-
PDF
- Split View
-
Views
-
Cite
Cite
Jean-Christophe Lagier, Camille Aubry, Marion Delord, Pierre Michelet, Hervé Tissot-Dupont, Matthieu Million, Philippe Brouqui, Didier Raoult, Philippe Parola, From Expert Protocols to Standardized Management of Infectious Diseases, Clinical Infectious Diseases, Volume 65, Issue suppl_1, 15 August 2017, Pages S12–S19, https://doi.org/10.1093/cid/cix403
Close - Share Icon Share
Abstract
We report here 4 examples of management of infectious diseases (IDs) at the University Hospital Institute Méditerranée Infection in Marseille, France, to illustrate the value of expert protocols feeding standardized management of IDs. First, we describe our experience on Q fever and Tropheryma whipplei infection management based on in vitro data and clinical outcome. Second, we describe our management-based approach for the treatment of infective endocarditis, leading to a strong reduction of mortality rate. Third, we report our use of fecal microbiota transplantation to face severe Clostridium difficile infections and to perform decolonization of patients colonized by emerging highly resistant bacteria. Finally, we present the standardized management of the main acute infections in patients admitted in the emergency department, promoting antibiotics by oral route, checking compliance with the protocol, and avoiding the unnecessary use of intravenous and urinary tract catheters. Overall, the standardization of the management is the keystone to reduce both mortality and morbidity related to IDs.
The management of infectious diseases (IDs) remains an important challenge for clinicians. Complexity of handling IDs suggests that ID experts were better at caring for IDs than other physicians [1, 2]. Also, stringent antibiotic stewardship programs have demonstrated a significant impact on ID outcome including life-threatening infections such as endocarditis [3, 4]. The compliance of clinicians with their established treatment protocols must be evaluated before reaching the conclusion of “failure” during antibiotic therapy [5, 6], as this critical point is difficult to analyze in multicenter studies [7]. Furthermore, device-associated infections including catheter-associated bloodstream infection and catheter-associated urinary tract infections are among the most frequently encountered life-threatening healthcare infections, which requires the avoidance of unnecessary indwelling catheter devices and an appropriate strategy of oral antibiotics [8, 9].
To highlight our contribution in the rational management of IDs, we present hereby 4 exemplary cases in the management of IDs in the University Hospital Institute (IHU) Méditerranée Infection in Marseille, France. First, we report our 30 years of expertise and management regarding intracellular bacterial infections such as Q fever and Tropheryma whipplei, based on both in vitro data and clinical outcome. Second, we described our 15 years of experience with a management-based approach for the treatment of infective endocarditis (IE), with important reductions in the mortality rate [3]. Third, we report the protocol of using fecal microbiota transplantation to decolonize Clostridium difficile and emerging highly resistant bacteria [10, 11]. Finally, since 2015, we have set up an acute ID unit dedicated to the standardized management of acute infections in patients admitted through the emergency department. We established protocols under the expertise of both emergency care and ID specialists to treat the most frequently encountered infectious syndromes, and promoting the preferential use of antibiotics through the oral route. We checked compliance with this protocol, and we monitored the need for both blood and urinary tract catheters in these and other hospital-based patients [12, 13].
Q Fever and Tropheryma Infections
Q Fever
Since 1985 to December 2015, we tested in our function of the French National Referral Center for Q fever 286273 samples from France and abroad for Coxiella burnetii [14]. Our current cohort of patients includes 1954 patient files and since 2007, 1784 are considered to have an active infection (1382 acute Q fever, 492 patients with persistent focal infection, 90 with both acute Q fever and a subsequently persistent focalized infection [15]). Infected patients were identified from all over France (including overseas territories such as La Reunion and French Guiana) and other countries (mainly Italy, the United Kingdom, and Israel). This allowed us to capture, mine, and analyze all the heterogeneity of clinical expressions and complications of the infection by different C. burnetii clones in different human populations. Our role as a reference center with a clinical personal experience of >30 years (D. R.), thorough analysis of our cohort database [15, 16], the inclusion of new technologies (specific polymerase chain reaction [PCR], 18F deoxy-glucose positron emission tomography combined with computed tomography [PET/CT]) [17, 18], the study of misleading classifications by other teams [19], and the questioning of our recommendations [19] led us to establish new standardized diagnostic criteria and therapeutic protocols (Supplementary Tables 1–9) [17].
Formerly, patients infected with C. burnetii were classified into only 2 medical conditions: acute and chronic Q fever. Our work on C. burnetii infections helped to accurately define 10 medical conditions linked to C. burnetii infection (Supplementary Tables 1–6), classifying 98.8% (1764/1784) of our patients considered as infected. These definitions contributed to the establishment of more specific treatment protocols (Supplementary Tables 7 and 8) in the context of primary infection and 5 treatment protocols for cardiovascular infections (Supplementary Table 9). Each of them corresponds to a specific treatment or management (Supplementary Tables 7–9), in the scope of laboratory-based personalized medicine.
By clarifying the clinical management of patients infected by C. burnetii, we demonstrated that the “chronic Q fever” term should be banned and replaced for “persistent focalized Q-fever infection” [17]. Indeed, endocarditis could occur during acute Q fever [15, 20], requiring a carefully adapted treatment. Moreover, the “chronic Q fever” term is a mix of very different medical conditions leading to inadequate management [18]. We believe that the newly proposed diagnostic criteria and treatment protocols [17] significantly and dramatically improved the care of C. burnetii–infected patients. Using these criteria and protocols available online (http://en.mediterranee- infection.com/article.php?laref=157&titre=q-fever-treatment), any ID specialist (and/or medical doctor) is able to classify and treat 97% of patients infected by C. burnetii. Obviously, an expert opinion remains essential for patients with unfavorable serological outcome (serological failure or relapse) despite careful protocol application or for the 3% of patients with a rare presentation. Reporting such cases to our worldwide expert center (failure, relapse, rare cases) will contribute to ongoing studies on the classification and treatment of these particular situations, and will lead to the proposition of new evidence-based protocols improving the global care for C. burnetii–infected patients.
Tropheryma whipplei
Tropheryma whipplei is the causative agent of Whipple’s disease, which is defined by the characteristic histological involvement in small-bowel biopsies (positive periodic acid-Schiff staining and or immunohistochemistry). This bacterium can caused localized chronic infections without digestive involvement, mainly endocarditis, encephalitis, uveitis, and osteoarticular infections [21]. Since the first culture of T. whipplei performed in our laboratory in 2000 [21], we have diagnosed >300 T. whipplei infections; some of these patients were referred to one of us (D. R.) for expert management. We are also sometimes contacted by patients or physicians for an opinion concerning a second-line treatment [22].
Once we made available for the first time the possibility of culturing T. whipplei, susceptibility tests were carried out that were able to explain why trimethoprim-sulfamethoxazole, the most frequent empiric treatment proposed, is frequently ineffective [7, 21]. First, T. whipplei proved to be naturally resistant to trimethoprim, and acquired resistance to sulfamethoxazole was frequent. A recent study has demonstrated that 25.9% of the T. whipplei strains were resistant to sulfamethoxazole [21]. In addition, the association of doxycycline and hydroxychloroquine was shown as the sole bactericidal treatment for T. whipplei. Second, the lifetime susceptibility of the patients with Whipple disease to T. whipplei highlighted reinfections caused by different genotypes, leading us to propose lifetime treatment and monitoring [23]. Indeed, we demonstrated by in vitro studies, then clinical outcomes studies, that a 1-year treatment with an association of doxycycline (200 mg per day) and hydroxychloroquine (600 mg per day), followed by lifetime treatment with doxycycline was the most appropriate treatment for patients with Whipple’s disease [7]. Lifetime surveillance including antibiotic serum dosages to monitor the patient’s compliance is required [7]. For endocarditis, the same lifetime treatment is required as we demonstrated that T. whipplei endocarditis can transform secondarily into classic Whipple’s disease [24]. We propose the same management for encephalitis because of frequent relapses. For other localized chronic infections, we suggest a combination of doxycycline and hydroxychloroquine for 12–18 months’ duration [7], followed by a lifetime surveillance. All these protocols are available for physicians on our website (http://www.mediterranee-infection.com/article.php?larub=65&titre=les-protocoles-therapeutiques).
INFECTIVE ENDOCARDITIS
Despite improvements in its management, IE is a deadly disease that remains associated with high mortality and severe complications. Antibiotics remain the central pillar in the treatment of IE, but it is of first importance to rely on an “endocarditis team,” including cardiologists, microbiologists, ID specialists, and surgeons with a very high level of expertise [3]. This collaboration allows an early cardiologic diagnosis (clinic, echocardiography, imaging, PET scan) [3, 25], a rapid surgical decision, and an early antibiotic treatment, adapted to the clinical and microbiological situation. In our center, the microbiological investigations of patients with clinical suspicion of IE are systematically performed with a specific “diagnostic kit” for endocarditis, including 3 sets of blood cultures and detection of specific antibodies directed against C. burnetii, Bartonella species, Brucella species, Aspergillus species, Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae, a detection of the rheumatoid factor, anticardiolipin, and serum concentration of specific immunoglobulin E using pig epithelium. We systematically perform cultures, molecular detection methods, and histopathological analysis on the surgically excised valves. When results of first-rank tests are negative, we systematically perform molecular detection of C. burnetii, Bartonella species, T. whipplei, Mycoplasma species, Streptococcus mitis, Streptococcus gallolyticus, Enterococcus faecalis, Enterococcus faecium, Staphylococcus aureus from ethylenediaminetetraacetic acid blood, and Bartonella species by Western blot.
First-line empiric antibiotic protocols (Supplementary Table 10) are systematically prescribed, according to the microbiological and clinical situation: ceftriaxone and gentamicin are used in Streptococcus species IE, and ceftriaxone and amoxicillin are used in Enterococcus species IE, as recommended by the 2015 European Society of Cardiology guidelines. Clindamycin and co-trimoxazole are used for S. aureus IE, whatever their sensitivity to methicillin and the clinical situation (native or prosthetic valve) [26]. In IE due to coagulase-negative Staphylococcus, whatever their sensitivity to methicillin, we use vancomycin and gentamicin. In blood culture–negative endocarditis (BCNE) cases, an evaluation of the epidemiological factors, the history of prior infections including cardiovascular infections, exposure to antimicrobials, clinical course, severity, and extracardiac foci of infection are to be considered. However, since 2002 we have used standardized protocols: In community- acquired BCNE, we have prescribed 6 weeks of amoxicillin and 3 weeks of gentamicin, whereas in hospital-acquired BCNE, we have used 6 weeks of vancomycin and 3 weeks of gentamicin. If fever persisted after 48 hours of treatment, liposomal amphotericin B was added to the protocol.
A recent evaluation of these BCNE protocols showed an 87% adherence to the protocol (all deviations were justified) and a global fatality rate of 5.1%, which is low compared to the literature review [4]. Although they are different from the European Society of Cardiology and American Heart Association “consensual” guidelines, our “expert’s recipes” have proven to be easy to apply to the vast majority of IE cases, efficient, and well-adapted to the local conditions [3, 4, 26].
FECAL MICROBIOTA TRANSPLANTATION
Clostridium difficile Infections
While the first traces of fecal transplant date from the fourth century ce in China, used to treat patients ingesting poisoned food or having severe diarrhea [27], fecal microbiota transplantation has seen an extraordinary revival following the publication of the first randomized trial demonstrating the superiority of this technique in comparison to the use of antibiotics in recurrent C. difficile infections [28], and then the recommendations of the European Society of Clinical Microbiology and Infectious Diseases [29]. Contrary to the idea that it is an infection with a low mortality rate, from 2012 to 2015, the lethality rate of C. difficile infections has been evaluated in France as ranging from 17.2% to 17.9% whatever the causative ribotype [30], corresponding to approximately 1800 deaths each year. In 2013, during a C. difficile hypervirulent 027 ribotype regional outbreak, the observed mortality was >50% at 1 month, and almost 75% of the deaths occurred during the first week of evolution, which made the recommended strategy totally ineffective for most of our patients [10]. This led us to propose to perform fecal transplants from the first episode, as soon as possible and in any case no later than 7 days following the infection [10]. The nasogastric route was chosen because it was the easiest, and we prescribed concomitant antibiotics to reduce the bacterial load (Supplementary Materials). The results were spectacular, with a 5-fold reduction of the number of deaths [10]. About one-third of the patients, however, needed a second fecal transplant [10]. Beyond the 027 ribotype, fecal microbiota transplantation has also demonstrated efficiency in first intention in severe infection of C. difficile, whatever the ribotype [31]. Indeed, we also reported fecal microbiota transplantation performed by nasogastric transplantation for 2 patients for whom colectomy was considered [31]. Our experience demonstrated the feasibility and success of fecal microbiota transplantation in early stages of severe C. difficile infections. At the moment we cannot provide comparative randomized studies with conventional approaches, but the difference in mortality appeared so compelling that to propose randomized studies was considered unethical, as in other life-threatening diseases [32].
Gut Decolonization of Multidrug-Resistant Bacteria
The colonization by emerging highly resistant bacteria and, in particular, by those Enterobacteriaceae producing carbapenemases is a growing public health problem in France [33]. Hospital patient care, regulated by a report of the High Council for Public Health, includes reinforced isolation measures, as well as screening and cohorting of contact subjects, which is expensive and frequently unfeasible [33]. Treating by antibiotics such colonized patients in the objective of decolonizing the multidrug-resistant strain is not only harmful but totally unnecessary. In this context, we proposed fecal microbiota transplantation in an 82-year-old woman for management of a long-term carriage of OXA-48 carbapenemase-producing Klebsiella pneumoniae [11]. She received a bowel lavage associated with 4 successive administrations of colimycin (2.5 MIU) and gentamicin (100 mg) according to the strain and the antibiotic susceptibility testing results to reduce the bacterial load [11]. We then performed, by nasogastric route, a fecal microbiota transplantation prepared from a healthy anonymous donor after testing for the absence of pathogens according to French recommendations [34]. Since this case report, we have treated 4 other colonized patients by fecal microbiota transplantation. Overall, the outcome has been suitable in 4 of the 5 cases (80%), with a mean follow-up of 98 days (10–155 days) (unpublished data). Finally, 10 case reports, including our own case, were published describing the decolonization of multidrug-resistant bacteria including extended-spectrum β-lactamase–producing, carbapenemase-producing Enterobacteriaceae or vancomycin-resistant enterococci [35–37]. Large studies, including cost-effectiveness studies, are needed to definitively demonstrate the efficiency of fecal microbiota transplantation decolonization of antibiotic-resistant organisms.
Although simplified by using the nasogastric route and freezing the microbiota [38], fecal microbiota transplantation remains a complex process with limitations for some patients. Our preliminary unpublished in vitro results using optimized freeze-dried microbiota protocols are encouraging. To the best of our knowledge, only 1 case report was previously published on the efficient use of freeze-dried, capsulized fecal microbiota transplantation in a patient suffering from relapsing C. difficile infection [39], but this way must be the future prospect of this treatment.
STANDARDIZED MANAGEMENT OF INFECTIOUS DISEASES FROM EMERGENCY ROOMS
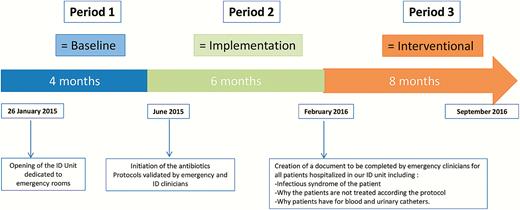
One of our ID units is exclusively dedicated to hospitalized patients originating from emergency rooms of our tertiary care hospital. The recruitment in this emergency acute ID unit is based on the presence of fever whatever the age, the supposed cause, and the underlying diseases. The standardized protocols were elaborated in a multidisciplinary approach following a review of evidence-based studies and using local bacterial resistance data. These protocols are available as part of the software used for drug prescription by the ID clinicians as well as by emergency room clinicians. One of our main concerns was to focus on the main infection syndromes detected in the emergency room, and also to promote a more comprehensive use of oral route of antibiotic administration rather than the intravenous route to avoid catheter-associated bloodstream infections (Supplementary Table 11). Since the beginning of 2015, 3 distinct periods have followed. Period 1 (baseline period) was a 4-month period starting at the opening of this unit until the establishment and acceptance of antibiotic protocols. Period 2 (implementation period) was an 8-month period starting from the initiation of the antibiotic protocols until the beginning of the interventional file. Finally, period 3 (interventional period) was an 8-month period starting with the creation of a compulsory document to be completed by emergency specialists and including justification for antibiotics, intravenous catheters, and urinary catheters (Supplementary Materials; Figure 1).
Baseline, implementation, and interventional periods. Abbreviation: ID, infectious diseases.
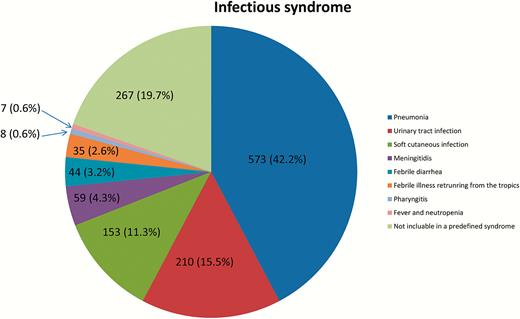
During our 20-month study, 1356 patients were hospitalized in our acute ID unit, including 281 patients in period 1 (21%), 544 in period 2 (40%), and 531 in period 3 (39%) with no difference in the main demographic characteristics during the 3 following periods (Table 1; Figure 1). The mortality rate ranged from 0.75% (period 3) to 1.4% (period 1) without any significant difference. Of the 1356 patients hospitalized in our ID unit, 1308 (96.4%) were hospitalized from the emergency room, 30 patients from intensive care units (2.2%), and 18 from diverse medical units. Overall, the mean duration of hospitalization was of 3.6 days (without significant difference between the 3 periods). Among the 1356 patients, 573 patients (42.2%) had pneumonia, 210 patients (15.5%) had a urinary tract infection, 153 patients (11.3%) had a soft cutaneous infection, 59 patients had meningeal syndrome (4.3%), 44 patients had febrile diarrhea (3.2%), 35 patients had febrile illness after they returned from the tropics (2.6%), 8 patients had a pharyngitis (0.6%), and 7 patients were febrile during neutropenia (0.6%) (Figure 2). Finally, 185 had a fever of unknown origin (13.6%), 36 patients had arthritis or osteitis (2.7%), 27 patients had eruptive fever (2%), 14 patients had febrile abdominal pain (1%), and 5 patients were hospitalized for another cause (0.4%).
Main Demographic Characteristics
| Characteristic . | Period 1 . | Period 2 . | Period 3 . |
|---|---|---|---|
| Baseline Period . | Implementation . | Interventional . | |
| Duration | 4 mo | 8 mo | 8 mo |
| No. of hospitalized patients | 281 | 544 | 531 |
| No. of male/female (sex ratio) | 171/110 (1.5) | 306/238 (1.3) | 304/227 (1.3) |
| Mean age, y | 63 | 64.9 | 61.8 |
| No. (%) of patients >85 y | 60 (21.3) | 117 (21.5) | 94 (17.7) |
| Hospitalization duration | 3.7 d | 3.7 d | 3.4 d |
| No. of deaths (mortality rate) | 4 (1.4%) | 6 (1.1%) | 4 (0.7%) |
| Characteristic . | Period 1 . | Period 2 . | Period 3 . |
|---|---|---|---|
| Baseline Period . | Implementation . | Interventional . | |
| Duration | 4 mo | 8 mo | 8 mo |
| No. of hospitalized patients | 281 | 544 | 531 |
| No. of male/female (sex ratio) | 171/110 (1.5) | 306/238 (1.3) | 304/227 (1.3) |
| Mean age, y | 63 | 64.9 | 61.8 |
| No. (%) of patients >85 y | 60 (21.3) | 117 (21.5) | 94 (17.7) |
| Hospitalization duration | 3.7 d | 3.7 d | 3.4 d |
| No. of deaths (mortality rate) | 4 (1.4%) | 6 (1.1%) | 4 (0.7%) |
Main Demographic Characteristics
| Characteristic . | Period 1 . | Period 2 . | Period 3 . |
|---|---|---|---|
| Baseline Period . | Implementation . | Interventional . | |
| Duration | 4 mo | 8 mo | 8 mo |
| No. of hospitalized patients | 281 | 544 | 531 |
| No. of male/female (sex ratio) | 171/110 (1.5) | 306/238 (1.3) | 304/227 (1.3) |
| Mean age, y | 63 | 64.9 | 61.8 |
| No. (%) of patients >85 y | 60 (21.3) | 117 (21.5) | 94 (17.7) |
| Hospitalization duration | 3.7 d | 3.7 d | 3.4 d |
| No. of deaths (mortality rate) | 4 (1.4%) | 6 (1.1%) | 4 (0.7%) |
| Characteristic . | Period 1 . | Period 2 . | Period 3 . |
|---|---|---|---|
| Baseline Period . | Implementation . | Interventional . | |
| Duration | 4 mo | 8 mo | 8 mo |
| No. of hospitalized patients | 281 | 544 | 531 |
| No. of male/female (sex ratio) | 171/110 (1.5) | 306/238 (1.3) | 304/227 (1.3) |
| Mean age, y | 63 | 64.9 | 61.8 |
| No. (%) of patients >85 y | 60 (21.3) | 117 (21.5) | 94 (17.7) |
| Hospitalization duration | 3.7 d | 3.7 d | 3.4 d |
| No. of deaths (mortality rate) | 4 (1.4%) | 6 (1.1%) | 4 (0.7%) |
Peripheral Intravenous and Urinary Tract Catheters
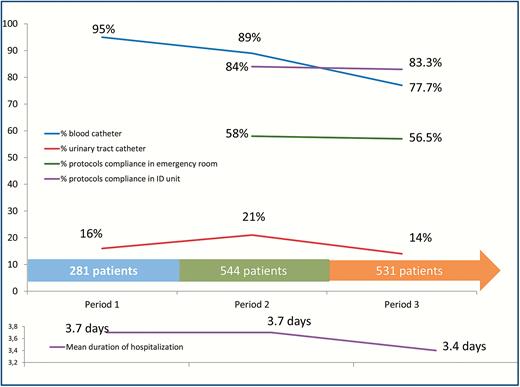
A total of 1167 patients (86.1%) arrived in our unit with a peripheral intravenous catheter. The proportion of patients hospitalized with a peripheral intravenous catheter decreased from period 1 (267/281 [95%]) to period 2 (487/544 [89.5%]) and period 3 (413/531 [77.8%]) (P < 10–6; (Supplementary Table 12; Figure 3). During the evaluable period (period 2 + period 3), the number of unnecessary blood catheters was 430 of 900 hospitalized patients (47.7%). The proportion of unnecessary blood catheters decreased significantly from period 2 (272/487 [55.8%]) to period 3 (158/413 [38.2%]) (P = .001; Supplementary Table 12). The number of unnecessary intravenous catheter was significantly higher overnight (307/609 [50.4%]) compared with patients hospitalized during the day (123/291 [42.2%]) (P = .02; Supplementary Table 13). Finally, among the 1167 patients with intravenous devices, peripheral intravenous catheters were removed in 659 cases (56.5%) during the first 24 hours of hospitalization (Supplementary Table 12).
Patient details across the baseline, implementation, and interventional periods.
Of the 1356 hospitalized patients, 235 had been given a urinary tract catheter (17.3%) (Table 3; Figure 3). The proportion of patients hospitalized with a urinary tract catheter decreased from period 1 and 2 (160/825 [19.4%]) to period 3 (75/531 [14.1%]) (P = .01; Supplementary Table 14). Among the 235 patients with a urinary catheter, the device was unnecessary in 52 cases (22.1%). The proportion of unnecessary urinary tract catheters decreased from period 1 and 2 (41/160 [25.6%]) to period 3 (11/75 [14.7%]) (P = .06) (Supplementary Table 14). The number of unnecessary urinary tract catheters was significantly higher in patients hospitalized overnight (33/144 [23%]) compared with patients hospitalized during the day (9/91 [10%]) (P < .01; Supplementary Table 13). Finally, urinary tract catheters were removed in 77 cases (32.7%) during the first 24 hours of hospitalization (Supplementary Table 14).
Protocol Compliance
Among the 1075 patients hospitalized during period 2 and period 3, 699 were hospitalized for one of the syndromes for which the protocols were established. Overall, the antibiotic protocols compliance in emergency rooms was observed for 403 patients (58.2%). From period 2 to period 3, the protocols were followed in 58% and 56.6%, respectively, of the cases in the emergency room. During period 3, the justifying management document was completed for 224 of 531 patients (42.2%). In our unit, the compliance was observed in 84.2% and 83.3% of the cases from period 2 to period 3, respectively (Figure 3). Among the 116 patients for whom the protocols were not followed, it was in 30 cases (25.8%) because of failure and in 29 cases (25%) because of contraindication (Supplementary Table 15).
Future Perspectives
The first lesson of our common program “from emergency room to ID unit” is that 80% of the patients with fever could be included in only 9 different and simple infectious syndromes. All these syndromes were treatable using a comfortable protocol including only 12 different antibiotic drugs, most of them (66%) by oral route, as recently recommended by the implementation of antibiotic stewardship program guidelines [40, 41]. We observed 56% of unnecessary catheters in period 2, this being close to the rate of 50% observed in a Australian tertiary emergency department [42]. Considering that S. aureus sepsis in our hospital has a lethality rate close to 20% [30], and that venous catheters are the major causes of these bacteremia, such a high proportion of unnecessary venous catheters is intolerable. We believe that the current priority to reduce healthcare infections is to monitor the use of unnecessary catheters [43, 44], and we observed an encouraging decrease from 56% to 38% during the last period. The same trend, though not significant, was observed for urinary tract catheters, and providing regular feedback to emergency room doctors about the high rate of unnecessary devices should be continued [12].
Despite the fact that our protocol was simple and established with a multidisciplinary local consensus, the level of compliance with our guidelines was lower than expected, notably in emergency rooms [45]. Probably only a limited number of well-trained physicians will assure the success of management-based approaches [3], but in our tertiary emergency department, 130 different physicians (including residents and medical doctors with various specialties) have a rotation in the emergency room and can prescribe overnight, compared with only 25 emergency specialists (residents and medical doctors) during the day. This can explain the lower overnight compliance that we observed. A significant higher overnight percentage of intravenous (P < 10–4) and urinary (P = .01) catheter prescriptions compared with the percentage by day was also observed (Supplementary Table 13). Beyond the number of prescribers, Goldstein et al have observed that the antibiotic stewardship team intervention rejection rate varied from 20% to 100% depending on the medical doctor’s specialty [6]. In addition, the behavior depends on individuals because 85.6% of the rejections of the antibiotic stewardship team proposals were from only 6 medical doctors including 3 ID doctors, 2 pneumologists, and 1 internist. This should lead to personalized efforts to increase compliance [6].
Role of Clinical Microbiology Laboratories
From our point of view, the clinical microbiology laboratories play a central role in optimizing the management of IDs. We followed for many years a technology-driven approach using, for example, mass spectrometry for colony identification, optimized quantitative PCR for diagnosis, or real-time genomics for isolate characterization [46, 47]. In addition, the management of IDs in our reference center was dramatically improved with the help of our point-of care (POC) laboratory [48]. POC laboratories, operating around the clock and 7 days a week, provide rapid diagnoses of ID within 2 hours, largely based on immunochromatography and real-time PCR tests [48]. In addition, these tests are combined into syndrome-based kits that facilitate sampling and modify rapidly the treatment management and the isolation of patients to reduce healthcare-associated infections [48]. Among the examples described above, the POC has played a central part in the management of the 027 ribotype C. difficile outbreak [10].
CONCLUSIONS
Through the standardization of our therapeutic protocols, our main objective for the management of infections in the IHU Méditerranée Infection is to require clinicians to follow established protocols including fighting against unnecessary catheters. From the initial expert’s protocols, we would generalize our standardized approach for the management of all ID patients hospitalized in our facility. To date, we only performed limited interventions in the scheduled management of main infection syndromes detected from emergency rooms. Increasing the communication and establishing a dialogue between ID units and emergency rooms, including personalized feedback for the clinicians working in emergency rooms, appears necessary to increase the compliance and to reduce unnecessary catheter use [6, 40]. Assessment of the real necessity of catheters needs to be permanent and should include nurses [49]. Finally, we should also focus our future efforts on overnight hospitalization and among non–emergency specialist physicians.
In conclusion, we have demonstrated through the examples discussed in this review that this pragmatic approach, followed over the course of 30 years, allowed us to reduce morbidity and mortality related to IDs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. We thank all the emergency physicians for their strong participation in this project. We thank Dr Frédérique Gouriet for reviewing the endocarditis section.
Financial support. This study was funded by Fondation Méditeranée Infection.
Supplement sponsorship. This article appears as part of the supplement “Emerging Concepts and Strategies in Clinical Microbiology and Infectious Diseases,” sponsored by IHU Méditerranée Infection.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
J.-C. L. and C. A. contributed equally to this work.
Correspondence: P. Parola, Unité de Recherche en Maladies Infectieuses et Tropicales Emergentes (URMITE), IHU Méditerranée Infection 19-21 Boulevard Jean Moulin, Marseille 13005, France (philippe.parola@univ-amu.fr).
- antibiotics
- bacterial endocarditis
- clostridium difficile infections
- communicable diseases
- drug administration routes
- emergency service, hospital
- feces
- hospitals, university
- id
- iduronate sulfatase
- q fever
- urinary tract
- infections
- bacteria
- morbidity
- mortality
- transplantation
- treatment outcome
- catheters
- microbial colonization
- tropheryma whipplei
- microbiome





Comments