05. Error bars represent the mean ± SD of triplicate samples.
Discussion
Pancreatic cancer is one of the most aggressive forms of cancer, with a poor prognosis and survival rate. CSCs are considered a good target for cancer research because they are responsible for cancer proliferation, metastasis and chemoresistance. PRDM14 is known to maintain pluripotency in ESCs and to be overexpressed in some cancers. In this study, we assessed whether PRDM14 plays a role in pancreatic cancer and its cancer stem-like phenotypes.
PRDM14 was overexpressed in pancreatic cancer tissues compared with normal pancreas tissues, but the expression level did not correlate with cancer stage or differentiation. According to previous studies, PRDM14 suppresses differentiation in ESCs and correlates with poor differentiation in non-small cell lung cancer (18,38). To confirm the relationship between PRDM14 expression and differentiation in pancreatic cancer, a larger sample size is needed, because only a small portion of our samples included the differentiation score. Meanwhile, the lack of correlation between PRDM14 expression and cancer stage observed here has also been reported in non-small cell lung cancer (38). PRDM14 has been reported to be associated with initiation of several cancers (21,39,40), suggesting that PRDM14 overexpression might occur before tumor initiation. Interestingly, we also observed high PRDM14 staining in non-neoplasmic cancer-adjacent tissues as well as cancer tissues in immunohistochemistry. Combination of PRDM14 overexpression with other events, such as mutations or changes in expression levels of other genes, might lead to tumor initiation.
Although the relationship between PRDM14 and miRNA regulation has not previously been reported, we observed changes in miR-125a-3p expression in PRDM14-knockdown pancreatic cancer cells. The tumor suppressor miR-125a-3p has been reported to be down-regulated in pancreatic cancer compared with normal controls, and has been suggested to be a good marker for detecting the disease (24). Regulation of these miRNAs may be a critical role of PRDM14 knockdown for tumor suppression.
Our data suggest that the increase in miR-125a-3p caused by PRDM14 knockdown decreased Fyn expression in pancreatic cancer cells. Fyn regulation by miR-125a-3p was recently reported in the regulation of cell proliferation and migration in the HEK293T cell line, and in ovarian and prostate cancers (28,29,41). SFKs, including Fyn, are known to be involved in diverse signaling pathways via cell-surface receptors and to regulate many biological events such as proliferation, adhesion, migration and differentiation (42). They have been reported to be overexpressed in various cancers (43,44) and previous studies have reported that Fyn inhibition by SFK inhibitors or siRNA targeting Fyn suppresses the proliferation, migration, and invasion phenotypes of pancreatic cancer cell lines (45–47). Moreover, Fyn expression is correlated with metastasis in patients with pancreatic cancer and Fyn knockdown reduces primary tumor and liver metastasis in mice (30). Taken together, these data indicate that Fyn is an important target for cancer treatment. However, the complexities of both cancer regulation and SFK-related signaling pathways cause difficulty in cancer treatment when SFK inhibitors are used as single agents. In addition, Fyn plays important roles in normal tissues, and Fyn knockout causes defects in neural function, and the disruption of Fyn and another SFK members results in T cell and osteoclast defects in mice (42,48,49). In contrast, PRDM14 is reported to be expressed in ESCs and primordial germ cells, but has no or scarce expression in adult normal tissues. Therefore, knockdown of PRDM14 is considered to have no/few side effects on normal adult tissues of patients; however, further experiments should be conducted to confirm the side effects and off-target effects prior to clinical use, although PRDM14 expression is considered to be absent/scarce in normal adult tissues and the siRNA was designed to produce minimal off-target effects in humans. Our results indicate that PRDM14, as an upstream protein in the Fyn-miR-125a-3p pathway, is a favorable target for pancreatic cancer treatment.
In summary, PRDM14 was overexpressed in pancreatic cancer tissues, and PRDM14 knockdown reduced some CSC phenotypes in pancreatic cancer cells. PRDM14 knockdown also increased miR-125a-3p expression, and subsequently down-regulated Fyn expression, which is reported to regulate cancer phenotypes in pancreatic cancer. Treatment using DNA-chimeric siRNA targeting PRDM14 also reduced liver metastasis in a mouse model. These results suggest that knockdown of PRDM14 reduced CSC phenotypes via miR-125a-3p and regulation of Fyn expression in pancreatic cancer, and PRDM14 may be a powerful target for the treatment of pancreatic cancer.
Supplementary material
Supplementary data are available at Carcinogenesis online.
Funding
This work was supported by funding from the “Practical Research for Innovative Cancer Control” of the Japan Agency for Medical Research and Development (H.T.) and Chugai Pharmaceutical Co., Ltd. (H.T., K.I.).
Abbreviations
- ALDH
aldehyde dehydrogenase
- CSC
cancer stem cell
- ESA
epithelial-specific antigen
- ESC
embryonic stem cell
- FAK
focal adhesion kinase
- PDAC
pancreatic ductal adenocarcinoma
- SFK
Src family of cytoplasmic tyrosine kinase
- siRNA
small interfering RNA
- SP
side population
- TMA
tissue microarray
Acknowledgments
We thank Naohiko Koshikawa and Daisuke Hoshino for helpful discussions.
Conflict of Interest Statement: None declared.
References
Author notes
The authors contributed equally to this work.